Final report for FNE21-977
Project Information
The purpose of this project was to determine if a broad-spectrum OMRI-certified insecticide can be used to control major pests of hops (including leafhoppers) to increase hop productivity without creating other pest management challenges or a reduction in beneficial arthropods. We used pyrethrin treatment in a one- and three-acre hopyard in several different regimes culminating in a final year of complete pyrethrin coverage. We sampled arthropods, plant biomass and yield, and leaf hopperburn to determine if spray regime or location (edge versus interior) impacted measures of hop production and management. There was evidence from all three measures that pyrethrin positively impacted management of hops pests and improved yield in hops, without resulting in other pest management issues. Thus, we will continue to use and evaluate pyrethrin to target pest populations and we advise other farmers to consider this approach. However, there is evidence that leafhoppers are able to rapidly re-populate small hopyards and potential evidence of selection for resistance to pyrethrin treatment. Thus, all applications of this approach should utilize arthropod sampling or scouting to monitor effectiveness.
This project sought to determine whether spraying Pyrethrin on hop field edges would reduce arthropod pest populations, while also minimizing negative impacts on beneficial predator species.
Objective 1) To determine if some hops pests (potato leaf hopper and damson hop aphid) establish at field edges first.
Objective 2) To determine if spraying a broad-spectrum lethal pesticide at hops field edges, in combination with less lethal insecticides throughout the whole field, can reduce populations of arthropods pests and prevent spread into the hop field interior.
Objective 3) To determine if this spatially adjusted insecticide application can minimize impact on beneficial predatory arthropods, and not lead to a spike in two-spotted spider mite populations.
Objective 4) To determine if hop yield is higher in subplots sprayed with pyganic vs. control and edge vs. interior.
To accomplish these objectives, we measured the number of pest and beneficial arthropods in each experimental condition. We hypothesized: 1) that spraying the field edge would reduce hops arthropod pests at the edge which would reduce their spread and establishment within the entire hopyard, and 2) that beneficial arthropods would be retained in the interior hopyard due to applying pyrethrin at the edges, limiting mortality to beneficials here only.
The most common and impactful arthropod pests on our hop (Humulus lupulus L.) farm are potato leafhopper (PLH), Empoasca fabae Harris, damson hop aphid (DHA), Phorodon humuli Schrank, and two-spotted spider mite (TSSM), Tetranychus urticae Koch. In high precipitation summers when downy mildew is our primary production challenge, these arthropods may have less impact. However, in drier summers these insects can exacerbate negative impacts already experienced by low precipitation. The spatial and temporal impact of these pests also varies within and across years. For example, while we saw high numbers of TSSM in 2018, they were nearly undetected in 2020, even though the extreme drought environmental conditions appeared very favorable to them. Secondly, we’ve also noticed that both PLH and DHA appear on the field edge first and that those plants show the telltale signs (e.g.leaf hopper burn) first. However, prior to this project we had not surveyed edge vs. interior, although the literature did corroborate this anecdotal observation (Emmen et al., 2004; Lorenzana et al, 2017). While TSSM do not appear to have the same ‘edge-first’ emergence pattern in a field, they can quickly increase population size following pyrethrin application, due to loss of beneficial TSSM predators. PLH temporality also depends on the prevailing weather and atmospheric conditions, since they are a migratory insect that appears in northern Maine during late June or early July. During 2020, PLH appeared to emerge earlier than prior years as an important pest, likely as impacted by the atmospheric conditions that brought significant southerly warm air to Maine from June 20 – June 30. As the record-setting dry summer continued, we entered ‘severe’ and then ‘extreme’ drought during the middle and later part of our season. Water stress, compounded by high populations of PLH, resulted in a 40% decline in hop yield compared to 2019. The impact of these arthropod pests is certainly exacerbated during drought years, but is also important in more average growing seasons. These are also issues faced by hop farmers across the Northeast (in fact, in Northern Maine we are likely 2-3 weeks behind others in the arrival of PLH).
As a certified-organic hops producer, our goal is to minimize the use of insecticides and maximize beneficial arthropod predator populations, which have been shown to be beneficial in controlling hop pests. We have used two insecticides to attempt to control arthropod pests (AzaMax and Trilogy), although we do not have experimental data on their effectiveness, and these products mainly suppress feeding or prevent molting. Another OMRI-certified product is pyganic, a Chrysanthemum-derived insecticide (e.g. PyGanic) that is broad-spectrum and has been shown to be effective on a wide range of arthropods. However, given its broad impact, it can also have a detrimental effect on beneficial arthropod predators. The consequence of using pyrethrin may in turn lead to a strong resurgence of arthropod pests that have a shorter generation interval than the predator species. Thus, prior to this project in our own hopyard we had only used pyrethrin during two seasons when absolutely necessary and, even then, at the end of the season when we would harvest before pests could reestablish.
