Final report for FS23-352
Project Information
As of the completion of Project FS23-353 we initially bred ten yaks and one jersey cow in March 3rd and 4th, 2025 at 70 and 96 hours after the CDR was removed. Four weeks later we pregnancy tested those bred plus a yak which was thirty days pregnant in early February using ultrasound. Of those bred in March three yaks had conceived as well as the Jersey cow. Two of the yaks later aborted. In our second round of breeding done April 11th and 12th, 2025 four of the yaks did not come into estrus within 96 hours: All of whom came in during the March breeding. Eight yaks were bred: Seven previously artificially inseminated and the naturally bred yak who had aborted since the February testing. These eight yaks were not pregnancy tested in the following months due to the physically stressful hot weather and fear of causing an abortion with the additional stress of an ultrasound. Activity using Estrotech patches, visually monitoring bull activity, and the individual yak cow’s behaviors strongly indicates four pregnancies: This would yield five yak pregnancies for the overall breeding round (March-April) which involved twelve yak cows (40% conception ) and one Jersey cow (100% ). Calving is not until November and December. [Results will be added to this report at that time.]
The second major objective of this project beyond improving the artificial insemination process (from collection of semen through insemination) was to perform a comparative growth study between yak and yak x angus so that beef producers can make informed decisions about introducing yak genetics to their operation. Results from a fifteen week study on a typical Eastern Kentucky fescue and mixed grass pasture the average weight daily gain of four yaks bulls was 0.595 lbs/day. The average daily hybrid gain (two bulls and two female) was 1.365 lbs/day with the two female hybrids having an average gain of 1.49 lbs per day with the two hybrid bulls averaging 1.24 lbs daily.
We had intended on including a comparative meat analysis which compared protein, fat content and taste of grass fed yak, grass fed angus and grass fed yak x angus sirloin to assist farmers in meat production decision making. However, because of the schedule of the University of Kentucky’s Angus breeding program, the angus cows to produce the hybrid could not be bred early enough in the overall study to have the hybrid calves at slaughter weight within the extended project period. This analysis will be completed during 2026 when the bull hybrids reach slaughter weight and the results will be provided to SSARE.
The final component of the project was to introduce existing and potential small acreage farmers, Black farmers and larger beef producer to yaks and yak genetics as a possible asset to their farm program. Over the two projects FS1-335 and FS23-352 we have held Yak conferences focusing on artificial insemination, yak husbandry and yak products (meat and fiber), run an article in a Cattleman’s Journal and even in Mother Earth News. In this project we have developed a self-run power-point:
"From the Himalayan Mountains to Eastern Kentucky
The Domestic Yak
An Introduction to Yaks and Yak Care'
which has been set up as a link through Google Documents. Information about the link and how to access it has been sent to Cooperative Extension Offices throughout the Southern Region, posted on the North American Yak Association’s website and Facebook, and sent to Historically Black College and University Agricultural Schools in the Southern Region.
The specific FS23-352 project objectives we worked on during this first year of the project are as follows:
- To successfully AI the angus at the University of Kentucky available for us use in this project as early as possible in the project so that we would be able to perform the comparative study described in the abstract.
- To review our semen collection protocol to determine where losses in sperm might be taking place before it is extended.
- Review our semen extending process to minimize the loss of motile sperm before freezing.
- To run side by side processing using several extenders to determine which extender yields the highest post thaw percent sperm motility.
- To determine if it is better fill straws before or after the initial cooling of the extended semen to 5C (41F) over 1.5 hours.
- To see where the freezing system used can be improved. This includes the actual freezing and going from having frozen straws in liquid nitrogen to having them in the LN storage tank.
- How to improve our actual insemination process to increase the conception rate.
- Participated in a SSARE sponsored Yak Conference, Project O522-157, held October 31, 2023.
The Project objectives worked on during the second year of the Project were:
- Further collection, extending and freezing of yak semen to test the process finalized during the first year and to make improvements.
- Dr. J. W. Lehmkuhler obtained permission from the University of Tennessee Department of Agriculture to use their Computer Assisted Semen Analyzer to better evaluate the post-thaw motility of the extended yak semen we produced and then to evaluate its viability over time.
- Perform at least one more round of artificial insemination using the Bos Indicus PG 5-Day + CIDR Fixed Time AI Protocol.
- Perform comparative weight gain study involving four yak bulls and the four hybrids produced during year 1 of the project.
- Develop an educational "product" which would be internet accessible to small acreage farmers, Black Farmers and larger beef producers to view on their own schedule which would serve as an introduction to the Domestic Yak, Yak products (meat and fiber) and basic husbandry required. The goal of this is to provide enough information for current and those interested in developing a farm to decide if the inclusion of yaks/yak genetics via AI is worth pursuing.
- A basic comparative meat study between grass fed yak meat, the yak x angus hybrid yak meat and gras fed angus meat which would look at protein, overall fat, moisture content and have a comparative taste component by a chef(s).
- And finally to develop a guide for persons interested Yak Artificial Insemination (Collection Semen through Insemination) based on what we have learned through Projects FS1-335 and FS23-352.
Cooperators
- (Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- (Researcher)
- (Educator and Researcher)
- (Researcher)
- (Researcher)
Research
A. Semen Collection
One of the key things we have learned in this project based on the fifteen yak bulls we worked with, is that yak sperm is very ambient temperature sensitive. Like beef bulls, and as shown in the FS21-335 final report, yak semen quality in terms of motility and morphology is dependent on the ambient temperature during the sixty days required for it to develop. In Eastern Kentucky, from the end of April to at least mid-October, even though yak bulls can technically get yak cows pregnant in the field, the quality of their sperm is very low in terms of percent motility and percent with good morphology…Too low to consider collecting for extending and preserving for artificial insemination. Over the past four years we have to use a threshold of 80% motility and 80% morphology at collection in order to have a sufficient post thaw viable sperm assuming the initial concentration was good. Furthermore, if a bull was sick or had other sources of stress during sixty days of sperm development, no matter what the ambient temperature was during that period, the quality of the semen can be negatively effected.
Note: The semen collected from four yak bulls at the end of October of 2025 had very low motility and morphology in three of the four bulls due to the extended high ambient temperature through the summer into the fall. When we collected again, in November, all the bulls’ sperm had a motility and morphology greater than 80%.
Over the past thirty plus years the development of quality beef bulls has included scrotal size, which is a heritable trait, as a key EPD. This is because the larger the scrotal size the greater the quantity and quality of the ejaculate as well as impacting the reproductive maturation of the heifers sired. This kind of research has not been done with yaks in the United States. In fact there has been no emphasis in breeding programs to increase scrotal size – and therefore, the quantity and quality of yak semen. However, based on the more than fifteen yak bulls we selected to collect semen from over the past two projects: If a yak bull’s testicular circumference was less than 27 centimeters, its semen was never good enough to collect for extending in terms of sperm concentration and semen volume collected.
When collecting yak semen, and all that takes place between collecting it and getting it in a 99-100 degree water bath to await being extended, TEMPERATURE CONTROL IS EVERYTHING.
