Progress report for FNE22-009
Project Information
We developed study parameters for 5 seed plots, and are refining templates for data collection and seed collection techniques, including best practices for viable native seed production. In the 2022 field season, we identified and collected ripe seed from robust wild populations of our 5 target species: Asclepias incarnata (swamp milkweed), Eupatorium perfoliatum (boneset), Eutrochium dubium (coastal Joe-Pye weed), Juncus tenuis (path rush), and Verbena hastata (Blue vervain). Our goal was to have genetic variation from a number of unique populations located within the Northeast for each species, to ensure a diverse locally adapted seed product. In 2023, we will propagate our wild seed-grown seedlings to landscape plugs and once rooted, install in the prepared foundation plots. Throughout the growing season we will record growth, care practices, seed development, and other relevant parameters. In fall 2023, we will collect seed, taking care to make several collections over the course of the seed production cycle to determine best practices. Seed will be evaluated for viability and germination tests will be conducted for each collection date. Data collection and seed collection will be replicated in 2024. In winter 2024/25 we will analyze our data, disseminate and publish our results in a digestible format. Through our study we will contribute best practices for native seed production for many stakeholders, including local farmers, community organizations, and nonprofit entities.
Plants grown from local ecotypic seed are genetically adapted to their environmental conditions, express resiliency in changing climates, and support a myriad of species that have coevolved in the landscape. To date, a number of native pollinators in the northeast U.S. have experienced dramatic population declines, including the federally endangered rusty patched bumble bee, yellow banded bumble bee, monarch butterfly, and several cuckoo bees (Forister et al. 2019). The main driver of insect declines is habitat loss and degradation, along with pesticides, invasive species, and climate change (Deutsch et al., 2008; Sánchez-Bayo & Wyckhuys, 2019). To halt and hopefully reverse observed declines, there is an urgent need to restore and enhance pollinator habitat (Hopwood 2008). Efforts cannot be limited to natural areas, but also need to include working lands, built landscapes, gardens, and roadsides.
Seed also offers genetic diversity as each plant grown from seed is genetically unique, buffering the resiliency of our gardens and landscapes. There are many native seed producers and programs throughout the country, but very few in the Northeast. Recent assessments of seed needs and the current status of the native seed market nationally (NASEM 2020), and in the northeast U.S. (Tangren & Toth 2020), solidify the need and successful growth potential of a native seed market in New England.
The demand for local ecotypic seed and plants grown from local ecotypic seed in the Northeast is growing rapidly. From grassroots community groups to large scale restoration projects to urban municipalities, the attention has turned to supporting native pollinators and ecosystem services. More and more nurseries are forming to meet the demands but the bottleneck remains with sourcing ecotypic seed (UNH Cooperative Extension article). Collection from wild populations alone cannot meet demand, and more importantly, not without inflicting stress and damage to the wild population. Our wild populations cannot bear the overharvesting of seed for ecotypic plant production on a large scale. We must incorporate innovative practices to produce sustainable, diverse ecotypic seed.
One elegant solution is the establishment of seed plots. Seed (or foundation) plots are cultivated rows or blocks of single straight species of native plants, grown from a diverse collection of wild collected local seed, for bulk seed production. Using seed from multiple populations of a species is shown to contain greater genetic diversity and may be more resilient to and adaptive under rapid climate change, with minimal risk of outbreeding depression in abundant species.
We will take a sustainable approach to the installation of our seed plots, using smothering techniques so as not to disturb soil structure and soil life. Plants will be planted, tended to, and harvested as seed matures, making sure to collect several times to capture the diversity of ripening seed. Seed will then be cleaned and stored appropriately according to species requirements. Data will be recorded at key points throughout the growing season to create a blueprint of best data management practices and to preserve genuine lineage.
We will explore production of several high demand herbaceous, perennial species such as Verbena hastata (blue vervain) and Schizachyrium scoparium (little bluestem) to determine best practices for bulk seed production, cleaning, storage and distribution. Local, sustainably produced ecotypic seed will be made available for use in production of genetically diverse native plants.
We aim to build upon the work of other groups in the northeast to test, document, and disperse best practice protocols for foundation plots to a wide variety of stakeholders, and create a standardized, easy to replicate model. Local farmers, land trusts, and other conservation or agricultural organizations will benefit from our research. Local farms can incorporate native seed foundation plots as a commercial crop to diversify their offerings as well as support beneficial insects and pollinators on their land. Land trusts and other land based conservation organizations can incorporate native seed plots to enrich their biodiversity and support wildlife and ecosystem services on their properties.
