Progress report for FNE25-105
Project Information
Thirty two packages of honey bees consisting of about 10,000 bees and a newly mated queen will be obtained from Gardner Apiaries, Baxley Georgia, a commercial package bee supplier. The packages will be divided into two groups of 16. Bees in the control group will be allowed to replace the package queen. In the experimental group, all attempts at queen replacement in the first five weeks will be thwarted (queen cells observed will be cut out, preventing the replacement of the packaged queen).
The primary end points measured will be
- the number of package queens still in their hives at five weeks after package installation
- the number of hives in each group surviving at the beginning of October (data collected across time and shown in a survival curve; end point for statistical analysis will be at the beginning of October).
Secondary end points will be
- the number of queen cells made in each colony (control and experimental) during the first 5 weeks after installation
- the “success” of surviving colonies (as shown by area of brood, population of bees, number of frames honey comb built)
How new beekeepers start a hive
With the development of colony collapse disorder in 2006 and the resulting nationwide attention it brought to the importance of honey bees for pollination, the number of people interested in becoming beekeepers increased dramatically. State wide, the number of registered beekeepers in Pennsylvania increased from 2010 in 2006 to approximately 6500 in 2023 (personal communication, Karen Roccasecca, PA State Apiarist). Locally, the Montgomery County (of PA) Beekeepers Association saw its beginner beekeeping class participant numbers rise from 10 to 100 after the public became aware of colony collapse disorder.
The most common way to start beekeeping is through the purchase of a package of honey bees consisting of about 10,000 worker honey bees in a wire mesh enclosed box with a new queen contained in a small cage that is located within the worker bee cluster (See Appendix A for photos of each of these.). In 2013 there were at least 23 package producers in the U.S. (https://www.beesource.com/threads/bee-oueens-and-package-provider-list.218154/). The number of packages produced in California, Georgia and other states is not tracked, but one producer, Wilbanks Apiaries, Inc., states that they produce 15,000 to 20,000 packages per year (https://farmflavor.com/georgia/georgia-crops-livestock/bees-knees-georgia/). Additionally, Gardner Apiaries may distribute 50,000 packages annually. This large number of packages indicates that thousands of beekeepers are purchasing packages to either replace winter losses or to start new colonies.
The queen enclosed in the package is not the mother of the worker honey bees but is completely unrelated to the bees in the package. Because of that, the worker bees in the package would immediately kill her if she was accessible to them, making it necessary to sequester the queen in her separate cage (See Appendix A) for several days before the worker bees will consider her “their” queen. (The queen cage is within the cluster of the package of bees, thus allowing the packaged bees to become familiar with her.)
Once accepted by the bees, the queen is released from her cage by the beekeeper so that she can start laying eggs. However, even after the queen starts laying eggs, the workers frequently attempt to replace her with a queen they make. Tarpy et al., 2021, reported that only 33% of package queens remain in the colony 12 weeks after package installation. More importantly, Tarpy et al. showed that twenty eight percent of package queens are replaced within the first 5 weeks of package installation. An additional 40% of the package queens were superseded (replaced by her daughters) in the following 5 weeks (weeks 6-10).
Tarpy demonstrated that adding a frame of brood increased package queen acceptance to about 75%. However, many beekeepers do not have a frame of brood to spare that they can provide to their new packages. Thus, this study is designed to determine if removal of replacement queen cells built in the first five weeks after the packaged is installed, a time when the worker bees in the hive are unrelated to the queen, increases the probably that packaged queen will be retained, leading to a high rate of colony survival and improved economic success for the beekeeper.
Impact on the hive of the early loss of a queen
Early loss of the queen threatens the survival of the package due to the short life span of the workers and the 3 weeks required to produce a new mated laying queen. The worker honey the bees only live about 6 weeks, and the bees in a package are at least one week old and possibly older. The original bees in the package must secrete wax and build comb, feed the queen so she can lay eggs, take care of the brood (larvae), and defend their nest before they die and are replaced by the new queen’s progeny.
If the packaged bees choose to replace the queen, the following honey bee development times must be considered. A queen requires 16 days to develop from an egg (See Appendix B). If the package bees attempt queen replacement, they will usually start to raise new queens from a 1 to 2 day old larva which will be available some 10 days after the package is installed. Starting with a 2 day old larvae (5 days from when the egg was laid), means that a new queen will emerge from her cocoon in about 11 days. Once the new queen emerges, she requires an additional 10 days (or more) to become sexually mature, mate and initiate egg laying. Thus, there will be a period of at least 21 days before any new eggs are laid. During this time the original package bees continue to age and die. Furthermore, once the new queen begins egg laying, it will take 21 days for the first worker bees to emerge from their cocoons. Therefore, early replacement of the package queen will set the package back by about 6 weeks. Few of the original package bees will survive this 6 week delay. The loss of workers due to early queen replacement, may doom the colony because their population becomes too small to carry out the functions essential for colony population growth and survival. Even if the colony survives, the small population means that the colony’s population will increase very slowly and may not have the population required to survive the following winter. Thus, it is imperative to learn how to prevent early queen replacement. This grant proposal will test the hypothesis that removal of early queen cells will reduce early queen replacement.