Given that yak bull semen had to be collected between late October (at the earliest) and the end of April due to the impact of ambient temperature on the quality of a bull’s, we were always collecting semen in cool to cold weather. And given that yak bull semen is 101 degrees in temperature, even in the summer a semen collection tube would have a far lower temperature than the bull’s semen: Let alone the ambient temperature on a late fall or winter day. Cold kills semen. So:
- The greater the temperature difference between the semen collection tube and the temperature surrounding the semen collection tube until the semen is placed in a 99-100 degree water bath; and,
- The amount of time the semen is in a collection tube with a temperature of less than 99-100 degrees
- => The more yak sperm will die before the extending process is even started.
In our situation, the semen was in the collection tube for minutes in the outside ambient air during the motility measurement and then being transported to a heated room (only in the 70s's) where it was then placed in a 99F water bath.
Holding the collection tube in one’s hand or gloved hand during collection and then holding it inside one’s coat (The traditional method) didn’t keep it warm enough to prevent significant amounts of sperm from dying.
Furthermore, at the other end of just getting the collected semen to the water bath, the now surviving sperm is cooler than the water bath. So when it is suddenly put in the water bath more sperm die from the heat differential.
The solution was quite simple. A simple chemical toe warmer was wrapped around the collection tube prior to collection to bring it up close to 100 F and then was protected in all the traditional methods as well to keep it as warm and protected as possible. Just this change made a significant difference in the final amount of viable extended sperm produced.
Remember: You will have loss at every step of the process.
- Collecting from the bull
- Every second the semen is at less than 99-100F until it is in the water bath.
- During the extending process every time it is out of the water bath until it has been extended since the room it is being extended in is at a temperature far less than 99-100F
- After the extended semen has cooled and is ready for loading straws: Here it is the reverse problem. You do not want the extended semen to warm up during the straw loading process.
- And after the straws have been frozen in the liquid nitrogen. You don’t want them to thaw while they are loaded in goblets and canes and finally placed in a tank of Liquid Nitrogen.
Each step in the process will have loss. The key is to minimize the loss at every step
The steps in semen collection we used and recommend are as follows:
- The bull is weighed too see if there could be a health issue that could impact perm quality and for record keeping
- The bull then advances to the squeeze chute where it is constrained.
- The collection equipment is ready and a toe warmer designed to heat to 102F is wrapped around the collection tube which is then put in an inside pocket of the person who will hold it during collection.
- The yak's temperature is taken - again if out of the normal range could indicate a problem with the sperm,
- The yak's scrotal circumference is measured and recorded. If the scrotal circumference is less than 27cm, the quality of the sperm: concentration, motility and morphology may not be high enough to continue.
- During this time the toe warmer applied to the collection tube should have brought its’ temperature close to 100F.
- The bull's Cowper's and Prostrate glands and the Seminal vesicle are very gently manually massaged for approximately two minutes.
The individual with the collection tube needs to be prepared to collect semen at this point since some yak bulls will ejaculate during the massage.
- A small bovine electro-ejaculator is lubricated and inserted and run on automatic intensity advancement. [This is like an internal muscle stimulator TENS unit for pain management.]
- Once the bull has settled down and developed a rocking motion the toe warmer encased collection tube should be held in the collector’s hand protecting it and in a position to receive the tip of the bull’s extended penis.
- Once the bull has deposited sufficient ejaculate in the collection tube the electro ejaculator is turn off and the collection tube is taken as protected as possible to have two pipettes ready to remove a drop of semen for a motility check by microscope and for a morphology evaluation. KEEP THIS TIME TO A MINIMUM. Once the drops have been removed the collection tube is taken to the water bath where it will be maintained at 99-100F.
- NOTE: When doing the motility check on the microscope, be sure the slide and cover have been warmed on a slide warmer before placing the drop of semen from the collection tube on the slide. The time between extracting a drop of the semen with the pipette until viewing by the microscope needs to be as fast as possible to reduce sperm death due to cold. The drop of semen taken for morphology evaluation doesn’t matter as it is the physical shapes of the sperm (eg, heads and tails) that matters.
- NOTE: The collection tube had the toe warmer around it and the collector's hand around it to keep it warm from before the electro-ejaculator cycle was started to when the semen was put in the water bath. The time from the collection to the water bath must be kept at a minimum.
KEY SEMEN COLLECTION GUIDELINES:
- KEEP SEMEN AS CLOSE TO 100F AS POSSIBLE DURING COLLECTION AND UNTIL IT IS PUT IN THE WATER BATH.
- IF MOTILITY OR MORPHOLOGY ARE LESS THAN 70%, THE SEMEN IS NOT GOING TO EXTEND WELL. Note, if the motility or morphology are low it does not mean the bull is "bad". It could be the result of the bull not having ejaculated for quite a while. We have learned to let the bulls we intend to use for AI in with several cows in estrus so they can have done some breeding prior to collection. A low motility and/or morphology can also indicate the bull was under some kind of stress during the prior sixty days while the semen collected was being formed or that that there is a health/nutritional issue.
B. Extending Yak
SemenIn this project we used a number of different extenders: TRIS extenders prepared by Dr, Lehmkuhler using formulas that had been successfully used for years with beef cattle and two purchased extenders from IMV Technologies: Optidyl – an egg based extender with powdered egg yolk included and BullXcell - an egg based extender which required the addition of egg yolk. In our use of the different extenders the egg based extenders (ones where egg yolk was added or that contained a powdered yolk in them) worked the best.
Note: With respect to adding egg yolk, the Researchers at the ICAR - National Research Centre on Yak in India were very clear about using fresh eggs. While eggs purchased at the grocery are fresh enough for consumption, they may not be fresh enough for making a high quality extender. Using the simple "how an egg sinks" method on eggs purchased at a grocery revealed than many were far from “fresh”. During this project we switched to farm eggs layed within twenty-four hours of making the extender verses store bought organic eggs used in the previous project FS21-335. The extender we have made under this project using truly fresh eggs has been much improved in terms of post thaw motility which, in part, could be partially due to egg "freshness". [Note: It is in the little details that the quality of the extended semen can be greatly impacted.]
Note: Making the BullXcell – Adding Fresh Egg Yolk
- In using a fresh egg yolk we first make sure the shell is clean using distilled water and then break the egg shell keeping the yolk inside it and drain off as much of egg white as possible. Once this is done we roll the yolk out of the shell on paper towels and roll it around on the towels so they can capture the fluid on the exterior of the yolk leaving a clean "dry" yolk. We then used a pipette to pierce the yolk and withdraw the amount needed from within.
- A warmer with a magnetic stirrer was used to mix the extender and added egg yolk. It is important that the prepared extender be a homogenous solution.
Note: It is important to note that some extenders will go bad within a certain amount of time. For example the Bullxcell Extender requires that the semen be added within ten minutes of making.
Key Steps in Extending the Collected Semen
- The collected semen needs to be placed in the water bath upon receiving.
- Once the collected semen has been put in the water bath a sample is taken to determine the sperm concentration per ml using an Accu-cell Bovine Photometer. Note: This device gives a sperm count per ml but does not differentiate between live and dead sperm. Therefore, if the concentration is 400 Million sperm/ml and the motility is 75%, one would assume a live sperm count of 300 Million sperm/ml.