If PLH and DHA are more likely to establish at field edges, then controlling them by spraying only in this zone could depress pest population growth while leaving a large area of refugia for beneficial insects and predators. This could potentially increase yield, reduce pesticide use, and retain beneficial predators. Thus, the secondary resurgence of pests that may occur post-pyrethrin application may be minimized while controlling the pest establishment at its source. By sampling both pest and predator populations, in both edge and interior areas of the field, and in pyrethrin vs. non-pyrethrin plots, our aim was to assess the impacts of a spatially explicit integrated pest management (IPM) approach. Further, by timing the first use of pyrethrin to the appearance of PLH, we can delay its potentially negative effects until PLH are present (and also well before we typically see aphids in any concerning level). If successful, this approach could result in minimized inputs and labor resources, maximized yield, and minimal impacts on beneficial arthropods. This solution of only spraying edges would also affect the economic threshold for these hop pests, considering that pyrethrin is a very expensive input, and its negative impact on beneficial arthropods could also result in a negative impact on yield.
Aroostook Hops LLC is the longest-established (est. 2009) and only USDA certified-organic hop farm in Maine (cert. 2014), run by a husband (Jason Johnston, Ph.D.) and wife (Krista Delahunty, M.Sc.) team. We grow 4-acres of five varieties of hops. We mainly pelletize our hops at our on-site processing facility, and package them in nitrogen-flushed Mylar bags. We sell our product to craft breweries, mostly located in Maine. We had the farm resources and equipment necessary to support the proposed activities for this project, mainly tractors and an air-blast sprayer. We have been leaders in researching sustainable organic hop growing practices and, with support from SARE, we have researched topics ranging from irrigation (FNE11-711), cover cropping and weed management (FNE12-742), and organic fertigation (FNE14-796).
Cooperators
- - Technical Advisor
Research
We have a 1-acre and a 3-acre hopyard with 4 varieties in each hopyard, with 5 varieties overall (Cascade, Centennial, Nugget, Willamette, Mt. Hood). As we considered experimental design, we weighed how to control for the experiment, while also accurately testing our hypothesis (with controls) versus spraying the entire edge. If edge spraying acts like a fence, then any unsprayed area may serve as an establishment and entry point. Thus, to test this possibility, for our 1-acre hopyard we sprayed the entire edge with pyrethrin, in order to compare edge vs. interior samples for arthropods, plant health, and yield. For our 3-acre hopyard, we established a randomized complete block design in order to test the effects of arthropod abundance in edge vs. interior and pyrethrin-sprayed vs. control(s). In order to accomplish this, we established spraying within 4 blocks of 250’ length across 5 rows of hops (60’ wide). Within each block we will randomly assign half the block as pyrethrin-sprayed and the other half will be a pyrethrin-control. Control treatments received water only, to control for the effect of the physical disruption of spraying that may cause PLH to relocate. The entire area of both fields were also sprayed with alternate applications of Trilogy or AzaMax. These are both neem-derived insecticides whose mode of action is anti-feedant or antivoltine (molt). These actions may help suppress population growth and have been widely used in organic operations such as ours, but are not generally lethal. We consider the pyrethrin treatment as additive, which is why we will spray AzaMax/Trilolgy in all rows/treatments as a baseline. Thus, we established five treatment combinations where we sampled arthropods, plant health, and yield. These include: pyrethrin (edge), pyrethrin (interior), pyrethrin-control (edge pyrethrin-control (interior), AzaMax/Trilogy (edge), and AzaMax/Trilogy (interior). The pyrethrin treated blocks are distributed north to south throughout our 500’ long field and span several varieties (Cascade, Centennial, Willamette, and Mt. Hood). Two of these blocks have lengths of rows located along edges (north and south), while two blocks are in the interior where edge samples are on the east or west end of the block, but the rest of the rows are within 'interior'. Thus, the design spanned our entire field and sampling at each cardinal direction. While the edges along our fields are not uniform, they consist of fallow field, forest, lawn or cropland (broccoli in 2021). Thus, there is minimized impact of adjacent crops that may either attract or trap PLH or other arthropods.
