Final report for FNE18-912
Project Information
Perennial groundcovers play a critical role in the forest ecosystem, yet are rarely employed on farms modeling ecological agricultural practices. This project evaluated the interspecies effects of three different treatments of woody and herbaceous perennial groundcovers as compared to a non-living woodchip mulch control in establishing a productive hazelnut (Corylus spp.) hedgerow system. Groundcovers were selected for low and spreading growth habits as well as their ability to accumulate nutrients, build soil and attract beneficial insects. Three different polyculture treatments and one control treatment were trialed in 30' blocks over 600' row feet beneath 200 newly planted hazelnut seedlings. Our results showed two of the treatments to be effective in reducing annual weed pressures and increasing soil health. The control performed best when measuring solely for hazelnut growth. We recommend that growers consider incorporating perennial groundcovers into their planting when striving for reduced labor costs and increased overall ecological health. This research was presented virtually at the 2021 NOFA Winter Conference.
The objective of our project is to determine if using herbaceous and woody perennial groundcovers during the establishment of hazelnut (Corylus spp.) shrubs in a hedgerow system will decrease weed pressure competition, improve soil health and impact the growth and nutrient composition of the hazelnuts. Over the course of the study we will observe the spreading rates and interspecies effects of plants selected for three different groundcover treatments: Soil building (S), Pollinator nectary (P), Nitrogen fixing (N) and a non-living mulch woodchip mulch control (C). We will also track growth rate of hazelnut canes as well as the soil organic matter and leaf tissue concentrations of nutrients (N, K, P, Ca, Mg, Fe, Cu, B, Mn, Z) over a four-year period. If a perennial living mulch can be established successfully, the need for annual application of other organic mulch would be nearly eliminated, greatly reducing the associated labor and costs of current hedgerow maintenance. We hypothesize that if well-selected for complimentary traits, these understory plants would function together to better accumulate and cycle nutrients, build soil organic matter, increase pollinator and predatory insect populations, and enhance overall ecosystem function and productivity.
Agriculture systems have evolved over thousands of years to maximize the size and productivity of a select number of edible plants for our consumption. The recent development of annual agriculture by means of regular soil tillage, monocropping, and chemical fertilizers has led to massive losses in the available nutrients and fertility of some of the most productive soils on the planet. Short term economic incentives and a lack of understanding around the biological soil food web has created a dearth of nutrients in our food crops and a host of pest and disease problems that increasingly must be managed with ever-stronger biocides and genetically modified organisms. Instead of continuing down the path of decreasing ecological health, many organic and alternative farmers are turning toward polycultural and diverse perennial deciduous agriculture systems to produce food in ways that restore and enhance soil health and ecological resilience.
There are many challenges in developing new systems of management in ways that will aid incremental transition efforts from annual to perennial agriculture; chief among these are concerns around establishment costs and comparatively lower yields during the establishment phase. Machine cultivation and harvesting are also more complicated in diverse perennial systems, requiring either more specialized equipment or more hand labor. Researchers at the Badgersett Research Farm are working to develop mechanical management options for large-scale commercial production of woody crops such as hazelnuts, chestnuts, and hickories for food, biomass, and oil production. However, there is still unexplored potential for smaller scale production of these crops in integrated orchards and farms.
The greatest challenge we have faced in establishing hazelnuts in a swale-and-berm hedgerow system is the need to manage and suppress the vigorous forest regrowth out of which the hedgerows were created. Weed management in hazelnut production is especially crucial for increasing nutrient availability, tree growth, and the efficiency of hand harvesting (Isık et al., 2014). One key principle of permaculture is the maxim, "the problem is the solution." In our case, the inability to mow with tractors provides an opportunity to explore alternative groundcover management practices that could lead to better overall health and productivity. Mulching with ramial woodchips has been our main strategy to date for covering the soil and maintaining a more fugally dominant soil microbial population. The limiting factor however has been the continued application of fresh woodchips for weed suppression in a large planting. We also seek to improve nutrient cycling and the structure and fertility of the soil to support a healthier, more diverse ecosystem and enhance crop growth and yields. Living mulches, cover crops, and perennial groundcovers have all been shown to positively impact these factors, yet knowledge on the integration of these management techniques in perennial systems is still insufficient for wider adaptation. More information is needed to better understand how intentional groupings or "guilds" of perennial plants can work together in a hedgerow system to achieve the goals of a holistic and more profitable management strategy.