- Once the concentration of sperm is known the amount of extender needed to protect the semen can be calculated. During the past project FS21-335 we extended to achieve a sperm concentration of 100 Million total (alive and dead) sperm per ml in order to try to account for motility and morphology so that the living sperm concentration in the extended semen would be at least 60 Million sperm/ml. To achieve this concentration required we often had to use the minimal amount of extender allowed: a 1:1 semen to extender mix.
Using this small amount of extender may have not been enough to properly protect the yak semen during freezing and later thawing to check post thaw motility and contributed to the low post thaw motility numbers we had been achieving during the FS21-335 project
In this project we have experimented with using extended total (alive and dead) sperm concentrations of 30 Million, 45 million and 60 million per ml. Over the six times we collected and extended semen the 30 Million and 45 Million total sperm per ml post thaw numbers have been excellent and better than the 60 Million total sperm /ml concentration post thaw results: Indicating that the reduced amount of extender needed for a concentration of 60 million sperm did not provide adequate freezing and later thawing protection for the sperm.
- The extender being used should be prepared and placed in the water bath to bring it up to the same temperature as the semen to be extended.
- Recall some extenders have a time limit within which the semen be added so be sure everything is ready once the time limited extender has been prepared and warmed.
- Remember that when you add the proper amount of extender for the desired concentration to the semen that you will be removing both the semen and extender from the water bath to extend. This should be done as quickly as possible to avoid cooling the unprotected sperm . Once combined the extended semen should be placed back in the water bath until it is time to cool the extended semen for packaging (in straws and frozen).
The following are the post thaw results of the three processed semen collections during the first year of Project FS23-352 using the two different IMV Technologies egg yolk based extenders: BullXcell (fresh egg yolk added) and Optidyl (egg yolk already contained in the premixed powdered extender).
Bull Extended sperm Freeze # BullXcell post thaw Optidyl post thaw
Concentration motility motility
Bull #47 30 million/ml 1 45-50% 35%
Sad Eyes 45 million/ml 1 55 % 40%
Phantom 30 million/ml 1 35 -40% 30%
Phantom 45 million/ml 1 45%
Phantom 60 million/ml 1 30% 66 minutes post thaw
Bull #47 45 million/ml 2 30% 25%
Sad Eyes 45 million/ml 2 50 % 45%
Phantom 45 million/ml 2 45% 35%
Bull #47 30 million/ml 3 40%
Bull #47 45 million/ml 3 35%
Sad Eyes 30 million/ml 3 45%
Sad eyes 45 million/ml 3 45%
Phantom 30 million/ml 3 40% 45%
- Note: All post -motilities were estimated by two researchers viewing sperm motility using a microscope
=>In this case the post thawed extended semen of two straws for each bull were mixed together and evaluated using the Computer Assisted Semen Analyzer.
=> Since whether a concentration of 30 or 45 million sperm per ml was determined to not be a factor in the post thaw motility, concentrations of 45M sperm/ml were used.
=>Column 3 [ thawtime] is the time motility was measured relative to being thawed:
0 -thaw time is 0 hours post thaw - when the frozen semen was first thawed
3 – thaw time is three hours post thaw
=>Total motility is the percentage of sperm actively moving in any direction
=>Progressive motility is the percentage of sperm moving forward in a linear or slightly curved path
=>Normal is the percentage of all of the sperm (dead and alive) without defects. Based on a sample of 100.
=>Primary is the percentage of all of the sperm with abnormally shaped heads, midpieces (corkscrew), or tails. These defects occur during spermatogenesis. Based on a sample of 100.
=> Secondary is the percentage of all of the sperm with defects that occur after formation typically during transport and storage in the epididymis or during ejaculation. Based on a sample of 100.
|
Bull |
Extender |
Thawtime |
Total motility |
Progressive Motility |
Normal |
Primary |
Secondary |
|
#47 |
Bullxcell |
0 |
46.85 |
23.65 |
89 |
8 |
3 |
|
#47 |
Bullxcell |
3 |
28.2 |
11.65 |
|||
|
#47 |
Optidyl |
0 |
32.2 |
15.7 |
91 |
5.5 |
3.5 |
|
#47 |
Optidyl |
3 |
1.05 |
0 |
|||
|
Helios |
Bullxcell |
0 |
21.65 |
9.95 |
87.5 |
5 |
7 |
|
Helios |
Bullxcell |
3 |
13.15 |
5.35 |
|||
|
Helios |
Optidyl |
0 |
3.7 |
0.6 |
78.5 |
8.5 |
13 |
|
Helios |
Optidyl |
3 |
2.3 |
0 |
|||
|
Klingon |
Bullxcell |
0 |
30.8 |
9.7 |
86 |
8 |
6 |
|
Klingon |
Bullxcell |
3 |
21.5 |
6.3 |
|||
|
Klingon |
Optidyl |
0 |
24.8 |
5 |
67 |
6 |
27 |
|
Klingon |
Optidyl |
3 |
7.4 |
0.7 |
|||
|
Phantom |
Bullxcell |
0 |
40.29 |
15.43 |
89.1 |
5.5 |
5.4 |
|
Phantom |
Bullxcell |
3 |
33.64 |
20.26 |
|||
|
Phantom |
Optidyl |
0 |
31.59 |
15.57 |
77.43 |
6.43 |
16.14 |
|
Phantom |
Optidyl |
3 |
3.51 |
1.2 |
|
||
|
|
|
|
|
|
|
|
|
|
Sad |
Bullxcell |
0 |
35.91 |
11.11 |
90 |
6 |
4 |
|
Sad |
Bullxcell |
3 |
7.97 |
3.52 |
|||
|
Sad |
Optidyl |
0 |
35.43 |
11.61 |
82 |
6. |
12 |
|
Sad |
Optidyl |
3 |
3.52 |
0.8 |
As an example consider Phantom’s Bullxcell extended semen results. All of the samples in the chart had a concentration of 45 million sperm/ml when extended.
- 1% of the sperm in this thawed semen sample were normal which is just over 40 million/ml. Remember this 40 million includes live and dead sperm. The dead sperm in the initial collection were never “sifted out” to they are part of the semen contained in the packaged frozen extended semen.
- The Total Motility (number of living sperm) upon thawing was 40.29% of the 45 million which is 18.1 million sperm. So, at insemination roughly 18.1 million sperm would be alive.
- The Progressive Motility, the percent of sperm that were moving in a forward direction (linear or slight arc) was roughly 15.43 % or 6.94 million sperm at the moment of insemination.
- Three hours later, the number of living sperm was 33.64% of the original 45 million sperm which is just over 15 million sperm still alive. During actual insemination there would be some further loss due to internal environment of the cow’s cervix/uterus where the sperm were deposited at insemination.