We will help to usher in a new period in native seed production by leveraging our proven leadership in the procurement, production, and safeguarding of plant materials, with a special focus on the collection and retention of genetic diversity and adaptation to local environmental conditions.
Cooperators
- - Technical Advisor
Research
Study Plot Design
Five foundation plots measuring 3’ wide by 80’ long were installed at Nasami Farm in a prepared area southeast of the building, on a south to north axis. The seed increase plots cover 1200 square feet total with each of the 5 beds holding ~ 200 plants per bed. Each 240 square foot seed increase plot represents one taxa, which allows for cross pollination and ensures a healthy seed set. We modified the design to integrate the Asclepias incarnata and the Verbena hastata plugs along a gradient to monitor and control pest pressure. Asclepias spp. often experience extensive pest damage from oliander aphid infestations. We designed a gradient planting of blocks of Asclepias incarnata and Verbena hastata into mixed blocks. The width of the beds takes into account the accessibility for maintenance and harvest by hand. Exact details and all work were documented, including diagrams and maps.
Site Preparation
In early August 2022, we prepared the site by removing invasive shrubs and mowing down existing vegetation. We covering the area with a combination of clear and opaque repurposed greenhouse poly to smother the vegetation without disturbing the soil structure or soil biota. We secured the poly with ground staples and cement blocks. In spring 2023 we removed the plastic and evaluated the site preparation. We found the area covered by clear plastic had successfully smothered the existing vegetation. The area covered by white plastic did not sufficiently smother the existing vegetation. We decided to mechanically remove the existing vegetation and level the area using a tractor with a box blade attachment, disturbing only the first few inches of soil. The plots were then demarcated and drip tape irrigation and landscape fabric were installed. Drip tape irrigation was installed in a matrix through each plot allowing for two distinct watering regimes based on taxa needs. Landscape fabric, a woven plastic fabric used to control weeds, was laid over the irrigation tape and staked down with washers and ground nails. The total area encompasses 1200 square feet for the plots with 3’ paths and borders between and around plots, for a total area of 3300 sq. ft.

Plug production
Wild seed of the five targeted taxa was collected from several robust populations throughout the northeast with the intent of capturing a large genetic diversity for each taxa. Data on seed has been recorded and tracked, including population size, location, date of collection, as well as seed viability, count, and weight. Each collection was assigned a unique code that will be used to track the individual population's traits. Seed were processed and stored according to taxa by trained staff at Nasami Farm, using a combination of seed cleaning techniques such as sieves, microscopes, winnows, and small hand instruments. Seed cleaning and storage procedures were recorded for each taxa. In mid-December 2022, seed were sown in 9” x 12” x 3” molded plastic seed flats filled with Sungro super fine germination mix® (SFGM), topped with a thin layer of SFGM and filtered sand. Seed flats were placed in an unheated greenhouse to undergo a cold stratification of >= 90 days to break dormancy. In spring of 2023, seed flats were moved into a heated greenhouse and germination was monitored and recorded. As seedlings sized up (true leaves appear), they were transplanted into Deep Sure Root ® 5” deep plug trays. Plants received regular care and maintenance which included regular application of organic fertilizer (Neptune’s ® fish fertilizer, 2-4-1) and beneficial insects as part of our Integrated Pest Management (IPM) plan. No herbicides or pesticides are used in our production. Plugs were well rooted within 6-8 weeks and ready for installation.
Installation & Maintenance
From August 1-15, seed-grown landscape plugs were installed by torching a planting hole into the fabric with a propane torch, with a spacing of 1 sq.ft on center. After an initial day of digging holes by hand with professional hori-hori knives proved difficult due to stones and rocks, a 2 in. x 16 in. auger drill attachment was purchased to complete the planting efficiently and with less manual labor. Plugs were watered in and monitored for health, establishment, and pollination. Staff conducted routine plant care throughout the season including weeding and pest management. As seed set, staff monitored for ripeness and viability, using hand lens, cut tests, and microscope work. Multiple collections, early, mid and late, were made to ensure physiological diversity. Qualitative and quantitative data was recorded for each collection. Harvesting of seed was done by hand, following established best practices. Seed were cleaned, processed, and stored at Nasami in our climate controlled seed storage room.