Background:
Alternative groundcover management systems have been developed by a number of commercial orchardists seeking better organic weed control techniques that improve soil nutrients and fruit yields without the negative side effects of chemical herbicides (Lipecki and Berbeć, 1997)(Teasdale, 1996). Several studies suggest that planting cover crops as living mulch between orchard trees can greatly reduce weed density, the number of weed species, and total weed dry biomass (Isık et al., 2014)(Mennan, et al., 2006). Furthermore, the use of cover crops have been found to not only control weeds but also improve soil fertility, improve soil structure, and enhance crop growth and yield (Farooq et al., 2011).
Many different groundcover management systems have been studied in orchards including mechanical cultivation, sward strip-planting, and organic and synthetic mulches. In one study, natural (hay, wood chips, recycled paper pulp) and synthetic (polypropylene film and polyester fabric) mulches were compared with mechanical tillage and residual herbicides as orchard groundcover management systems in two New York apple orchards (Merwin, et al., 1995). The researchers found that soil concentrations of K, P, NO3 and Mg were greater in natural mulches. In another study, total soil N and C, C-to-N ratios, other essential plant nutrients, and overall apple tree growth were also greater in wood chip mulch after 16 years (Atucha et al., 2011). Others have also found groundcover management systems to have increased the species richness, abundance, and diversity (H′) of arthropods, even when used with frequent pesticide applications (Sirrine et al., 2008).
While these studies point to promising results - higher nutrients and healthier soils - by reducing the use of herbicide and tillage in perennial orchard systems and increasing the use of organic mulches and cover crops, less is known about the effects of establishing woody perennial groundcovers as part of a holistic groundcover management system. Much of this research is still preliminary, anecdotal, or theoretical, and has lacked significant enough data to warrant larger scale studies or trials. Some reasons may include the potential interference of woody perennials in mechanical management or harvesting on large scale commercial farms, or the assumption that perennial groundcovers may overly compete with primary trees and shrubs and be difficult to manage long term. However, there is some evidence that with careful selection, perennial groundcovers can significantly enhance the health of an orchard and reduce or nearly eliminate the need for the frequent cultivation, mowing, or mulching of conventional orchard management systems (Phillips, 2011).
While there is little research available on hazelnut hedgerow systems, the general principles of perennial design can be applied to assess plant compatibility and achieve certain results. The design of effective plant guilds draws primarily on observations made in naturally-occurring forest environments. A guild is defined as a "group of species with dissimilar community niches that form networks of mutual aid" (Jacke and Toensmeier, 2005). The functional interconnection between different species (for example, nut trees and squirrels) lead to systems of mutual support and interdependence, something that is observable in all diverse ecosystems. In addition, whenever multiple species occupy the same community niche and share the available resources, the resulting redundancy can help maintain the stability of the system and even use "more resources more fully by exploiting more niches" (Jacke and Toensmeier, 2005). For instance, a fruit and nut guild designed by Weiseman et. al. includes spearmint, wild ginger, and violets in the understory of pear, almond, and fig trees (Weiseman et. al., 2014). Ben Falk at Whole Systems Research Farm in VT favors comfrey, alliums, blue false indigo, and mushrooms beneath his fruit trees (Falk, 2013). Michael Phillips adds to that a combination of lovage, horseradish, daffodil, and lemon balm (Phillips, 2011).
At New Forest Farm in Wisconsin, Mark Shepard demonstrates how the productive potential of all niches in an ecosystem can lead to a very profitable enterprise. Even if the species in the guild are not all edible for humans, they can still perform a number of useful functions such as providing fodder, nectar, wildlife habitat, mulch, medicine, and essential oil and fixing nitrogen or accumulating other macro/micronutrients from the subsoil below (Weiseman et. al, 2014). Moreover, research suggests that the understory vegetation of a forest, despite its limited biomass, accounts for a disproportionately high percentage of nutrient concentrations and nutrient cycling, further highlighting their usefulness when incorporated into perennial agricultural systems (Muller, 1978). This suggests there is ample potential, in both health and in productivity, in studying the potential application of perennial groundcovers in a holistic hazelnut orchard management system.
References:
Atucha, A., Merwin, I. A., and Brown, M. (2011). "Long-term Effects of Four Groundcover Management Systems in an Apple Orchard." HortScience 46(8), 1176-1183.
Farooq, M., Jabran K., Cheema Z.A., Wahid A., Siddique K.H. (2011). "The role of allelopathy in agricultural pest management." Pest Management Science, 67, 493–506.
Isık, D., M. Dok, K. Ak, I. Macit, Z. Demir and H. Mennan (2014). "Use of Cover Crops for Weed Suppression in Hazelnut (Corylus avellana L.) in Turkey." Comm. Appl. Biol. Sci, Ghent University, 79(2), 105.