- The number of sperm that would still moving in a forward (linear or slight arc) direction apart from environmental losses after three hours would be 20.26% of the 45 million which is just over 9 million sperm
NOTE: The “increase in progressively motile sperm” at three hours post thaw can be partially due to the sperm having had more time in a warm environment since post thaw to adjust since the immediate post thaw measurement. The major difference is due to how the test is run: The post thawed sperm is placed in a water bath and a sample is taken immediately and evaluated for progressive motility. Three hours a later a second sample is taken from the original post thawed semen in the water bath and evaluated for progressive motility: Not the original sample evaluated three hours later. The fact that two different samples (same extended semen, but within the thawed semen there is not uniformity of quantity and quality of sperm in terms of motility and progressive motility) were taken gave rise to an unexpected increase in the three hour progressive motility as compared to the one hour progressive motility
NOTE: Look at what happens when Optidyl is used to extend Pantom’s semen. After three hours the progressive motility is 1.2 % - a half million sperm not counting losses due to the internal environment.
C. Cooling the Extended Semen
Once the semen has been extended the next step is to cool it down to 5C (41F) at the recommended cooling rate of 0.25C per minute.
To cool the semen to 5C we use a two cubic foot laboratory refrigerator which has a digitally controlled temperature. There was no way to set a cooling rate so what we had to do was determine its cooling rate by placing different volumes of 37C (water bath temperature) water in the refrigerator precooled to 5C and using a digital thermometer with a wire probe record how the interior temperature dropped over time: In reality we were determining the volume of the bath water we needed to have in a beaker with the extended semen so that the total extended semen and bath water would cool at 0.25C/min. Once this “total volume” which would cool at .25 C/minute was determined, the volume of bath water added to the total extended semen was the “volume of water which would cool at ).25C/min minus the volume of extended semen. The total cooling time was (37C-5C)/0.25/min = 128 minutes.
- NOTE: IN determining the volume which would cool at 0.25C/min. we did not have to wait the entire time for the refrigerator to cool to 5C but only long enough to see how many degrees it was cooling per minute once a constant cooling rate developed.
Once we had a volume of water at 37C that cooled at approximately 0.25C per minute we were done. In our case it was 400 ml. Knowing this, for example if we were going to cool a total of 40 ml of extended semen we would put its container in a beaker of 360ml of water for cooling in the refrigerator.
NOTE: We chose not to package the extended semen before cooling because to do so would require us to remove the semen from the bath water temperature of 99F (37C) into the air temperature of the room we worked in which would result in an uncontrolled cooling rate for the first twenty five or more degrees (F): From 99F to room temperature F.
We set the refrigerator in a cold room we constructed which could be cooled to 5C (41F). This way the chilled semen could be removed from the refrigerator, and packaged in straws, and then frozen in the cold room without “warming the cooled semen due to a higher room temperature.
The following took place in our cold room:
- Cooling the semen in the laboratory refrigerator at a rate of 0.25C/min
- Filling 0.5cc straws with the chilled semen. [Note the straws needed to be in the cold room while the semen was being cooled so they would be at 5C as well.] The straws were filled using a “semen straw filling nozzle” which allows fifteen straws to be filled simultaneously.
=>NOTE: Different colored straws were used for each bull. This is important as well as coding by goblet color and labeling the top of the canes since when selecting specifics straws from the Liquid Nitrogen storage tank to use for insemination it is critical that the straws brought out of the Liquid Nitrogen that are not going to be used are out of the LN for as short a time as possible to minimize thawing and then refreezing the thawed sperm which would kill them.
- After filling the straws an air pocket at the open end of each straw was made using a using a “semen straw comb”
- The straws were then sealed using glass sealing balls that had been warmed on a coffee cup warmer. The warming of the sealing balls made the “pushing the straw down over the sealing ball” easier.
=>NOTE: Glass sealing balls were used rather than metal sealing balls since they seemed to hold their position better than the metal balls when placed in Liquid Nitrogen.
- After all the extended semen had been sealed in straws they were placed on freezing racks and cooled in the refrigerator at 5C for an additional three hours.
After the additional 3 hour cooling the straws are frozen in Liquid Nitrogen, placed in goblets and canes, and then placed in a LN semen storage tank..
NOTE: Loading straws after extending before cooling verses loading after cooling in the cold room.
Semen straws can be filled after extending rather than in the cold room: The extender will protect the sperm from the change in temperature from the warming bath 99F to the ambient room temperature.
- Once you remove the extended semen from the bath what you don’t want to do is have rapid decreases in temperature or raise the temperature of the extended semen. If there is a temperature change it must be to a lower temperature at a rate less than 0.25C/min. Increasing the temperature of the extended semen will damage the sperm.
The extended semen is then cooled in the straws to 5C at a rate averaging about 0.25C/minute and then keep it at 5C for an additional three hours before freezing.
First: Compute 400ml – the total volume of extended semen in the straws (# of straws x .9 x straw volume). The .9 is added to account for the reduction in the amount of semen in each straw due to the air bubble created at the open end. THIS IS THE AMOUNT OF WATER THAT MUST BE COOLDED ALONG WITH THE EXTENDED SEMEN.
Second: Since you want the extended semen in the straws to cool at the same rate as the water you are colling with the straws, depending on the number of straws you are cooling, sandwich the straws in a sealed baggie between two baggies partially filled with water, the temperature of the room the straws were filled in, totaling the calculated amount of water (one below the straws and the other on top). The assumption is that by the time the straws are filled the extended semen has cooled to the temperature of the room in which the straws were filled. The straws should be placed in a sealed baggy between the two baggies containing water to prevent them from getting wet in case one of the water-filled baggies leaks.
NOTE: One must be sure that the water-filled baggies above and below the baggie with the semen straws surround all of the straws with water: If not, the extended semen in the straws that are exposed to the air will cool too quickly and be damaged.
We experimented with two methods of filling the straws:
- Filling the straws with extended semen room the water bath (37C=99F) in the room and then placing them in a baggie and sandwiching them between two baggies with water the temperature of the room the straws were filled so that the total volume of water in baggies plus (the number of straws x 0.9 x volume of a straw) = 400ml and then cooling them in the refrigerator at 0.25C/min to 5C followed by three hours at 5C in the refrigerator; and,
- First cooling the warm extended semen at 0.25C to 5C (128 minutes), packaging the chilled semen in the straws and then cooling the straws at 5C for an additional three hours.
We tested the post thaw motility results of extended semen packed both ways and found that cooling the extended semen and then packaging in straws verses packaging the extended warm semen at room temperature followed by cooling. By microscopic examination of the thawed semen, the researched believed the extended semen which was packaged in straws at 5C had slightly higher motility.
D. Freezing the cooled straws
Once the straws cooled for the additional three hours at 5C the extended semen is ready to be frozen in Liquid Nitrogen.
In this project we used two freezing techniques:
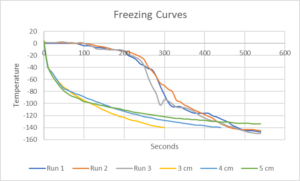
FIRST SYSTEM: This was the same technique used in Project FS21-335. We had a simple pulley system to control the descent of the freezing rack holding the semen straws into a Styrofoam container (21x13x10.5 inches (inside LxWxH) with 1.5 inch thick walls and a removable friction fit cover) into which we had poured enough liquid nitrogen to completely submerge the freezing rack and straws. The system was a hand crank operate pulley which lowered what looked like the lift system on the front of a forklift made of wood with two right angle brackets large enough for the freezing rack to securely sit on as shown.

