Final report for FNE19-936
Project Information
This project sought to reduce algal biofouling on a shellfish farm through the use of crop shading. Two types of shades were deployed on oyster cages during the 2019 growing season. Shades sat between the cage pontoons, covering sun-facing surfaces. Panel-shades were constructed of opaque ABS plastic and sat above the waterline. Mesh shades were constructed from sheets cut from HDPE oyster bags. Mesh shades sat against the cage just below the waterline.
Each month after the outset of fouling, shades were removed and the percent of each cage covered with algae and invertebrates was calculated.
The opaque elevated-panel shades greatly reduced all categories of algal fouling over the course of the growing season, virtually eliminating fouling by green and brown algae (1% coverage or less) and reducing red-algae-fouling by over 50% compared to the control.
Outreach was conducted in-person with a co-op of farmers, neighbors, and aquaculture researchers, as well as online though farm specific social media channels.
This project sought to prevent macroalgal bio-fouling on floating oyster cages using two different shading techniques. The first technique used solid-panel shades elevated just above the surface of the water. The second used submerged mesh shades fastened to the sun-facing portions of oyster cages.
Success of these techniques was gauged by:
1) Amount (area) of algal bio-fouling on shaded cages compared to an untreated control
2) Amount of other (invertebrate, etc) bio-fouling on sun-facing surfaces
3) Species composition of fouling organisms
4) Qualitative difficulty of handling cages in each treatment
5) Durability of each treatment in the field
The fouling of aquaculture gear with non-crop species provides one of the greatest sources of labor on an oyster farm. Colonization of oyster farming equipment by algae and invertebrates reduces crop growth rates and can increase mortality. To address this problem many shellfish farmers have adopted floating cage designs that allow for periodic air drying as a fouling control. Though highly effective for controlling soft-bodied invertebrates (such as tunicates, worms, and larval forms of shellfish), it is much less useful for controlling macroalgae, which have evolved to tolerate periodic drying at low tides. Fouling by macroalgae can pose unique problems on a shellfish farm. It is quick-onset, fast growing, heavy/difficult to handle, resistant to common cleaning techniques, and can lead to the settlement of other fouling organisms.
Biofouling is commonly controlled with 1) Hand-removal, 2) Air-drying, 3) Mechanical removal (tumbling or pressure washing). Hand removal of fouling organisms is both incredibly slow and labor intensive. It involves brushing or scraping equipment and oysters and is only a viable anti-fouling strategy on the smallest of farms. Many species of macroalgae can quickly regrow from small remnants left behind from hand-cleaning.
Air drying is effective against certain pest species that are less tolerant to time out of the water than oysters. This is typically achieved in one of two ways: Growing-cages that can be flipped to hold oysters out of the water, or a dedicated dock / land-based processing area (both are expensive options). If done for too short a time, air drying is ineffectual. Drying for too long risks crop mortality along with pest mortality. Air drying is only effective against a subset of fouling organisms and often requires additional hand-cleaning or mechanical tumbling to remove hardier species- including many types of macroalgae, which have evolved to withstand periodic air-drying at low tides.
Mechanical tumbling using motorized equipment can be very effective at removing fouling organisms from oysters (though not from oyster bags or cages). Unfortunately, equipment is expensive, loud, and is costly to maintain. Most tumblers use gas engines/burn hydrocarbons. Noise from mechanical tumbling and pressure washing can create conflict with nearby land-owners and can disturb wildlife.
The natural distribution of many macroalgae are highly light dependent (with species tied to a specific depth and light-period). This project used cage-shading a means to prevent algal colonization of oyster cages by introducing dark conditions unfavorable to algal growth. Crop shading provided an environmentally-friendly, passive, and prophylactic approach to mitigating algal bio-fouling that has the potential to benefit all shellfish farmers using floating-cage systems, as well as other ocean-users and noise-sensitive wildlife.
I am the owner of Winnegance Oyster Farm, located on the New Meadows River in southern Maine. The farm is in its sixth season of operation. I began harvesting in September of 2015 and switched from part-time to full-time work at that point.
My farm is comprised of four limited-purpose aquaculture licenses (LPAs) encompassing a 1600 sqft footprint of state-owned subtidal waters. At the beginning of the 2019 season, I had approximately 140,000 oysters, 120,000 hard-clams, 25,000 bay scallops, and 1200 green sea urchins on the farm. Oysters reach maturity in a little over a year on my site.
I sell the bulk of my oysters through shellfish dealers. Total oyster sales in 2019 were approximately 71,000 pieces. I expect to have a similar number available to buyers in 2020, though demand and sales will likely be affected by the coronavirus pandemic. I anticipate ~15,000 clams reaching maturity over the course of the season (though numbers are unclear for experimental crops).