Lipecki, J. and Berbeć, S. (1997). "Soil management in perennial crops: orchards and hop gardens." Soil and Tillage Research, 43(1-2), 169-184
Mennan, H., Ngouajio, M., Isık, D., Kaya, E. (2006). "Effects of alternative management systems on weed populations in hazelnut (Corylus avellana L.)." Crop Protection, 25(8), 835-841.
Merwin, I.A. and Stiles, W.C. (1994). Orchard Groundcover Management Impacts on Apple Tree Growth and Yield, and Nutrient Availability and Uptake. Journal of the American Society for Horticultural Science, 119(2), 209-215
Merwin, I.A., D.A. Rosenberger, C.A. Engle, D.L. Rist and M. Fargione (1995). "Comparing Mulches, Herbicides, and Cultivation as Orchard Groundcover Management Systems." HortTechnology, 5(2), 151-158.
Muller, R. N. (1978). "The phenology, growth and ecosystem dynamics of Erythronium americanum in the northern hardwood forest." Ecological Monographs 48:1-20.
Oliveira, M.T. and Merwin, I.A. (2001). "Soil physical conditions in a New York orchard after eight years under different groundcover management systems." Plant and Soil, 234(2), 233-237.
Sirrine, J.R. , D.K. Letourneau , C. Shennan , D. Sirrine , R. Fouch , L. Jackson , A. Mages. (2008) "Impacts of groundcover management systems on yield, leaf nutrients, weeds, and arthropods of tart cherry in Michigan, USA." Agriculture, Ecosystems & Environment 125(1-4), 239-245.
Teasdale, J. R. (1996). "Contribution of Cover Crops to Weed Management in Sustainable Agricultural Systems." Journal of Production Agriculture, 9(4), 475-479.
Weisman, et. al (2014) Integrated forest gardening: the complete guide to polycultures and plate guilds in permaculture systems. Chelsea Green Publishing: White River Junction, VT. pp. 35, 219
Phillips, M. (2011) The holistic orchard: tree fruits and berries the biological way. Chelsea Green Publishing: White River Junction, VT. pp. 31, 107
Jacke, D. and Toensmeier, E. (2005). Edible forest gardens: ecological vision and theory for temperate climate permaculture, vol. 1. Chelsea Green Publishing: White River Junction, VT. pp. 149, 164
Falk, B. (2013). The resilient farm and homestead: an innovative permaculture and whole systems design approach. Chelsea Green Publishing: White River Junction, VT. p. 156
Nutwood Farm is a 7-acre ecologically integrated farm growing hazelnuts, chestnuts, walnuts, and other perennial edible tree crops in Cummington, MA. Our goal is to provide delicious, nutrient dense foods for our community while enhancing the fertility, diversity and resilience of the ecosystem we inhabit. We are passionate about helping to shift our local food system towards regenerative agriculture and working to develop long-term community food sovereignty and bold economic sufficiency in our community.
Nutwood Farm was established in 2015 by Sara Tower and Kalyan Uprichard. Sara holds a BA in Environmental Studies and brings her research and non-profit work experience to the project management role to supervise the budget, data collections, and outreach components. Prior to co-founding Nutwood Farm, she apprenticed on several vegetable farms and worked as a program coordinator for New Lands Farm, a refugee and immigrant farming project in Springfield and Worcester, MA, where she oversaw the community farm site and provided technical assistance to 40 new American farmers growing small market gardens. Kalyan previously worked as the building and grounds manager at Earthdance, an artist retreat center in Plainfield, MA, where he oversaw the 100-acre property including the orchard and gardens. He brings his extensive knowledge of diverse and native plant species and their propagation/cultivation needs to design, implement and maintain the trial plant guilds.
Cooperators
- - Technical Advisor (Researcher)
- (Educator and Researcher)
Research
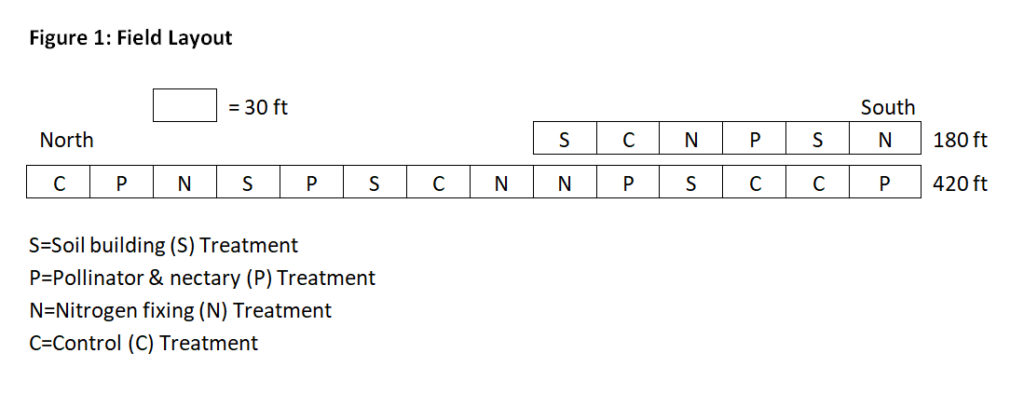
This project evaluated three different experimental perennial groundcover treatments (S, P, N) and one control (C). Each treatment was planted in 30 ft segments beneath 10 newly planted hazelnut seedlings spaced 3 feet apart. The four treatments were replicated 5 times in a random order for a total of 600 row ft. See Figure 1.