Insecticide applications were conducted beginning in late June or after the first emergence of PLH in 2021 and 2023, but in early June in 2023. We alternated AzaMax and Trilogy , and applied pyrethrin/water to treatment plots. Spraying occurred in the evening to reduce impact on beneficial insects as well as to minimize the photodegradation of pyrethrin (per the label). We used moderate to high amounts of insecticides per the label, i.e. AzaMax (2 quarts/50 gallons), Trilogy (1 gallon/50 gallons), and PyGanic (0.5 oz./gallon) utilizing our blast sprayer. The blast sprayer and tractor were calibrated and operated at a constant RPM to deliver consistent volume and range of spray. Based on past experience, our sprayer may spray up to 50 feet, but most spray hits plant or ground within 30 feet. However, given that potential drift, we planned our blocks to be 60 feet wide. This way we could spray on each side toward the interior of the block to ensure relatively even coverage within the block, and to prevent drift to outside of the block within the AzaMax/Trilogy control subplots. There was also non-treatment rows between each treatment block to act as a buffer.
Arthropod sampling was conducted through using sticky traps and leaf observations. Sticky traps were used to monitor beneficial and pest arthropods by placing them at edge (six feet from field edge) and interior (80 feet from field edge) locations six feet from the ground with the hop rows in between two bines. One sticky trap per treatment/block in both fields resulted in 20 sticky traps collected. Sticky traps were evaluated using a 10x-60x dissecting microscope with a mounted camera. Beneficial arthropod predators identified and quantified include Stethorus punctum, Anthocoridae, Geocoridae, Nabidae, Chrysopidae, Hemerobiidae, Coccinellidae, Syrphidae, Parasitica, and Araneae. Common hop pests (TSSM, PLH, total aphids, and DHA) were also counted.

In addition to sampling arthropods, we also assessed physical impacts of PLH on hops leaves, e.g. hopperburn. based on a modification of a scoring system used in alfalfa. We scored hopperburn according to the following ordinal score, where 0:no sign of leaf yellowing or necrosis, 1:<10% leaf yellowing, mainly at margins, 2:10-25% leaf yellowing, but no necrosis, 3:>25% leaf yellowing and <10% necrosis, 4:>25% leaf yellowing and >10% necrosis, and 5:>50% of leaf area is yellow or necrotic.
While we focused on arthropod abundance and hop plant effects as dependent variables for data analysis, we also measured yield on a systematically selected set of 6 bines per treatment, surrounding the sticky trap sample location. We were able to measure one bine at a time for both plant total biomass and hops cone yield for each treatment.
In 2023, we continued one more season of a subset of the experiment by conducting additional spraying and sticky trap measurement in three experimental rows of Cascade. The purpose was to gather another season's data on arthropod patterns, but also to start sampling earlier, since leafhoppers especially seemed to be appearing earlier than in the past and as conventional extension or MOFGA guidance had suggested. We sampled ten times from late May through early September, and applied five rounds of pyrethrin to these experimental rows (along with the rest of the yard).
This study was completed over three seasons since the variation in weather and thus arthropod abundance is stochastic, and thus one year may be very different from another. The third year of the study was completed in 2023. We collated all arthropod, hops leaf, and yield data to make comparisons across the treatment groups (including year as a factor) using general linear models and repeated measures analysis (JMP version 14 software).
We predicted that pyrethrin treatments would have lower pests and beneficial arthropods than the pyrethrin control (water); we predict that interior plots would have fewer PLH and aphids than edge plots but may not differ in beneficial arthropods. We also predict that interior AzaMax/Trilogy control plots will have more arthropod pests than pyrethrin edge subplots.
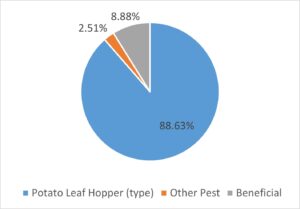
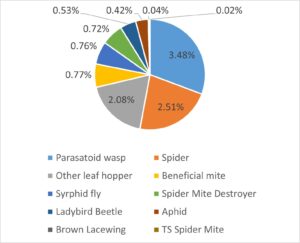
Overall, 5,294 arthropods (insects, spiders, and mites) were identified based on the targeted list of pest, predators and other beneficial arthropods. The vast majority of these (88.6%) were those assumed to be Potato Leafhopper (i.e. we labelled them ‘PLH type’). Beneficial arthropods represented 8.9% of the identified specimens, with parasatoid wasps and spiders as the top two categories of beneficials. While other pest species were detected there was a notable lack of aphids or two-spotted spider mites (TSSM) In our experience, anecdotally TSSM mainly become a problem during July and into August during a dry and hot summer. Aphids are often not a major problem, but may appear very late in the growing season just before and during harvest in September.