My educational and professional background is in environmental biology, though I have also worked in horticulture and the seafood industry. I received USDA SARE grants in past years- FNE15-848; studying tide-powered methods of cleaning and tumbling oysters and FNE16-877 / FNE17-901 exploring polyculture of littleneck clams and eastern oysters.
Cooperators
- - Technical Advisor (Educator)
Research
Two types of shades were constructed in May 2019 and were installed on the sun-facing portions of oyster-cages- between the pontoons, where the heaviest algal fouling is observed. Seven replicates of each design were tested. The position of each treatment on the farm was rotated after each sampling period to account-for/mitigate positional fouling patterns.
In the “elevated panel” treatment, frames were constructed from opaque sheets of ABS plastic. Each frame was fastened to an oyster cage with shock-cord. The frames sat on floating risers to ensure that the shades would sit above the surface regardless of cage-weight. This spacing will ensured adequate water/food flow to the oysters. The elevated shades are designed to prevent the onset of algal fouling.

In the “submerged mesh” treatment, shades were constructed out of HDPE mesh and were fastened directly to oyster cages with shock-cord. Depending on the load of the each cage, shades sat in 2-4 inches of seawater. The mesh allowed water-flow from the surface to the oysters below. Unlike the elevated shades, the shades in this treatment were not designed to prevent the colonization of algae. Instead, they were intended to collect fouling algae on a lightweight and easily removable substrate that shaded the cage below.

An untreated control treatment was tested over the same period.
All cages were manually cleaned and dried in the first half of May to provide a uniform starting point when shades were deployed on May 18. Fouling was measured on July 1, Aug 4, Sept 2, Oct 5, and Nov 9. The first measurement was delayed due to an unusually slow onset of spring fouling. Fouling was photographed during each measurement period.
Though Braun-Blanquet rapid cover estimates were initially proposed for this project, the wire grid of oyster cages provided a natural quadrat for more granular measurements (though this slowed field sampling). The percent cover figures reported in this project were calculated using this more intensive method. The same observer took all measurements.
Both experimental treatments and the untreated control group were subjected to normal farm operations (periodic air-drying to remove invertebrate pests and monthly thinning for harvest).
Results:
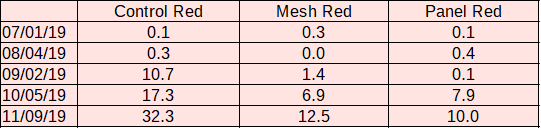
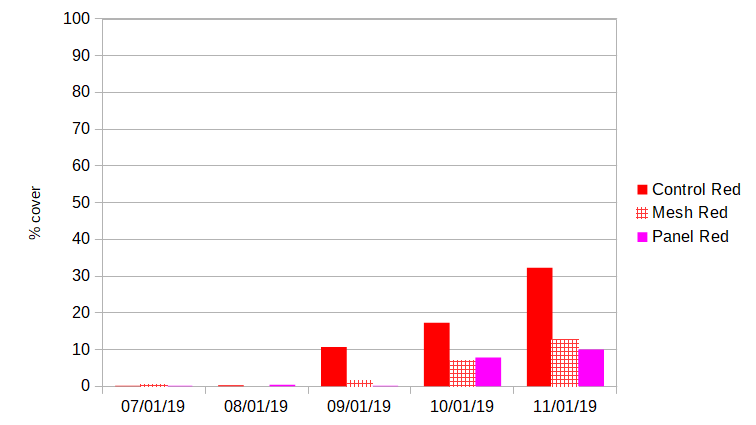
Red algae: An invasive fibrous red algae (Dasysiphonia japonica) began to grow on the farm in abundance in late August. Sun-facing surfaces across treatments saw less than 1% coverage by red algae until this point. By the Sept. 4 measurement, sun facing surfaces on control cages averaged 10.7% red algae coverage, while cages with mesh shades saw 1.4% coverage and panel shades saw 0.1%.
Red algae continued to flourish until the end of the oyster growing season. During the Oct. sampling period, red algae fouled 17.3 % of sun facing surfaces on control cages, 6.9% on mesh-shaded cages, and 7.9% on panel-shaded cages. In Nov. red algae fouled 32.3 % of sun facing surfaces on control cages, 12.5% on mesh-shaded cages, and 10.0% on panel-shaded cages.