A digital thermometer that could read below -140 C and had a wire temperature probe which we could attach to the rack without the exposed probe wire touching the rack was used to monitor the temperature of the straws as it was lowered according to the following protocol:
Starting temperature 5C => the brackets holding the freezing rack need to be able to raise approximately five inches above the walls of the Styrofoam box to keep the rack above the LN vapor in the box in order to start the process at 5C.
- Five minutes to go "smoothly" from 5C to -10C
- Four minutes from -10C to -40C
- Two minutes from -40C to -100C
- Three minutes from -100C to -140C
- We held the rack at -140C for one minute and then plunged it into the liquid nitrogen.
The closer the rack was to the liquid nitrogen the more a slight lowering would greatly decrease the temperature. The Temp vs. Time graph shown is from the first three of five times we froze the semen using this protocol.
NOTE that even though the descents were not smooth and had significant differences in rate of decent at each step, all of the resulting post thaw motilities were very close to one another.
SECOND SYSTEM: To test the necessity of a controlled temperature descent we used a second system to freeze the semen, This system consisted of a rectangular insulated metal container 10x10x8 inches which was filled two-thirds full of liquid nitrogen. A small rack that could hold about twenty semen straws was mounted on a rectangular metal frame which was mounted on two Styrofoam pontoons that would float the rack carrying the straws on the liquid nitrogen. The frame was designed to set how high the straws would be above the liquid nitrogen. The adjustment was from 2 to 5 centimeters. The temperature probe of the digital thermometer was attached to the rack holding the straws in order to monitor the temperature of the air surrounding the straws.
Once the frame was adjusted to the desired height the straws should be above the liquid nitrogen, the frame with the straws was placed on the liquid nitrogen and the temperature monitored. When the temperature decreased to -140C the frame was manually turned over and the straws submerged in the liquid nitrogen.
The chart below shows the post thaw motility of straws frozen using both methods. In both cases the straws were taken directly from the liquid nitrogen and placed in a semen straw warmer set at 99F for one minute before being cut in the center. A drop of the thawed extended semen was placed on a warmed slide with a warmed cover and then examined using a microscope for percent motility.
=>NOTE: Whenever motility was being evaluated at least two individuals separately estimate the percentage of mobile sperm.
Post Thaw Motility - Comparison of Freezing Methods
Bull Timed Lowering Floating Rack Method – Height Above LN
on rack into LN 3cm above LN 4cm above LN 5cm above LN
Sad 45% 50% 45% 40%
Straw – 30M sperm/ml, Bullxcell
Sad 45% 45% 25% 50% Straw – 30M sperm/ml, Optidyl
Sad Eyes 40% 40% 25% 30%
Straw – 45 M sperm/ml, Optidyl
#47 45-50% 50% 45% 25% Straw – 30 M sperm/ml Bullxcell
#47 35% 25% 25% 25%
Straw – 30M sperm/ml Optidyl
#47 30% 45M 20% 40% Straw - 45M sperm/ml Bullxcell
#47 25% 25% 25% Straw – 45M sperm/ml Optidyl
Phantom 45% 45% 35% Straw – 45M sperm/ml Bullxcell
The following figure shows the temperature of the rack the extended semen straws were placed on during the two methods of freezing. The gray, blue and red curves are for three freezing runs using the timed lowering of the rack through the LN vapor and into the LN and the yellow, light blie and green curves show the temperature change using the float and plunge method with the colors indicating how high above the LN the rack was floating until the temperature at that height decreased to -140C.
E. Loading Frozen Semen Straws into LN Storage Tank for Preservation
Under SSARE Project FS21-335 after the straws were submerged in the LN at the end of the freezing process we would use tweezers and pick up the straws, place them in a Goblet which was already attached to a Cane and lower it into the LN tank. Every time a new straw was added the Cane would have to be raise out of the LN tank high enough to place the straw in the Goblet and then lowered again. Even being done in the cold room at 5C/41F the straws were going from -196C into a 5C environment long enough to place them in a Goblet and Cane and to then lower the Cane back into the LN tank at minus 196C. Furthermore, since each Goblet held five straws, the straws already in Goblet were being raised up into 5C air and then back into minus 196 LN until the Goblet was filled with five straws. While this was done as quickly as possible, the straws were exposed to multiple temperature variations from minus 196C to 5C for at least 8 seconds (The average time it took to fill a Goblet). The stress on the frozen extended sperm resulted in decreased overall motility and progressive motility.
The solution was simple:
- When freezing the straws, instead of filling the Styrofoam container with enough Liquid Nitrogen to immerse the straws, fill it a depth of 1.5-2 inches of Liquid Nitrogen.
- Once the straws are submerged on the rack in the Liquid Nitrogen at the end of freezing process, remove the rack leaving the straws on the bottom of the Styrofoam container.
- After several minutes select several straws for post thaw results. Remove them one at a time placing the straw in a semen warmer at 99F and move to the room with the microscope.
- After one minute in the warmer, cut the straw in the middle and place a drop of the extended semen p on a warmed slide with a warmed slide cover placed over it.
- The thawed extended semen is the examined by at least two people (experienced in estimating motility) who estimate the motility and who do not share their result until each person has examined it.
- This should be done for each variation of semen frozen (eg. bull, extender type, concentration).
- After determining post thaw motilities, the straws in the Liquid Nitrogen in the container can be loaded in prelabeled Goblets making sure that the Goblet is entirely submerged while being filled using 16 inch tweezers. After the Goblet is filled it is left in the Liquid Nitrogen until all the needed Goblets are filled.
- After all the Goblets are filled, a Cane, which holds two Goblets, is submerged in the Liquid Nitrogen and filled with two Goblets. The entire process being done with the Cane and Goblets submerged.
- The loaded cane is then removed from the Liquid Nitrogen and placed in the semen storage tank as quickly as possible. Note: Since the Goblets are closed on one end, they will contain a small amount of Liquid Nitrogen with the straws which will help reduce any temperature change during the few seconds it takes to move the loaded Cane into the storage tank.
E. Insemination
The critical issue in the actual artificial insemination of yaks is knowing when to inseminate. During the FS21-335 Project and the beginning of this project when we artificially inseminated the angus at the University of Kentucky with frozen yak semen we followed a slightly modified a standard seven day CIDR protocol used for beef cattle.
In this modified protocol we eliminated the injection of GnRh at the time of CIDR insertion, left the CIDRs in for seven days and upon removal injected the yaks with 5ml of Lutalyse. Approximately 72 hours after the removal of the CIDRs, the time used in the beef industry, we inseminated the yaks and followed with a second insemination at approximately 96 hours.
Yaks do not show many signs of estrus. However, during the insemination of each yak cow Dr. Anderson was able to assess if the cow was close to estrus by the “slickness” the vaginal wall, smell and ease of insertion of the insemination gun into the cervix. However, we did not know if they were in standing heat or if ovulation had occurred.