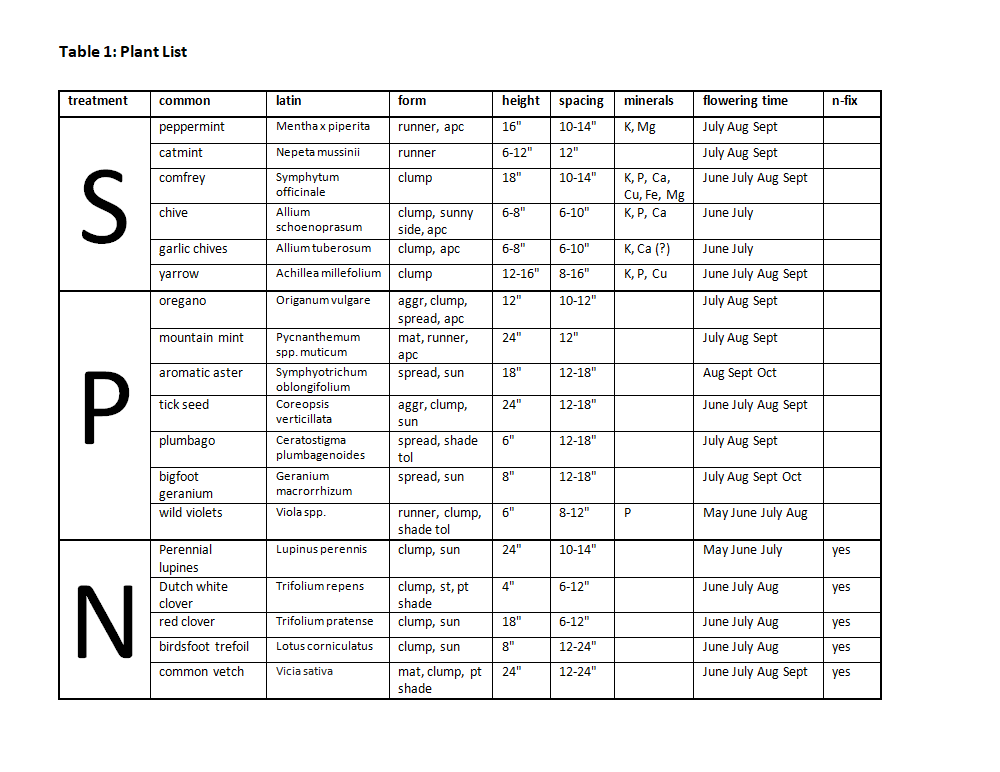
The groundcover plants used were selected based upon specific functions advantageous in perennial polycultures. For the two primary soil building and pollinator polycultures we narrowed down a much larger plant list using the following criteria:
- Not a sterile cultivar or overly hybridized for use in landscaping
- Relatively available and easy to acquire from a nursery at an affordable price
- Must be hardy to zone 5 at least.
- Anything planted from seed could not need more than 60 days of cold stratification (we only had 3 months before the warm growing season began)
- Preference for edible plants over similar functioning plants that were not edible i.e. peppermint vs. ajuga mint
Each polyculture contained a mix of types of groundcovers such as creeping, spreading, clumping, etc., as well as varying tolerance to sun, shade, wet and dry conditions. Other characteristics we took into account included form, height, nectar production, flowering time, insect habitat, nutrient accumulation and cycling, edible or medicinal qualities, and potential income generation. The final treatments were designed to maximize one of three goals: soil mineral accumulation (S), nectar production for native pollinators (P) and nitrogen fixation (N), all within the desired parameters of weed suppression and field management. See Table 1.