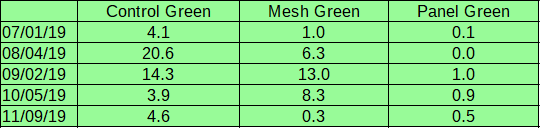
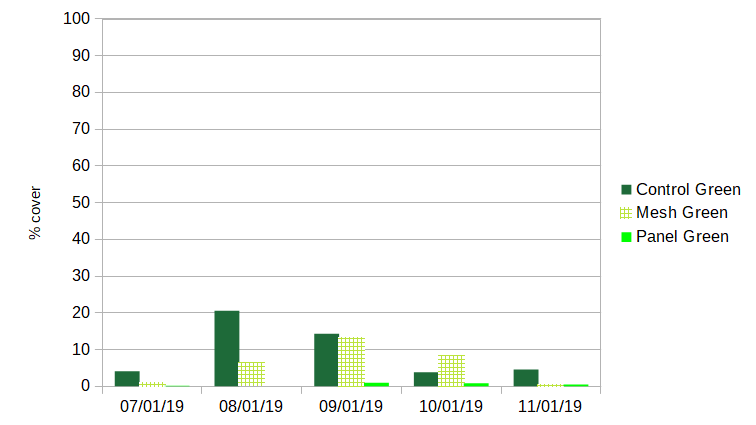
Green algae: Green algae was present on cages throughout the season. Two forms of the Ulva were consistently observed- fibrous and ribbon-like. An additional leaf-like sea-lettuce (Ulva lactuca) was observed during August sampling. In all sampling periods, panel shades strongly deterred fouling by green algae, with a maximum coverage of 1% (compared to 20.6% for control cages and 13.0% for cages with mesh shades).
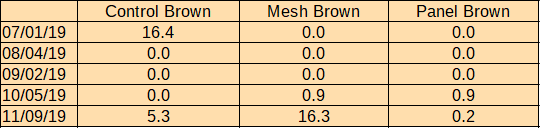
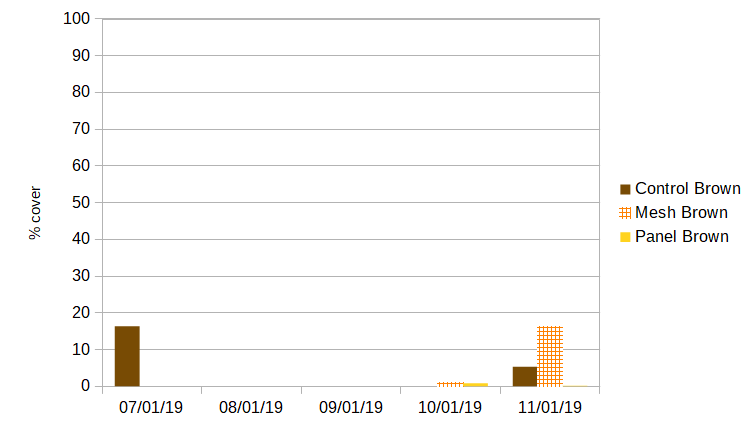
Brown algae: An invasive un-branched tube-like brown algae was present on all cages prior to the experiment. After manual removal and air-drying in May, it rebounded on control cages, covering 16.4% of sun-facing surfaces by the start of July. It was not present on shaded cages in either treatment during this period. This brown algae died-off across the farm in July. No new brown algae fouling was observed until mid Sept. During October measurements, small amounts of kelp were found fouling shaded treatments (.09% for both). Kelp and the invasive brown algae were observed during November measurements on all treatments- control 5.3%, mesh 16.3%, and panel 0.02%.
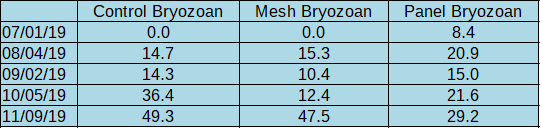
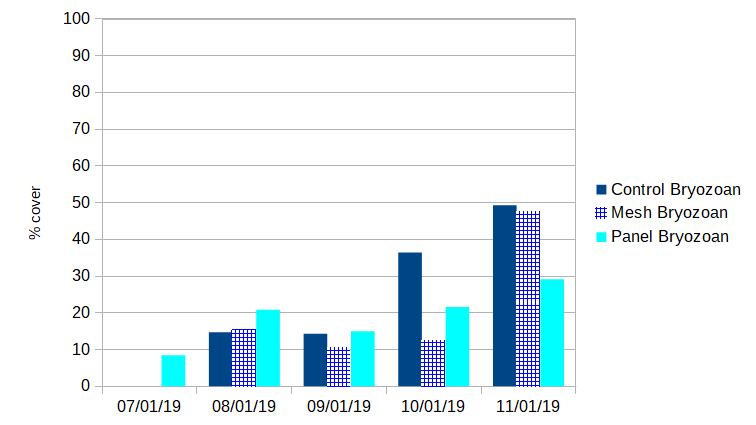
Invertebrates: Over the course of the shading experiment there were several invertebrate “sets” (hatches/colonization events). Normal air-drying practices prevented large infestations. A feathery bryozoan accounted for the vast majority of invertebrate fouling throughout the season. Though it first showed up under panel shades in July (8.4% coverage), it eventually colonized all treatments. It did not appear to flourish in the comparative absence of algae under shades. The bryozoans were easy to remove though air drying, though they reestablished each month.