For many years we have assumed that PLH has been a major pest and we have had multiple samples identified through the local extension office. However, during this study we noticed some very early and very late leafhoppers, and deployed sticky traps to sample them. These emerged during early May well before the typical arrival of these migratory species in northern Maine, and were also found in late October well after southward migration. Through visiting UMaine extension’s insect diagnostician for a day with many samples, it was determined that the leafhoppers that occur throughout and after the entire growing season were likely not PLH, but a similar-appearing leafhopper in the same (revised) genus as PLH, Hebata. It is likely that this is not a migratory species, but one that overwinters as adults. Further samples have been sent to a leafhopper expert at the University of Illinois for positive species identification. This is an important finding that other farmers and extension specialists should consider when determining pest species of hops. However, the damage caused by these leafhoppers does not appear different from the ‘hopperburn’ associated with PLH. And, in fact since this species may become a pest on hops earlier in the growing season, it may be an even more serious pest than PLH.

Patterns of arthropod abundance revealed some of the hypothesized patterns, and allowed assessment of project outcomes, but overall did not show strong evidence of edge versus interior differences in leafhopper abundance or major differences in arthropod abundances based on pyrethrin treatment or control conditions. During 2021, the east yard which was sprayed along the entire perimeter only did not bear any differences in leafhopper abundance based on edge versus interior samples. There was a significant decline (p=0.0027) in leafhopper abundance by ordinal date, with fewer than half the count of leafhoppers in early August compared to late June and July. Similarly, the west yard had a significantly (p=0.001) lower count of leafhoppers as the season advanced, and also showed more leafhoppers in interior versus edge samples (p=0.045). For 2022, there were no patterns observed for leafhoppers in the East yard, but the west yard had more leafhoppers in pyrethrin treatment versus control (p=0.0023), interior versus edge (p=0.0004), and date, i.e. July greater than August (p=0.001). Higher abundance in interior as well as in pyrethrin treatments were contradictory to predictions. This may be due to a real contradiction to our predictions; alternately, it may actually indicate that there is more plant matter to feed on in those conditions. There were few patterns observed in beneficial arthropods counts – the only significant pattern was a higher abundance (p=0.0181) of beneficials in the west yard during 2021.
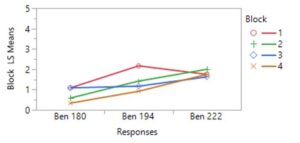
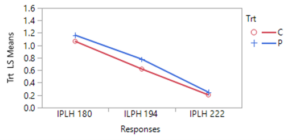
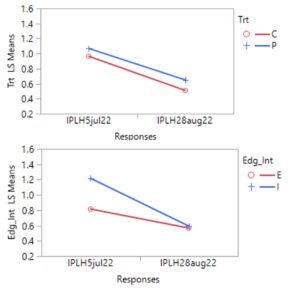
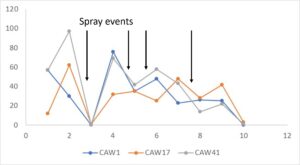
During 2023, more frequent applications of pyrethrin throughout the entire hops farm demonstrated the potential impact of this treatment on leafhopper abundance. Of four spray events that were closely connected to pre- and post- spraying sticky card collection, 3 events resulted in a significant (or nearly complete) reduction in leafhoppers, while one event led to little evidence of change. Patterns of beneficial arthropods varied similarly. However, this response may also suggest selection for resistant (to pyrethrin treatment) leafhoppers, since the first spray was so effective and subsequent spray events resulted in a lower magnitude or equivocal leafhopper abundance response.
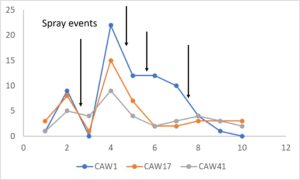
Hopperburn was monitored and then measured at the end of the growing season prior to harvest – generally there was extensive evidence of hopperburn, and damage typically is greatest at lower parts of the plant. During 2022, there was almost uniformly the highest level of hopperburn damage noted at the 4’-6’ sampling range across all varieties and treatments (see figure). However, during 2023 hopperburn values were much lower in general and did not differ by edge and interior locations.