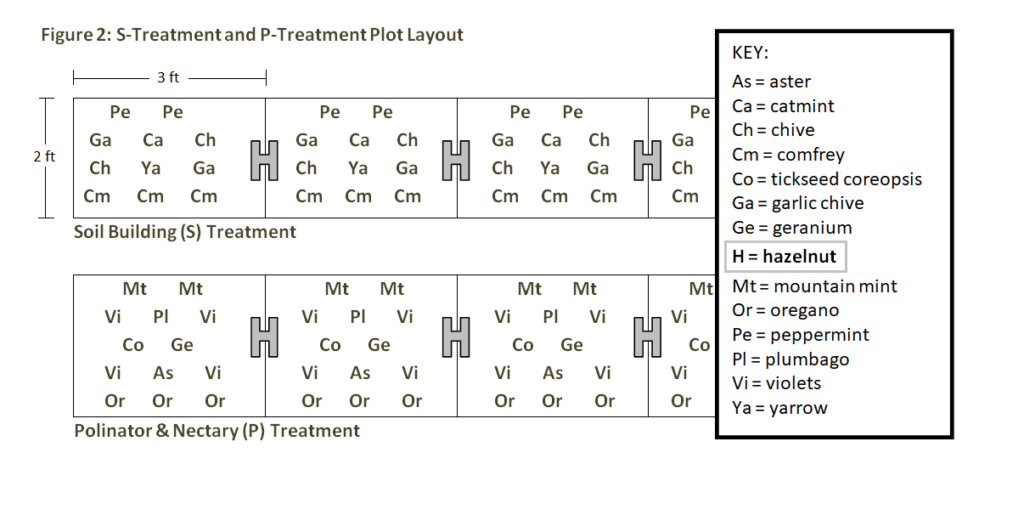
200 bare root seed-grown hybrid hazelnuts whips were planted in the study site on May 15th and 16th, 2018 and mulched with 2 inches of ramial woodchips. Over the next six weeks, we filled the treatment plots with the selected seeds and seedlings. The S- and P- treatments were planted with plugs according to a "pattern within a pattern," repeating an identical 3 ft block of plants 10 times over the length of each 30 ft treatment. See Figure 2. The nitrogen fixing (N) treatment was direct seeded with an even mixture of pre-soaked and inoculated species. The control (C) treatment was left covered with only the ramial woodchip mulch at a depth of 2 inches.
Measurements
Measurements were taken between May of 2018 and October of 2021. They consisted of a baseline soil test and annual fall soil tests, two leaf tissue analysis in August of 2019 and 2021, and annual summer field measurements to track hazelnut and groundcover growth.
Soil samples were collected at 6" depth in two areas within each treatment and combined by treatment type (S, P, N, C). Samples were dried for a day and sent to Logan Labs, LLC for assessment using Mehlich III analysis. Leaf tissue samples were collected on the four middle hazelnuts in each treatment plot and also combined by treatment type (S, P, N, C). Approximately 50 total leaves from each treatment were sent to the UMass and Penn State Leaf Tissue Labs for analysis.
In the field, the diameter of the leader cane at 4 inches from soil level and plant width (bird's eye) were measured on the four middle hazelnuts in each treatment to minimize crossover effects of adjacent treatment plots in comparing overall hazelnut growth. The spread and density of ground covers around each measured hazelnut were rated using whole integers on a scale of 1 to 5, 1 being minimal/ineffective and 5 being complete coverage/no weeds.
At the onset of our study, we also intended to collect data on the population (numbers and diversity) of pollinator insects in the study area using yellow sticky cards. However, this proved to be difficult to carry out with limited entomology expertise and some data was ultimately lost due to frequent summer rains.
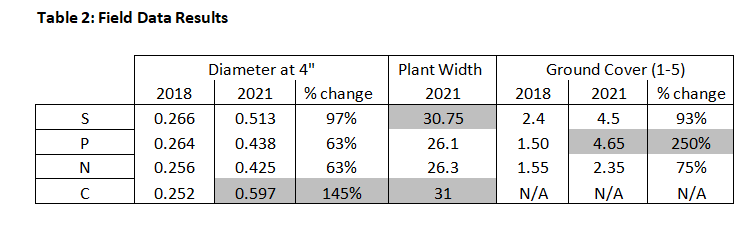
Our results demonstrated that perennial groundcovers can be very effective in reducing annual weed pressures, and may also improve soil heath and ecological function. Using the rating scale (1-5), the best overage ground coverage was observed in the P-treatment with a mean of 4.65 followed by the S-treatment with a mean of 4.5. See Table 2. Both S- and P-treatments developed dense clumps of woody perennials that effectively outcompeted annual weeds as well as many woody stump sprouts and brambles. In year 1, we spent 20 total person-hours hand weeding on five separate occasions to manage weeds in the treatment plots. By years 3 and 4 of the study, virtually no manual labor was needed to clear unwanted herbaceous competition from the S- and P-treatments.