Data collection
Data was recorded throughout the collection, propagation, and installation processes to determine best practices and replicability. Variables such as germination rates, plant height, vigor, survivorship, planting date, and environmental conditions were recorded throughout the study. Recorded in tabular form allows for quantitative analysis, which will better inform our process as well as our ability to make recommendations for establishing successful native seed production using seed increase plots.
Identifying and coordinating seed collection for a variety of population sites proved time consuming, coupled with poor seed set due to environmental conditions, such as drought. Thankfully, we were able to collaborate with several other native plant conservation groups, Wild Seed Project in Maine, and the Ecotype project in Connecticut to secure additional collections.
The seed plot site preparation became a part of our 2022 intern Penelope Rose's project. She conducted research into our study and prepared the site by removing invasive and wood vegetation with a weed wrench, mowing and flagging the area, and with additional help from nursery staff, securing sheets of greenhouse poly to smother the vegetation without disturbing the soil. The site is in a highly visible location close to our retail shopping pad and garnered lots of questions and discussion- not only about seed plots, but no-till site preparation as well.
In December of 2022 we sowed seed from our wild or ecotypic seed for each of the 5 taxa. For each taxa we had 3 distinct collections, except for Eupatorium perfoliatum for which we had 4 distinct collections. In spring 2023 we transplanted germinated seedlings into landscape plugs with a goal of 250 plants per species. Final counts differed due to survivorship, germination, and spacing constraints. In August 2023, our total plantings for each taxa were:
Asclepias incarnata 246
Eupatorium perfoliatum 234
Eutrochium dubium 237
Juncus tenuis 249
Verbena hastata 250
By collections:
| Species | Collection | County | Ecoregion | Quantity planted |
| Asclepias incarnata | 2022-0276-E | Fairfield Cty, CT | 59 | 100 |
| Asclepias incarnata | 2022-0203-W | Franklin Cty, MA | 59 | 99 |
| Asclepias incarnata | 2021-0150-W | Hampshire Cty, MA | 59 | 48 |
| Eupatorium perfoliatum | 2022-0167-W | Franklin Cty, MA | 59 | 36 |
| Eupatorium perfoliatum | 2022-0193-W | Barnstable Cty, MA | 84 | 99 |
| Eupatorium perfoliatum | 2022-0291-W | Cumberland Cty, ME | 59f | 48 |
| Eupatorium perfoliatum | 2022-0169-W | Hampshire Cty, MA | 59a | 51 |
| Eutrochium dubium | 2022-0198-W | Barnstable Cty, MA | 84 | 93 |
| Eutrochium dubium | 2022-0194-W | Newport, RI | 59 | 47 |
| Eutrochium dubium | 2022-0292-W | York Cty, ME | 59f | 97 |
| Juncus tenuis | 2022-0110-P | Franklin Cty, MA ecotype | 59 | 99 |
| Juncus tenuis | 2022-0192-W | Barnstable Cty, MA | 84 | 51 |
| Juncus tenuis | 2022-0197-W | Barnstable Cty, MA | 84 | 99 |
| Verbena hastata | 2021-0309-W | Worcester Cty, MA (NWS) | 59 | 100 |
| Verbena hastata | 2021-0195-P | Suffolk Cty, MA ecotype | 59f | 100 |
| Verbena hastata | 2022-0161-W | Hampshire Cty, MA | 59 |
50 |
We saw pest pressure from oliander aphids on Asclepias incarnata in late August- September, and herbivory from monarch (Danaus plexippus) caterpillars. As our plugs were still young and not established, we carefully removed the monarch larvae and relocated them to established Asclepias spp. nearby. We then treated aphid infested Asclepias incarnata plugs with Dr. Brommer's soap at a dilution of 1 Tbsp.: 32 oz. water on two occasions. The applications were done with a backpack sprayer with a drenching nozzle at the end of the day.
We also saw pest pressure along the eastern edge of the plots, closest to the open meadow. A number of Eutrochium dubium were predated by cottontails. We lost a total of 7 original plants but were able to replace them with existing surplus seedlings of corresponding collections. We then applied several applications of chili mixture (3 Tbsp. chili; 1 gallon water; 1 tsp. dish soap as surfectant) at end of day when no rain was forecast to deter predation.