The fact that we did not know exactly when they were in estrus, and more exactly if ovulation had occurred contributed significantly to poor and inconsistent breeding results. The other factor was the quality of the semen we were using.
The quality of the frozen semen was tested by using unfrozen extended semen on a group of ten yak cows. With the unfrozen semen we had a conception rate of 40%. While with frozen semen we have had conception rates between 0 and 25% using the same sequencing protocol. So inconsistent semen quality (% post thaw motility) was a key issue as well as timing.
When we inseminated the angus we had a 66% conception rate with frozen semen (with good post thaw motility) which was excellent. Of course the reason it worked so well is we were breeding angus for which the seven day protocol works very well.
- So good quality semen inseminated at the correct time did provide good results.
This left two things to be resolved:
- Improved and consistent percent post thaw motility – which we resolved in the first year of this grant by eliminating semen exposure to temperature changes from Collection (use of warmers on the collection tube) to how we got the frozen semen into the Storage tank – described previously.
- Knowing when to inseminate
Dr. Anderson researched different sequencing protocols being used at the yak research centers in China and India and, based on their insemination results, recommended we use a five day protocol developed for Bos Indicus which consists of giving 5ml of Lutalyse at the time of CIDR insertion and a second 5ml injection of Lutalyse when the CIDR is removed. GnRh would be given after an insemination if the yak did not give signs of being close to estrus.
=>NOTE: The purpose of the GnRh was to induce ovulation within twelve hours so it could be inseminated by remaining viable semen. This works for beef cattle and was assumed it might work for yak.
To test the Bos Indicus Sequencing protocol we ran four trials in which we sequenced three yak cows following this system and kept them in a corral around which we had positioned four game cameras. Twenty-four hours after removing the CIDRs we brought a bull into the corral with the cows and turned the cameras on letting them run for 120 hours post CIDR removal.
- In all four trials estrus, as determined by how the yak cows reacted to the bull’s mounting, ranged from 60 hours to 110 hours.
- Furthermore, there was no clustering of estrus occurring around any specific time(s) although the majority were between 70 and 100 hours.
We still did not know when a given cow was in estrus within the overall time range of 60-110 hours.
To solve this we took a friendly bull that was no longer consistently producing semen good enough for the project and Dr. Prater performed an epididymectomy, removing the epididymus which holds the sperm before entering the vas deferens. In a bull the epididymectomy is far easier on the bull and less invasive than a vasectomy. This provided us with a completely intact bull who was not fertile.
This bull would serve two purposes:
- It is known from beef cattle that having a bull present with cows helps stimulate and "cluster" estrus; and,
- The behavior of the yak cows to the mounting by the bull would signal when cows were in estrus. We could tell this by placing Estrotech patches made for this purpose just above the tails of the yaks. These patches turn a bright red from the pressure and movement of being mounted by the bull.
Our first attempt to use the Bos Indicus sequencing system with the "teaser" bull and use of tail patches was done on March 13th, and 14th, 2024 after we had made the three successful semen collections with high post thaw motility results. Twenty-two yaks were sequenced along with a Jersey cow and half Jersey heifer.
Approximately 48 hours after CIRD removal one yak started showing signs of being near estrus as detected by her movements using the Cattle Manager System which will successfully predict estrus in beef cattle based on their movements.
By 60 hours after CIDR removal the bulls in an adjacent pasture were all along the fence-line. The teaser bull had mounted the one yak who showed signs of estrus earlier according to Cattle Manager. This was known by the patch having been rubbed red.
At 70 hours post CIDR removal 5 yaks had been in or were in estrus (red patches from the bull’s mounting). All of the yaks were bred except a small 400 pound three year old female. We were going to wait to see if she would come into estrus. The Jesey and half Jersey heifer were also bred but had exhibited no signs of estrus.
At 96 hours post CIDR removal 12 more yaks had red tail patches signaling having been or being in estrus. All the yaks who had not been in estrus before were bred at this time including the young small female which still showed no sign of estrus and a twenty year old cow who also stilllshowed no signs.
[The Jersey and half Jersey also showed no sign of being in heat by internal examination which would be expected since this was a Bos Indicus protocol and they would respond to the seven day Bos Taurus protocol.]
Dr. Anderson, based on how the remaining two yak cows which had not yet come into estrus felt when inseminating them, estimated they would come into estrus in at about 108 hours post CIDR removal – ten hours after being inseminated at 96 hours post CIDR removal.
=>Note: Two ½ ml straws were used at each insemination. All of the straws contained a minimum of 30M extended sperm and the lowest post thaw motility was 30% so at least 9 million motile sperm per insemination.
=>Note: Using the information from the Computer Assisted Semen Analyser the Progressive Motility of the post thawed extended semen was between 15% and 23% for the straws tested. Assuming two ½ ml straws with concentration of 30M sperm/ml and a progressive motility of 15% result in approximately 4.5 million or more progressively motile sperm at the time of insemination.
The twenty-two artificially inseminated yaks, the Jersey cow and half Jersey heifer were pregnancy tested on April 18, 204 using ultrasound:
8 yaks initially conceived: 36% conception rate
2 aborted post conception
Jersey & half-Jersey heifer: 50% conception rate
The remaining open yaks were rebred April 20 and 21 after being given Lutalyse on the 18th to bring them into estrus for a second round of artificial insemination.
16 yak cows rebred
Half jersey heifer rebred
Due to the heat of the summer it was decided to check pregnancy used Estrotech patches with a teaser bull present and not ultrasound because the stress of the heat combined with that of the ultrasound could induce an abortion.
4 yaks conceived second round of breeding (4/18)= 22% conception rate
Half – Jersey never came into estrus
Overall conception rate for the March-April AI: 12 conceptions out of 22 yaks = 54%, non yaks – 50%
=>The much-improved conception rate as compared to those of the first project FS1-335 can be attributed to improvements in processing the semen resulting in consistently better post thaw motility, using the Bos Indicus sequencing protocol, and having a better estimate f estrus through use of the Estrotech patches and the teaser bull whose presence might also have caused clustering of estrus in some of the yak cows.
We ran a second test of the Bos Indicus Artificial Insemination Protocol utilizing the Estrotech patches and teaser bull to better identify when the yaks were in estrus. The first insemination was done March 3, 2025. Eleven yak cows and a Jersey cow and half Jersey heifer were inseminated. Five of the yak cows were inseminated with semen collected from the bull Helios: A new bull we hadn’t collected from before. The remaining six yaks and the Jersey cow were inseminated using semen collected from a bull (Phantom) we had used previously and whose extended semen had consistently good post thaw motility and had resulted in previous AI pregnancies.
Of the five yaks inseminated using the semen collected from Helios: 0 conceptions (0%)
Of the six yaks inseminated with the semen from Phantom: 3 conceptions (50%)
Of the two non-yaks inseminated with Phantom’s semen: 1 conception (50%)
There were two early abortions post conception.