In terms of cane diameter and plant width, the hazelnuts in C-treatment grew the fastest with a mean increase in diameter of 0.252 to 0.597 inches (145%) and mean plant width of 31 inches. The second fastest growth was found in the S-treatment with a mean increase in diameter of 0.266 to 0.513 inches (97%) and mean plant width of 30.75 inches. The P-treatment and N-treatment were tied with a mean increase in diameter of 63% and mean plant width of 26 inches. These results were not significantly altered by losses (6.2%) or dieback and resprouting (27.5%) in the hazelnut planting.
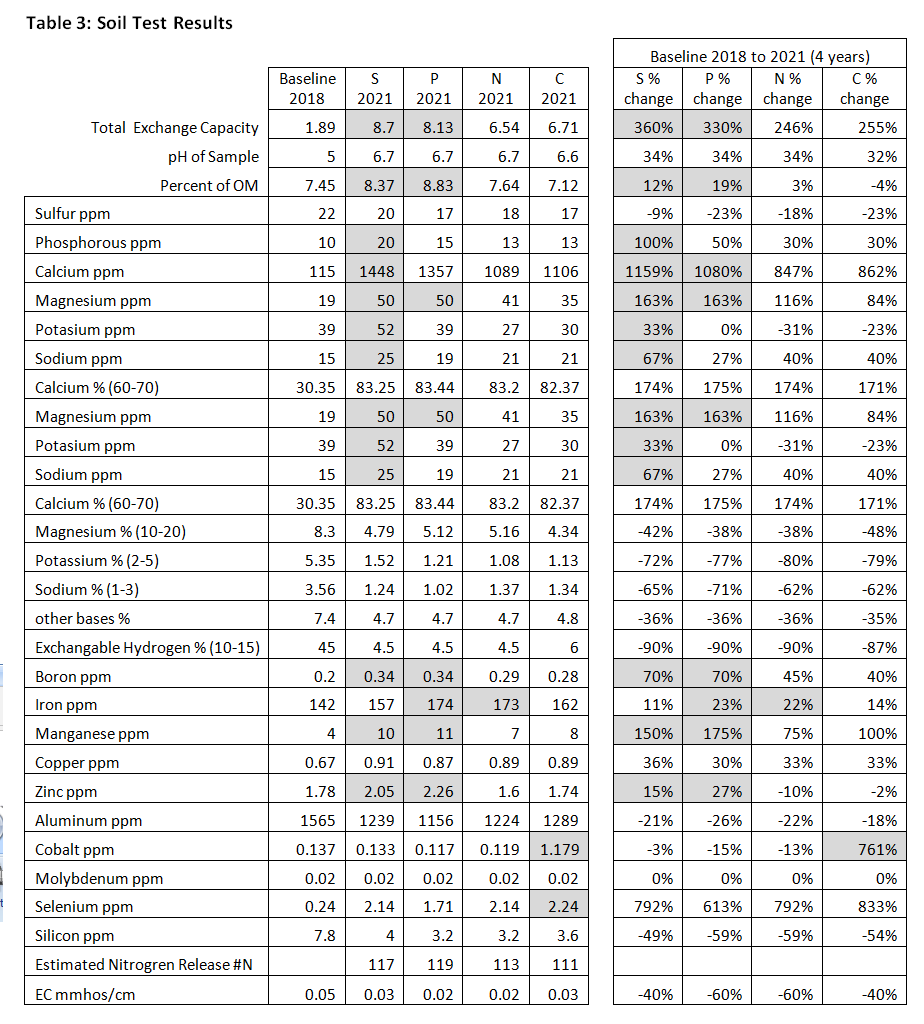
Soil and leaf tissue analyses showed the S- and P-treatments to have a moderately beneficial impact on soil and hazelnut plant health. Baseline soil tests in May of 2018 right after tilling and planting showed highly disturbed acidic soils with low nutrient availability and very low exchange capacity. We applied high-calcium lime evenly to all treatment plots in October 2018 to alkalize the soil, which succeeded in raising the pH of the study area from 5.0 to 6.7 in 2021. Final soil tests also showed an increase in percentage of soil organic matter in P from 7.45 to 8.83 (19%), followed by S (12%) and N (3%). The C-treatment actually decreased in soil organic matter by -4%. Similarly, cation exchange capacity (CEC) increased dramatically in the S-treatment from 1.89 meq/100g to 8.7 meq/100g (360%), followed by P (330%), C (255%) and N (246%). See Table 3.