Discussion:


Discussion:
Elevated-panel shades were effective at preventing all types of algal fouling throughout the growing season. The effects were most pronounced with green algae, which live highest in the intertidal zone and require the most light. The empty space below panel shades did not become a haven for problematic invertebrate species like mussels or sea-vases. The feathery bryozoans that did colonize this space during some months were easily controlled with a brief air-drying, and did not make oyster cages more difficult to handle.
Panel shades easy to handle, but the spacers used to keep them afloat were bulky- making them difficult to store on the boat on the rare occasions they needed to be removed from the field (major windstorms). Shades were relatively durable despite constant exposure to wind and waves. Over the course of the experiment one panel was lost during a major windstorm, and one required repair.
Removal of fouling by swapping-out mesh shades was only consistently effective with red algae (though this was by far the most problematic type of algal fouling in 2019). Green and brown algae were still able to colonize cages beneath mesh shades. Mesh shades did not increase fouling by hardy invertebrates (mussels, sea-vases, barnacles) and did not have the desired effect of displacing/chaffing-away bryozoans.


Mesh shades required more labor and boat/land space than panel shades. They needed to be dried or swapped out once they had collected algae. Heavily fouled mesh was heavy and unwieldy (though much less so than a fouled oyster cage). Mesh shades were very durable, with no loss or damage occurring over the course of the experiment.

This project sought to mitigate algal biofouling through the use of crop shading. Over the course of the growing season, shaded oyster cages were less fouled and easier to handle than more control cages. Invertebrates did not overtake the portion of cages kept clear of algae.
Panel shades performed better than mesh shades, though both treatments reduced reduced colonization of the fibrous red algae that accounted for the majority of added weight on oyster cages. Panel shades were also easier to handle, did not themselves foul, and did not require dedicated boat-space for drying.
Shading was clearly effective and I adopted its use on the rest of my farm. I deployed panel shades to prevent fouling on cages situated in fouling prone positions (end cages). Panel shades were also effective at killing/removing algae already on cages. This effect was noticeable after one week (though was more effective after two weeks).

Shaded cages provided a large time savings over manual algae removal, and made air-drying (for invertebrate removal) less labor intensive and more effective by reducing cage weight (lighter cages were easier to lift and sat higher above the water during drying).
Education & Outreach Activities and Participation Summary
Participation Summary:
Information was shared in person with members of an aquaculture co-op, farm-neighbors, and partners involved in other research projects. From these interactions, 22 individuals expressed interest in trying this technique on their own farms or aquaculture research sites.
Web/social media outreach was conducted throughout the run of the experiment on farm-specific accounts (followed largely by other farmers). Project related posts on Facebook and Instagram had 2275 "reads" and 349 "active-interactions" (likes, shares) between May and December.
Upon approval, the full report will be posted on the Winnegance Oyster Farm website- with links shared through social media and industry-specific list-serves. http://www.winneganceoysterfarm.com/
Learning Outcomes
Approximately forty-five farmers received in-depth descriptions of the experiment (awareness), with 22 saying they were interested in testing it on their own farms.
Project Outcomes
I've adopted the techniques developed in this project and have freely shared them with my peers. The techniques developed here have been useful in other unrelated research projects I'm involved with- reducing fouling of nursery-bags in a clam-oyster polyculture projects.
The technique has clear quality of life benefits, greatly reducing strenuous scrubbing and lifting.
The planned use of rapid Braun-Blanquet methods was unnecessary for the relatively small number of samples and a single observer. More intensive measurements were adopted to give higher data resolution.
Future iterations of the panel shades will be less bulky and more "stack-able" to improve ease of use. A less bright material would also be preferable to reduce visual impact (though no complaints were voiced about the experiment).
Panel shades were easier to use than mesh shades. They also did not require drying between deployments. Mesh shades would be useful to collect algae as food for other species, but panel shades were otherwise preferable.
Further research could address a number of topics:
-Changes in oyster growth and mortality in shaded cages
-Effects of different shade materials
-Usefulness of algae collected on mesh shades to herbivorous crops (snails, limpets, urchins, etc.)
This techniques developed in this project could benefit any shellfish farmers that use floating equipment and experience algal biofouling (across regions).