Cooperators
- - Technical Advisor
Research
Bees and hives
Thirty two three pound packages of Italian hybrid (Italian crossed with Carniolan and Buckfast) (Michael Gardner, personal communication) honey bees including a marked 2025 queen were obtained from Gardner Apiaries (Baxley, GA) and transported to southeast Pennsylvania by Honey Hill Farm, LLC. The day after their April 11, 2025 arrival, each package was assessed for potential Varroa mite infestation using an alcohol wash carried out on ½ cup of bees in a Ceracell varroa test jar. Two successive 1 min washes were performed, with pouring the alcohol trough a nylon coffee filter each time. The total number of varroa on the filter was counted, and the allocation of the packages to the four study groups was done by a stratified randomization.
Following randomization, the packages of bees were placed into hives comprised of all new equipment: 10 frame wooden Langstroth wax-dipped hive boxes, either deep (16 hives) or medium (16 hives), with Acorn beeswax coated plastic foundation in the wooden frames. Each hive was identified with a unique number for record keeping purposes. The 16 deep hives were placed in one apiary location, and the 16 medium hives were situated in a separate apiary location (The two apiaries, located in zip codes 19454 and 18944, are about 13 miles apart, both in southeast PA). Each colony began with a single brood box (medium or deep), additional boxes of foundation being added when 70% of the box on the hive had been drawn. The hives were arranged so that hives in the two study groups at each location were alternated in sequence, with the front of the hives in each group differentiated with stripes of colored tape (vertical and horizontal, blue and yellow). The brood chamber, three medium or two deep boxes, was topped with a queen excluder, and medium depth honey supers were added as needed. All hives were fed with 1/1 sugar syrup and a small strip of Global Patties (Airdrie Alberta, Canada) pollen supplement for the first five weeks of the study.
Study design and hive management
To evaluate whether cutting supersedure cells could reduce queen supersedure, the hives were divided into two groups, a control group (C) in which supersedure cells were noted but not cut and an experimental group (E) in which supersedure cells were counted and cut. These two groups were replicated at each location, so that there were four groups each containing eight hives: medium control (MC), medium experimental (ME), deep control (DC), and deep experimental (DE). The four groups followed the same protocol throughout the study, only small variations occurring.
When swarm cells (queen cells on the face of the comb) were observed, they were counted and cut (destroyed) in all groups. Since many of the usual swarm mitigation procedures would confound the aims of this study, an approach that reduced the hive’s field force was employed: after the swarm cells were cut, the queen was kept with her hive and the hive was moved to another location in the apiary.
Queen status and hive development were monitored at weekly intervals for the first 12 weeks and at three-week intervals for the remainder of the 27-week study. At each inspection, data were obtained on the number and kind (supersedure or swarm) of queen cells present, the number of queen cells cut, the presence of eggs and larvae, the presence of a marked or of an unmarked queen, the area of sealed brood, the number of boxes on the hive, the number of frames covered with bees, and the number of frames of foundation that had been drawn. Each hive was managed independently; no empty frames, frames of brood, frames of honey or other resources were interchanged among the study hives or with non-study hives. Hives that superseded their queens were so noted, and hives becoming queenless were lost to the study.
Brood area was measured using a hive frame fitted with a stringed grid (Robyn Underwood, personal communication; see photo) that was laid on top of each frame, so that the number of grid squares occupied by sealed brood could be quickly counted. Later in the study (week 8), the large quantity of brood in the hives necessitated a more rapid approach to quantifying the sealed brood. From this time point onward, the area of sealed brood observed on each side of each frame was estimated as 0, 25, 50, 75, or 100%. Brood area by each of these methods was converted to cells of sealed brood, so that the data from each initial data gathering method are expressed in the same unit, cells of sealed brood. The expression of brood area as cells of sealed brood also standardized data from medium and deep boxes to a common unit. In the frames used in this study, a medium frame contained 2378 cells/side, and a deep frame held 3690 cells/side.
Varroa mite management
The level of varroa mite infestation was monitored at least monthly once hives were well established. Bees on brood frames were gently shaken into a dishpan and ½ cup of bees was scooped up and placed into an alcohol containing mite wash jar (Ceracell Varroa Test Bottle, Ceracell Beekeeping Supplies, Ltd., New Zealand) and swirled. After a minute, the basket containing the bees was lifted out of the alcohol and the alcohol contents of the jar was poured through a nylon mesh coffee filter. The filtered alcohol was poured back into the mite wash jar with the bee basket replaced and the wash process was repeated. The mite count from the combined washes was recorded and converted to mites per 100 bees by dividing the number of mites seen by 3.