The Computer Assisted Semen Analyzer POST THAW results done using frozen semen samples for Helios and Phantom taken from the processed straws in the March and April inseminations were:
Extender used: BullxCell
Bull Total Motility at thaw Progressive Motility at thaw Progressive Motility at 3hrs
Helios 21.65% 9.95% 5.35% = 2.4M sperm
Phantom 40.29% 15.43% 20.26% = 9.1M sperm
[Total sperm contained per 0.5 ml straw: 22.5M Two straws per insemination.]
Unfortunately we did not have this information for his March-April round of insemination. If we had, we would never have used the extended semen from Helios and used the BullxCell from Phantom or Sad: Both of whom had much higher progressive motility at 0 and 3 hours post thaw.
For the April insemination after determining which yaks were open or pregnant from the March 3-4 insemination by ultrasound we gave all the open yaks except two 5 ml of Lutalyse as the yaks were already synched from the Bos Indicus protocol used for the March 3rd- insemination. The two not given Lutalyse showed no Corpus Luteum and as such the Lutalyse would have been ineffective.
Of those given Lutalyse (had Estrotech patches affixed with the teaser bull present):
- One yak showed estrus by Estrotech patch and teaser bull behavior at 5pm, April 13th – so she was inseminated more than twelve hours after showing estrus;
- Six of the remaining eight yaks were showing good signs of estrus based on the Estrotech patch and teaser bull on the morning of the 14th ; and,
- Two did not start to show any signs of estrus until the morning of April 15th.
The result was
- 1 yak inseminated more than 12 hour post estrus (but this would be during ovulation)
- 6 yaks in estrus (as indicated by Estrotech patch and teaser bull) inseminated April 14-15:
- 2 yaks just starting to how signs of estrus on the morning of April 15th and inseminated before full signs of estrus.
Dr. Anderson, the Reproduction Specialist, could tell by examination that the first yak was post estrus and said the six by internal examination were in estrus. These seven yaks would have had the greatest chance of conception.
=>The two yaks who were just starting to show signs of being mounted according to their Estrotech patches and their unwillingness to stand for the teaser bull probably would not have conceived since according to the CASA analyses of the semen three hours seems to be the approximate limit of having sufficient progressively motile sperm to inseminate (although the sperm would survive longer in the reproductive track than in a test tube at 99F.)
Our belief is that the insemination protocol [Bos indicus, Estrotech patches, teaser bull first round followed by Lutalyse, Estrotech patches and teaser bull] worked very well in telling us who was really ready to breed. The problem, as noted, was the semen.
=>Even though this project ends September 30, 2025, we have scheduled another round of insemination first week of December, 2025 wit the second round done approximately thirty days later. This time we will use semen from Phantom and the Bos Indicus – Estrotech patches-teaser bull protocol. The results from this round of insemination will be attached to this report.
- Research on The Hybrid Yaks
The plan of the project was to obtain information preliminary pieces of data concerning yak x angus hybrids.
- Artificial insemination pregnancy rate using extended yak semen and the standard beef AI seven-day protocol;
- Weight gain information: How weight gain of yak x angus hybrids compared to that of yaks; and,
- Quality of meat in terms of fat content and protein of grass-fed yak x angus as compared to grass-fed yak.
=>As noted previously, six University of Kentucky angus cows were artificially inseminated with extended yak semen with known good post thaw motility. Four of the six, 66%, became pregnant and calved in the spring of 2024 with birthweights between 50 and 60 pounds. Yak birth weights run between 25 and 40 pounds.

Hybrids at approximately one month.
=>A fifteen week comparative weight gain study on typical eastern Kentucky mixed pasture grass which was approximately two-thirds fescue endophyte infected fescue was held May 3rd to August 15th, 2025. The results were as follows:
Weight Weight % Increase
Hybrids May 2, 2025 August 22, 2025 in Body Weight Daily Gain
M201 F 572 738 29% 1.49lb
M202 B 631 775 23% 1.3lb
M203 F 464 630 36% 1.49lb
M204 B 570 700 23% 1.18lb
Yaks
Yak AB B 462 535 16% .66lb
Yak MB B 458 535 17% .69lb
Yak CB B 453 503 11% .45lb
Yak TB B 389 453 16% .58lb
Hybrid heifer at 15 months Hybrid bull at fifteen months
NOTE: We were not be able to do the meat analysis based on one of the hybrid bulls within the time frame of this grant. Once one of the hybrid bulls is ready to process for meat, a sample will be taken and analyzed and the results included as an addendum to this report.
Research Results and Discussion
This project had two overall objectives:
To improve the artificial insemination process developed under FS21-335 to the point it will yield good conception rates where the conception rate is calculated based on the result of two inseminations: The initial Bos Indicus sequencing protocol induced estrus insemination followed in approximately thirty days with a Lutalyse induced estrus for those yaks who had not conceived as a result of the first insemination. This means of defining the conception rate is the same as used for beef cattle: Overall conception rate after two cycles of artificial insemination.
To produce yak-angus hybrids could:
- Use for a comparative growth rate study with yaks; and,
- Compare the protein and fat content of the grassfed yak x angus hybrid meat with that of grassfed yak and angus.
The purpose being to see if there might be an economic gain for farmers to use yakxangus/yakxbeef hybrids in their operations.
We knew from a SSARE funded study, FS JEFFS STUDY, completed during this study that yaks consumed forage at roughly the same rate as a percentage of body weight as beef cattle. As such the forage consumption rates as a percentage of body weight for the yaks and yak x angus would be the same.
First Objective: Artificial Insemination Process
At the end of our first artificial insemination development for yak project, FS1-335, we had demonstrated the feasibility of artificial insemination for yak genetic improvement and on farm use but the motilities of the produced extended semen pre freezing were inconsistent as were the post thaw frozen extended semen motility percentages. Under this grant we have significantly improved our process from collection through filling the semen storage tanks after freezing by eliminating sperm loss due to exposure to surrounding air temperatures different than that of the semen at that moment in the entire process: From the bull the sperm were dying at collection because the air temperature was not 99-100F (ejaculate temperature)….all the way to loading the Goblets and Canes and placing them in the Liquid Nitrogen cooled semen storage tanks in which the air surrounding the frozen straws was 5C while the straws were at -196C.
Initiating temperature protection for the sperm throughout the entire process resulted in consistently good extended sperm motility. The following results are from a collection in February, 2025.
Notice that the semen extended with Bullxcell has 5-15% greater motility than the same semen extended with Optidyl.
With good extended sperm motility percentages the post motility percentages increased once the temperature controls to protect the sperm were put in place that would protect the sperm from taking the cooled extended semen out of the refrigerator to placing the frozen straws in the Liquid Nitrogen semen storage tanks.
=>The access and use of the Computer Assisted Semen Analyzer at the University of Tennessee Institute of Agriculture allowed us to more precisely estimate not only post thaw motility but progressive motility at post thaw AND progressive motility three hours after being thawed. The following are results of for semen collected after the temperature controls had been put in place and we were consistently getting good post thaw motility when viewed under a microscope.