Relative to the other treatments, soil the S-treatment increased the most in macronutrients (K, P, Ca, Mg) and trace minerals (Na, B). Soil in P showed moderate gains in some macronutrients (Ca, Mg) and several trace minerals (B, Fe, Mn, Zn). Soil in N gained fewer nutrients overall except for iron. Soil in the C-treatment did not show relative gains in any nutrient except for cobalt.
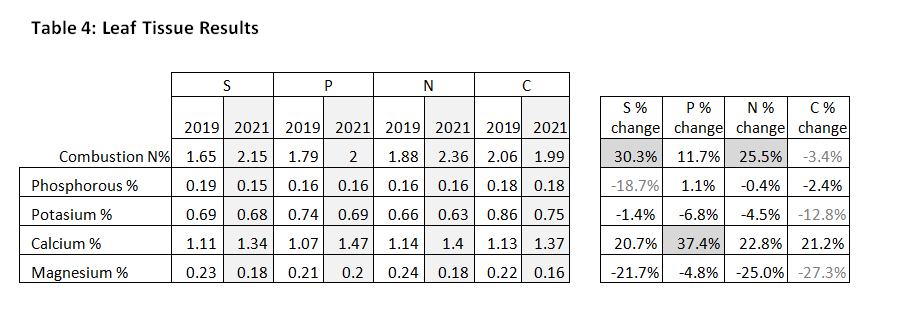
Leaf tissue tests give a snapshot of the nutrients immediately available to the hazelnuts. Between the August 2019 and August 2021 samples, we saw a 30% increase in available N in the S-treatment, comparing favorably to a 26% increase in available N in the N-treatment. Hazelnuts in the P-treatment had the highest overall availability of nutrients (Ca, Mn, Cu, and Zn). In the N-treatment, only copper showed a high relative increase of 53%. In the C-treatment, no elements showed relative increases and N, K, Mg, Mn, B all declined more than in any other treatment. See Table 4.
Discussion:
This study illustrated both the benefits and challenges of incorporating perennial polycultures in a productive farm system. Conventional concerns about eliminating competition to maximize yields may have some merit as hazelnut growth was strongest in the control treatment where all weeds were removed regularly throughout the growing season. However, the continual labor required for that method of field management may negate the benefits of promoting faster cane growth, which is not necessarily correlated with earlier or higher productivity. Even if it were, maximizing the growth of a single crop simply may not be the most important factor in designing more resilient farm systems. Other considerations such as managing for multiple yields to more rapidly increase soil health or generate multiple streams of income are examples of what is possible when designing a polycultural cropping system. As a primary crop, hazelnuts are a long-lived perennial reaching 10-15' in height that over time will shade out any root zone competition. They also do not begin bearing nuts until year 3 or 4, reaching maturity around year 8. Creating a perennial system that increases soil health while eliminating the need for manual, mechanical or chemical weed control and allows for a more hands-off approach in the establishment years may be incredibly advantageous, at least until perennial agriculture becomes more incentivized by market forces and integrated into the mainstream food supply.
In conducting our study, we used a management technique affectionately known as STUN or "sheer total utter neglect." This means we did not artificially irrigate or coddle any dying plants and instead let the forces of nature decide who would thrive. The plants that survived and thrived over the course of our 4-year study were: comfrey, yarrow, peppermint, chives, oregano, mountain mint, coreopsis, geranium, trefoil, lupines and vetch. The plants that did not survive were: catmint, garlic chives, aster, plumbago, and violet. Red and white clovers proved slow to spread and were ultimately overtaken by other weeds in years 3 and 4. Yarrow and mountain mint had a long flowering time and attracted many pollinators, but grew much taller than anticipated, at times closing canopy over the seedling hazelnuts. The lupins and vetch survived somewhat but did not spread or contribute much to the N-treatment. And, while effective, we may regret planting so much oregano In our field as it spreads quickly and forms a very dense and impenetrable root mat that is very difficult to remove.
Several of our findings from this study stood out to us as noteworthy. The first was that soil organic matter in the C-treatment actually decreased 4% from the baseline measurement. It is likely that additional applications of woodchip mulch in year 3 and 4 may have helped to protect and build more organic matter, although it would have added another significant input of field labor. The overall nutrient availability in the C-treatment was also lower than in the other treatments. Although hazelnut growth appeared to be strongest in the control, the S-treatment was a very close second and may have ultimately prevailed had the study continued for another few years. Finally, although we designed the N-treatment specifically to maximize N fixation, the S-treatment ultimately contained more available N in both soil matter and leaf tissues. This is a complex discovery that merits further investigation.