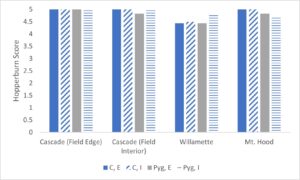
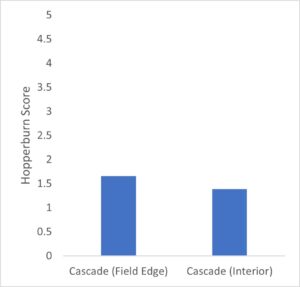
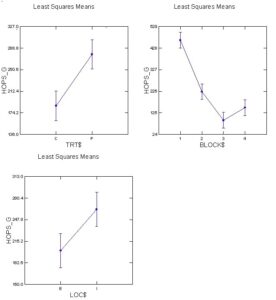
Positive effects of pyrethrin treatment were observed on several measures of hop productivity. Here we report the general patterns based on a general linear model analysis of hops plant mass (kg) and hops cone wet mass (g) from six plants per treatment. In general, the experimental blocks differed as expected with cascade blocks having higher mass of both cones and plant than other blocks (varieties). In some cases treatment with pyrethrin versus control was significant in favor of higher masses of hops and plants for pyrethrin treatment (2021), but was only marginally significant (p=0.054) for plant mass in 2022 and not significantly different for hops mass in 2022. Location (interior versus field edge) also differed consistently with interior plants and hops samples higher than exterior samples (p<.05 in 3 of 4 cases). In the second, 1-acre field where we sprayed the entire circumference in 2021 and sprayed the entire field in 2022, we found significant differences between varieties (Cascade > Nugget), no effect of year, and during both years both hops and plant masses were higher in edge versus interior samples. Given the differences in pyrethrin spraying patterns between yards opposing results for plant mass and yield by location (edge versus interior) may be related to pyrethrin treatment. Overall there multiple pieces of evidence that pyrethrin treatment has positive impacts on yield.
We sought to accomplish four main objectives, as summarized below with specific conclusions, followed by our broader conclusions and outcomes.
Objective 1) To determine if some hops pests (PLH and DHA) establish at field edges first.
In general, our sampling and analyses did not determine differences between edge and interior abundance of leafhoppers (and almost no aphids were detected). However, during 2023 our first sampling (1 sticky trap per row) had the highest abundance in one edge (n=97), second highest from an interior row (n=62) and least abundance (n=30) along an edge row adjacent to early successional forest. Following pyrethrin treatment there was only 0-1 leafhopper detected in these same plots. Three days later, sticky traps had the lowest in the interior row (n=32) followed by the two edge rows (n=76 and 69). This is the only evidence that leafhoppers establish at the edge first. However, given the small scale of our hopyard and the high mobility of this species, it may be that movement to optimal foraging habitat is rapid. In fact, the plant biomass and yield data (more detail below) provides evidence that interior plants produce more biomass and cone yield than edge plants.
Objective 2) To determine if spraying a broad-spectrum lethal pesticide at hops field edges, in combination with less lethal insecticides throughout the whole field, can reduce populations of arthropods pests and prevent spread into the hop field interior.
Based on either spraying a perimeter only of our 1 acre yard (2021) or spraying half of treatment blocks in the west yard (2021-2022), it is clear that a partial spraying approach does not substantially reduce populations in edge versus interior or pyrethrin treatment versus control. Any potential impact following pyrethrin treatment would likely be rapidly overcome by movement of unaffected individuals. This conclusion is supported by our 2023 experiment during which early treatment led to almost complete decline of leafhoppers in the three rows sampled. However, that this was followed by rapid resurgence of leafhoppers (as well as beneficials) suggests that there is an abundance of source population – which may be related to landscape factors, time of year, or arthropod movement patterns. Positive impacts of pyrethrin spraying on hops yield, plant biomass, and hopperburn provide corroborating evidence that pyrethrin spraying may have a positive impact on hops production, even though most evidence collected here (based on mainly mobile adults) did not find last impacts on leafhopper abundance.
Objective 3) To determine if this spatially adjusted insecticide application can minimize impact on beneficial predatory arthropods, and not lead to a spike in TSSM populations.