To determine change and rates of change in genetic makeup in the target plant populations over time and across species, we plan to conduct genetic analysis on our baseline wild seed and plants, as well as subsequent generations. This will help inform growers when to replenish stock for renewed diversity and resilience.
For this effort, in early September, we collected leaf tissue samples for later genetic analysis (funding dependent) from each of the unique wild collections. One to several leaves were collected (aim of 3x3 cm of material) per plant and placed in a small labeled glassine envelope. Random leaf samples were made from 20 plants of each collection for the 5 taxa and sent to our research botanist, Dr. Jessa Finch for documentation and storage. For seed, we reserved 50-100 randomly selected seed from each original collection.
Three of our five species produced seed in this first season of our seed increase plots. We made at least 3 timed collections, early, mid- and late season to ensure physiological diversity. These first generation seeds were given a unique code to represent the new population created by the cross pollination of the wild collections:
| Species | collection code | collection dates | weight | viability |
| Eupatorium perfoliatum | 2023-5174-E (G-1) | 10/19/2023 | 0.88g | 10 of 10 good |
| Juncus tenuis | 2023-5250-E (G-1) | 8/29, 9/15, 11/3 | 13.37g | good viability, consistent |
| Verbena hastata | 2023-5174-E (G-1) | 10/5/2024; 10/19/2024; 11/3/2023 |
20.91g | 10 of 10 good |
Seeds will be stored for future use and a sample of these seeds will be sent for genetic testing following the above protocols.
In 2024, we monitored and recorded plant survivorship, height and vigor, as well as phenological information such as budding, bloom time and seed set. We noticed phenological differences across ecotypes within species for Eupatorium perfoliatum and Eutrochium dubium. Maine ecoptypes for both species bloomed and set seed earlier than the Massachusetts, Rhode Island, and Connecticut lineages, although they all had overlap in bloom times which allowed for cross pollination. Our goal is to create a northeast mixed genetic species with greater plasticity for resiliency throughout the region. Overall the plants grew strong, a benefit of growing to plug size in the greenhouses first. We had a wet spring and did not utilize the irrigation soaker hoses until mid-summer when precipitation receded. We did utilize the soaker hoses through most of the remaining summer due to drought like conditions, as the plants were in their first year of establishment.
We began collecting seed in July with our Juncus tenuis seed, and continued right through late November. We made weekly to bi-weekly collections to capture the genetic diversity of early to mid to late ripening seed. We have begun processing the G-1 seed for storage, exact quantities for the 2024 season will be tabulated and presented in the final report. We continued to see aphid damage to our Asclepias incarnata plants, despite our interplanting with Verbena hastata. In addition, we found the Verbena hastata grew tall and earlier than the later emerging Asclepias incarnata and may have impacted their growth through shading. We also saw herbivory of the Asclepias incarnata by monarch caterpillars (their host plant) so we chose not to treat the plants with castille soap, instead we mechanically hand-squished aphids.
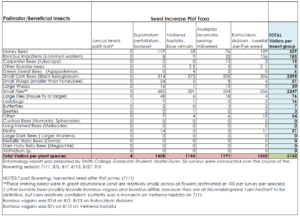
We were also interested in collecting data on pollinator and beneficial insect interactions within our native plant seed production plots to highlight the additional benefits of pest management and pollinator services accompanying native plant production. An entomology report was prepared by Smith College Graduate Student, Mariel Dunn. Six surveys were conducted over the course of the flowering season: 7/11, 8/2, 8/7, 8/13, 8/27, and 9/5.
Nasami Ecotype data sheet_MDunn.xlsx - Total_Table
Education & Outreach Activities and Participation Summary
Participation Summary:
We began working with consultant Matt Garrambone of Beechwood Environmental, Inc. Matt is assisting us with all aspects of our seed processing facility and contributes to our seed increase plot program. In November (11/8) we held our first sold out workshop on native seed plot production with 28 participants. Participants ranged from established nurseries to local farmers to interested landowners and managers. We discussed the need and demand for local ecotypic native seed, proper seed collection and breeding protocols, and seed plot design and installation. We finished the workshop with seed cleaning demonstrations. We plan to conduct 1-2 more workshops in the coming months as the interest was so great.
Information on our workshop was shared on social media and in our monthly newsletter.