Mite treatment was initiated when mite counts reached a threshold of 3 or more mites per 100 bees(2). The newly approved VarroxSanÒ (Vita Bee Health, Basingstoke, United Kingdom) oxalic acid sustained release product and FormicPro (Nod Apiaries, Ontario, CA) were used at their label doses, the former at 4 strips and the latter at 2 strips per hive, both applied in the brood nest.
The 27 week data collection time point (October 25, 2025) marked the completion of the field portion of the study, and hive management reverted to the practices of each investigator in preparation for winter.
Hive development
Of the thirty-two packages installed, two were found to have queens laying only drone brood. These two hives (one in the ME and one in the DC group) were removed from the study, which proceeded with a total of30 hives.
Of the 30 hives initiated with mated queens, two (one in each control group) were found to be queenless with no signs of eggs or larvae nor any queen cells present from week 1. Since all queens were alive at the time of their release, it is presumed that these two hives rejected their queens before they were able to lay any eggs.
Two other hives (in the experimental group) in which queens were not found present at the week 1 inspection had supersedure cells. Cutting these queen cells (according to the study protocol) unfortunately resulted in these hives becoming hopelessly queenless, as their queens had already been eliminated.
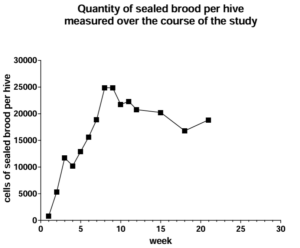
Development of the remaining 26 hives progressed, as evidenced by their production of sealed brood (Figure 1), which peaked at about 25,000 cells of sealed brood at weeks 7-8 (the first half of June). The amount of sealed brood measured in the hives was slightly less (about 20,000 cells of sealed brood) in the latter part of the study.
Queen cells-supersedure and swarm
Because the focus of this study was on queen supersedure, we observed and recorded which colonies made supersedure queen cells and how many they produced. Having not anticipated that, in addition to supersedure cells, the hives would produce swarm cells, the protocol did not include a procedure for dealing with swarm cells. With a view to both how any beekeeper would mitigate swarming (cut queen cells and reduce hive population—by making a split, for example) and adhering to the confines of the protocol that required that each hive be maintained independently, the following protocol was developed: irrespective of the study group of the hive, the swarm cells would be cut, and, to reduce the field population of the colony (and hopefully mitigate swarming potential), the hive moved to a new location within the apiary.
Figure 2
Thus, we observed and recorded which colonies made queen cells, 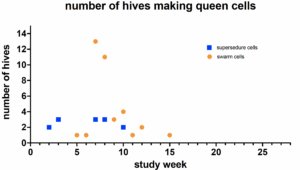 as well as their number and kind (supersedure-on the face of the frame, or swarm-at the bottom of the frame). Figure 2 shows the number of hives making either kind of queen cell over the course of this study. At a given observation time, both supersedure and swarm cells were found in some hives, whereas in other hives, only one or the other kind of queen cell was observed. Almost all hives (23/26) made queen cells at some point in the study, and some hives repeatedly produced queen cells (queen cell production in each hive ranged from 0-4 separate observed instances of queen cell production).
as well as their number and kind (supersedure-on the face of the frame, or swarm-at the bottom of the frame). Figure 2 shows the number of hives making either kind of queen cell over the course of this study. At a given observation time, both supersedure and swarm cells were found in some hives, whereas in other hives, only one or the other kind of queen cell was observed. Almost all hives (23/26) made queen cells at some point in the study, and some hives repeatedly produced queen cells (queen cell production in each hive ranged from 0-4 separate observed instances of queen cell production).
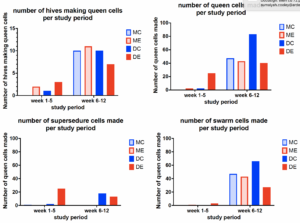
Figure 3.
As shown in Figure 3, the number of hives making queen cells (top left panel) and the number of queen cells made (three other panels) were greater in the second period of the study (weeks 6-12) than in the first period (weeks 1-5), as weeks 7 and 8 were the time of peak of swarm cell creation (bottom right panel). Further, a greater number of swarm cells (186) were made than supersedure cells (56), and a greater number of hives made swarm cells (19) than made supersedure cells (7).
The study design called for destruction of supersedure queen cells in the experimental but not the control group (see study design above). To evaluate the effect of this intervention on the supersedure of package queens, the study data on creation of supersedure cells and their management was tracked to the outcome of the supersedure attempt. In the control group DC, the two hives that made supersedure cells went on to supersede the package queen (see Table 1). In contrast, none of the seven hives in the experimental group whose supersedure queen cells were cut was successful in superseding its queen. Cutting queen cells was found to significantly decrease the likelihood of queen supersedure (Fischer’s Exact test, p = 0.0278).