There were two major variables in our study that we expect had an impact on our results. The first was the site used in the study, which was broken into two uneven hedgerows in our newly cleared field, a Christmas tree farm that was logged and abandoned in the mid-1990s. Sloped terrain, sandy acidic soils, variable soil moisture, and two unprecedented seasons of heavy rainfall in 2018 and 2021 has made perennial tree crop establishment challenging, and we still have a lot of work to do to balance our soil mineral composition and biological health. The second major limitation was the hazelnut nursery stock used in the experiment, all of which were hybrid seedlings with highly diverse genetics. For this reason it is impossible to completely correlate hazelnut and groundcover growth to our experimental treatments.
Nevertheless, we feel that our observations were still valuable due to the significant lack of research in this area of inquiry. When we designed the groundcover treatments, we had little data on how each plant species interacted with others, or whether we would later come to regret the use of an "aggressive" groundcover in our field. Although labor intensive to design and plant each treatment, we came away with useful suggestions on a polyculture that we ourselves would use in future plantings to support overall soil health and minimize labor in field maintenance.
If rapid hazelnut growth and productivity is the only goal of the farm system, woodchip mulch may be the best field management method to suppress weed competition. However, it requires regular weeding and multiple reapplications of mulch which may be too labor intensive or expensive depending on the size and scale of the operation. Alternatively, a mixture of perennial groundcovers in the hedgerow system offers the opportunity to reduce overall labor in the establishment years while increasing soil health and ecological function. While hazelnut growth may be nominally impacted by the presence of other perennial root systems, there is evidence to support that such diversity may be beneficial to the hazelnuts through increased organic matter, soil exchange capacity, and nutrient cycling over the long term.
Based on our observations, we recommend using the following plants for further study in designing a hazelnut polyculture: comfrey, peppermint, coreopsis, geranium, and trefoil. We observed these groundcovers to be hardy, useful, and effective, and would be interested to track interspecies effects, measure growth and collect nutritional data for this treatment in a future planting.
Education & Outreach Activities and Participation Summary
Participation Summary:
We presented at the Northeast Organic Farming Association (NOFA Mass) Winter Conference 2020. Our virtual workshop was titled, "Growing Nuts: Land Management and Soil Building," and featured an overview of our establishment techniques and low-carbon maintenance of our perennial tree crops, and included a thorough report of our SARE-funded research to date. The workshop lasted 90 minutes and engaged around 40 virtual attendees. To obtain a recording of the workshop, please contact NOFA Mass, https://www.nofamass.org/.
We also hosted several farm visit/work days with local community members and university students including UMass Amherst, Smith College, and Greenfield Community College.
A research article for the Nutshell (published by the Northeast Nut Growers Association) is currently being submitted for publication in 2022.
Learning Outcomes
-
Soil testing and analysis
-
Leaf tissue sampling and analysis
-
Familiarity with groundcover species
-
Groundcover management
-
Soil building
-
Experimental design
-
Research presentation skills
Project Outcomes
This project gave us a unique opportunity to explore a concept that is not well understood in either conventional or organic no-till farming. Without taking on any significant risks, we were able to experiment with a number of different groundcover species and make real time observations about their habits and usefulness in applied perennial farm design. We now have a greater understanding of the ideal polyculture to use when establishing new hazelnut hedgerows and will share this information widely with aspiring new growers of this important perennial crop.
Over the course of our study, we greatly refined our experimental design and data collection systems to capture the most relevant information. We discovered half way through our study that some information, such as the number of root suckers, was not as indicative of hazelnut health as plant width. We also found that collecting and identifying insects to assess pollinator populations was extremely difficult and we were ultimately unable to include this element in our research. In general, we probably could have reduced the amount of data we collected annually to simply capture baseline and year 4 results. If we were to revise our methodology, we would likely wish to perform the same study using hazelnut cultivars (genetic clones) instead of seedlings. This limiting factor made it difficult to draw definite conclusions about the impact of different groundcover treatments on hazelnut growth. However, we do feel that we collected enough evidence to warrant further experimentation and research into the potential benefits of utilizing perennial groundcovers in orchard and hedgerow systems on overall soil and ecological health. Even small gains in soil organic matter may become critical adaptations for farms in an increasingly unstable climate. We certainly plan to integrate our chosen groundcovers into more areas of our farm and will continue to share our findings widely with other nut farmers and tree crop growers in the Northeast.
Information Products
- PASA Farm Innovations Poster 2022 (Fact Sheet)