While we did find some evidence for a negative impact of pyrethrin spraying on beneficials, we did not generally find differences in beneficials by treatment versus control. However, overall the beneficial population was relatively small (about 10% of the leafhoppers). One general pattern across both 2021 and 2022 was a decline in overall leafhopper abundance but an increase in beneficials. Thus, while we may not have detected treatment effects, it may be that beneficials increasing through the growing season do relate to the decline of leafhoppers. During these three seasons, we detected very low abundance of TSSM (only one from all sticky cards), and little evidence of them during scouting. Thus, we did not experience a problem with TSSM, but this does not rule out this possibility.
Objective 4) To determine if hop yield is higher in subplots sprayed with Pyrethrin vs. control and edge vs. interior.
Both plant biomass and hops yield were significantly higher in pyrethrin versus control and in interior versus edge plants. This was a strong and significant result that supports the use of pyrethrin treatment to attain higher yield.
Overall, we found several lines of evidence that pyrethrin treatment can reduce leafhopper populations, even if only briefly, as well as positively impact hops yield. We did not find major evidence for a negative impact on beneficials, of a proliferation of two-spotted spider mites, or other unanticipated effects. Thus, we have concluded that pyrethrin is a useful tool in the management of leafhoppers (though we can’t say much about other pest species) on hops, and we expect to continue its use when it may be most effective at reducing pest populations (e.g. from early June through early July in our region). However, given the cost of the material, farmers should carefully consider the magnitude of the yield impact on their farm. While we did not see a major impact on beneficials, we are also a small hops farm with 1 and 3-acre hop yards surrounded by meadow, early successional forest and other habitats that may support beneficial arthropods. Thus, the results of our on-farm study may be different on a larger hop farm, or where adjacent habitats are other monoculture crops.
A major result of our study is that our farm is not impacted by the PLH but by a morphologically similar leafhopper in the genus Hebata that likely overwinters and thus appears much earlier than migratory leafhopper.
Education & Outreach Activities and Participation Summary
Participation Summary:
We created a one-page research summary to share on our website and any interested farmers, educators, or service providers SARE_AroostookHops_ShortReport_May2024. We shared this document with four faculty, extension professionals, and crop and conservation specialists in Maine and Vermont; we also encouraged them to share this document and that we could follow up with more detailed information. We hosted one tour in August, 2023 of several professionals from the Maine Organic Farmers and Gardeners Association, and discussed this project along with the broader challenges of organic hops production. We spent a half-day with a UMaine Extension entomologist reviewing a variety of specimens, with a focus on leafhopper identification. This led to interactions with two other entomologists (one undergrad at Delaware and one faculty in Illinois). We are open to more consultations or presentations since these results are likely not only of interest to hops production, but also to the broader issue of inseticides in organic production, as well as leafhopper management.
Learning Outcomes
Use of broad-spectrum insecticide in certified organic hops; management of leafhoppers generally; correct identification of leafhopper pest; potential impacts on beneficial arthropods of broad-spectrum insecticide.
Project Outcomes
Leafhoppers are a major pest of hops as well as many other crops, and managing these in certified organic production is fraught with concerns about effectiveness, impacts on beneficials, or exacerbation of other arthropod management issues. Through this project we have become more comfortable using pyrethrin in our context based on its impact on leafhoppers and hops growth and yield, while also not negatively impacting beneficial arthropods or resulting in increases in other arthropod pests.
We think it is a success that our project identified that potato leaf hopper is NOT the leafhopper pest we are dealing with. Other northern farmers should take note of this finding, and seek species-level identification of their predominant leafhopper pest species.
One key aspect of the project success was that we used different (and increasingly intensive) pyrethrin spray applications over three years. In the third year we used many more spray events flanked by pre- and post- sampling that revealed interesting and useful patterns about leafhopper abundance and impacts of pyrethrin. The overall approach of measuring arthropod abundance, hopperburn, plant biomass, and hops yield provided different scales of information - if we had not measured and found that plant biomass and yield is positively impacted by pyrethrin treatment, we may not have detected the positive impact of pyrethrin based on sticky trap monitoring alone. We did mostly answer the questions we set out to study, however given the stochasticity of pest issues we still can not be certain whether pyrethrin use may not contribute to a potential increase in aphids or two-spotted spider mites since neither were abundant in the three years of this study. We will continue to communicate and promote careful use of pyrethrins, along with monitoring pre- and post- treatment. Given the results of the 2023 intensive pyrethrin applications, i.e. that some applications did not greatly reduce leafhopper abundance, additional work may need to focus on potential selection of resistant individuals. These results would be of interest to hops farmers across North America, but also to any organic farmers would deal with leafhopper management issues or have used or contemplated using pyrethrin.