Table 1. Cutting supersedure cells reduced the supersedure of package queens in colonies initiated from packages of honey bees. (Supersedure cells were cut in the experimental group but not in the control group.) The queen outcome in the C vs E group was significantly different: Fischer’s Exact test, p = 0.0278.
|
location |
study group |
# hives making supersedure cells |
# of hives superseding their package queen |
|
1 |
C |
0 |
0 |
|
E |
1 |
0 |
|
|
2 |
C |
2 |
2 |
|
E |
6 |
0 |
Cutting the large number of swarm cells made (186 cells in 19 hives) and relocating the hive within the apiary appeared to impede swarming, as we did not observe queen replacement in any of the hives that made swarm queen cells. Some hives had swarm cells cut and were relocated on multiple occasions in the study.
Queen supersedure-consequences
During the course of this study, two hives could be clearly identified as superseding their package queen, the new queen being observed in one hive at week three and the other at week 10. The hive that superseded early in its development suffered a major setback in its brood production, a condition that persisted even late in the study; it is doubtful if this hive has the resources to sustain itself through the coming winter. In contrast, the hive that superseded at week 10 showed a brief decrease in its brood production, but rebounded by weeks 15-18.
Conclusion
The results of the present study support the simple manipulation of cutting supersedure queen cells as an effective tool to prevent the supersedure of package queens. Preventing queen supersedure is especially desirable early in the development of a colony from a bee package, a time when the colony may lack the resources to overcome the setback attendant to queen replacement. Incorporation of this manipulation into establishment of colonies from packages is may increase the success of colony establishment from package bees.
Discussion
Development of hives
Beginning new honey bee colonies from commercially obtained packages is a standard beekeeping method in widespread use, and others have documented package development in locations in North America. In a study monitoring hive development from commercial two-pound packages of bees, conducted over two successive years in central Manitoba, Canada (Harris 2008), the new hives were resourced with frames of pollen, honey and sugar syrup. The peak area of sealed brood was about 18-20,000 cells, the peak coinciding, like ours, with the peak honey flow. The area of sealed brood declined thereafter, reaching zero by early October. Although the shape of the brood area curve in the Canadian study is compressed compared to ours due to the more northerly location and shorter floral season, the shape and peak of the brood area across time curve approach ours. As in our study, brood boxes and honey supers were added across the study, in their case producing honey that was harvested twice.
Another study documenting hive development from packages of honey bees was conducted in North Carolina (Tarpy 2021). Using six frame nuc boxes (with one or two position(s) occupied by a frame feeder), this study monitored hive development from three-pound packages of bees across a 12 week period. Coincidentally, this study and ours purchased bees from the same supplier, Gardner Apiaries. The shorter time frame of this North Carolina study precludes a full-season view of brood development, but the peak area of sealed brood (3.5 frames-deep or medium?) occurred about 5-6 weeks after package installation and continued at about that level until the end of the study at 12 weeks. The smaller area of sealed brood observed in the N.C. study compared to our study and that of Harris may be due to differences in the size of the hives into which the packages were placed (full sized hives with boxes added as needed vs. one nuc box in the study of Tarpy et al.), to differences in feeding (pollen, honey and sugar syrup in the study of Harris; sugar syrup and pollen supplement in the present study vs sugar syrup only in the study of Tarpy et al.) or to environmental factors, as the peak brood area in the study of Harris and the present study occur at the time of substantial honey flow, reported to be extremely poor (Eric Talley, personal communication) in the Tarpy study.
References
Harris JL. Development of honey bee colonies initiated from package bees on the northern Great Plains of North America. Journal of Apicultural Research. 2008;47(2):141-50.
Tarpy DR, Talley E, Metz BN. Influence of brood pheromone on honey bee colony establishment and queen replacement. Journal of Apicultural Research. 2021;60(2):220-8.
Conclusion
The results of the present study support the simple manipulation of cutting supersedure queen cells as an effective tool to prevent the supersedure of package queens. Preventing queen supersedure is especially desirable early in the development of a colony from a bee package, a time when the colony may lack the resources to overcome the setback attendant to queen replacement. Incorporation of this manipulation into establishment of colonies from packages is may increase the success of colony establishment from package bees.
Education & outreach activities and participation summary
Participation summary:
At the apiary site, we posted a few educational signs regarding honey bees and the study. (see enclosure). Passers by, people walking int he Natural Lands Trust preserve on a road adjacent to the apiary site, were interested int he study, were interested in the study and were glad to see honey bees on the property.
A short overview of the study was presented at the Pennsylvania State Beekeepers Association annual meeting in November 2025. the slide set used is enclosed.