Obs ThawTime Bull Concentration Extender Date % Motile Dead Progressive Slow Viable
1 0 Phantom 45 B 45406 33.1 34.4 16 6.1 65.6
2 0 Sad 45 B 45406 55.3 26.5 23.7 13.3 73.5
3 0 Phantom 45 B 45386 15.2 57.8 6.3 5.4 42.2
4 0 Fortyseven 45 B 45373 50.8 17.6 23.2 10.2 82.4
5 0 Sad 45 B 45373 52.1 30.1 12 19.9 69.9
6 0 Phantom 45 B 45373 47.3 30.4 15.8 11.5 69.6
7 3 Phantom 45 B 45406 53.2 17.7 30.1 7.6 82.3
8 3 Sad 45 B 45406 3.8 49.3 0.9 2.4 50.7
9 3 Phantom 45 B 45386 0.9 56.5 5 0.9 43.5
10 3 Fortyseven 45 B 45373 29.4 23.5 13 4.6 76.5
11 3 Sad 45 B 45373 21. 43.6 8.3 7.4 56.4
12 3 Phantom 45 B 45373 38.1 26.7 21.9 7.6 73.3
Extender used: Bullxcell
Lines 1-6 are at 0 hours post thaw
Lines 7-12 are at 3 hours post thaw
Definitions => Motility: sperm moving in direction or spinning
Progressive: Moving in a set direction or a gentle arc
Slow: Slower than average
Viable: Alive- included moving and more of a vibration in place
Note the drop in progressive motility from 0 to 3 hour post thaw. After reviewing all of the data available from the semen collected once we had corrected the temperature losses, analysis of the CASA data determined that in general 3 hours post thaw was the point beyond which there was insufficient progressively motile sperm to have a good chance of conception.
For beef cattle the assumption is that inseminated sperm is sufficiently viable for twelve hours after insemination to result in conception. The CASA results point towards 3 hours being the post insemination period of viability for extended yak sperm. THIS IS VERY IMPORTANT because rather than having a twelve hour breeding window for conception as in beef cattle, yaks have roughly three hour window for conception.
=>This narrow 3 hour window explains: 1. Why the actual conception rate using artificial insemination apart from semen quality is low; and 2. How important knowing when the yak cow is in estrus to get the insemination time correct.
The following table shows the parallel results when semen from the same collection extended with BUllxcell shown was extended using Optidyl (which included the egg yolk in powdered form) rather than requiring a fresh eggyolk as Bullxcell did. Obs ThawTime Bull Concentration Extender Date % Motile Dead Progressive Slow Viable
- 0 Phantom 45 O 45406 9 31.3 11.1 3.4 6 8.7
- 0 Sad 45 O 45406 43 5 20.3 10.5 61.5 0 Fortyseven 45 O 45373 35.3 27 16.3 8 73
- 0 Sad 45 O 45373 6 36.7 14.4 9.1 63.3
- 0 Phantom 45 O 45373 36 3 19.8 7.2 57.7
- 3 Phantom 45 O 45406 2 40.8 0 0.9 59.2
- 3 Sad 45 O 45406 9 43.9 . 0 0.7 56.1
- 3 Fortyseven 45 O 45373 40.1 0 .1 59.9
- 3 Sad 45 O 45373 6 46.8 0. 1.4 53.2
- 3 Phantom 45 O 45373 9 35.3 0 . 0.9 64.7
Extender used: Optidyl
Lines 1-5 are at 0 hours post thaw
Lines 7-10 are at 3 hours post thaw
While the 0 hour post thaw motility and progressive motility for semen extended using the Optidyl extender was quite good, at three hours the percent of motile sperm had dropped to under 2% no sperm were showing progressively motility.
=>When all of the CASA data for the semen extended using Optidyl was analyzed it was clear that the Optidyl extender did not protect the sperm post thaw for sufficient time to inseminate. The conclusion from the study is that Bullxcell, using a true fresh egg, is a good extender for extending yak semen to be used for artificial insemination.
After we were able to extend and freeze the yak semen and have good post thaw motility as measured by the microscopic observation, the focus was on determining estrus to maximize the conception rate. Unfortunaely we did not have the CASA three hour post thaw progressive motility data on the bulls or know that the Optidyl extender did not protect the sperm post thaw and therefore should not be used until after our final round of insemination.
We did know from the results of the first grant FS1-335 that the key to achieving higher conception rates (assuming good semen) was knowing when the yaks were in estrus. A switch from the seven day standard beef synchronization protocol to the Bos Indicus five day protocol which utilized Lutalyse at CIDR insertion and removal, combined with:
=>The use of Estrotech patches; and, =>A teaser bull – not only to help confirm estrus by the yak cow’s responses to his mounting, but by his presence causing a clustering effect in terms of the yak cows coming into estrus (if like beef cattle)
made to maximize our ability to detect when the yak cows were truly in estrus rather than by the condition of the interior walls of their vagina.
Furthermore, digital camera recording of four different yak cows with a bull present they had been synchronized using the Bos Indicus protocol gave us information about the expected time period over which estrus would most likely take place: Between 60 and 110 hours post CIDR removal.
=>In our March-April, 2024 insemination which utilized our improved extended semen from two bulls who consistently had the best (microscope observed) post thaw motility results, we had an overall conception rate of 54% for the two inseminations.
=>In our March-April , 2025 insemination we utilized the semen from a bull who consistently had very good microscopic post thaw motility and a new bull who we had never collected from. The semen collected from both bulls was extended and frozen with good motility at post thaw (by microscope).
- As stated in the Research Section of this report, the frozen extended semen from bull with a track record of good post thaw motility was used to inseminate six yaks and a Jersey cow in the March (first) insemination. When the cows were checked in April, three yaks had conceived (although two were undergoing an early abortion) a month later) and the Jersey cow had conceived: A 50% conception rate for the yak cows.
- The frozen extended semen from the new bull (Helios) whose semen also had good post thaw motility under microscope was used to inseminate five yaks: 0 conception rate.
When we pregnancy tested them using ultrasound after the first insemination round we gave 5ml of Lutalyse to of the open (and aborting) yaks. Three days later, using the Estrotech patches and teaser bull to determine estrus as in the first round of insemination, we rebred all but two the open yaks and an additional yak which was not part of the first round: A total of 9 yaks were bred. The two who were not bred did not show any signs of estrus. The semen we used was from the new bull (Helios).
We did not try to ultrasound the inseminated yaks thirty days post insemination or later throughout the summer out of concern we would cause a stress induced abortion due to the heat and our activity. A pregnancy test using ultrasound was conducted September 24, 2025 on the yaks inseminated in April. Being so long after the insemination date we do not know the conception rate since abortions between the insemination date in April and September 24th could have occurred.
After we had done the second round of the March-April 2025 insemination we received the CASA results on Helios. Two samples of his semen extended with Bullxcell were analyzed post thaw and three hours post thaw and two samples extended with Optidyl were analyzed three hours post thaw.
Bullxcell: 0 hr Post thaw Motility 0 hr Post thaw Progressive 3hr post thaw motility 3 hr progressive
Sample 1 6.6% 1.5% 1.4% 0.2%
Sample 2 36.7% 18.1% 24.9% 10.5%
Optidyl: - - 1.9% 1.7%
- - 5.5% 1.%
The CASA analysis using all of the data from the bulls we collected from revealed that Optidyl did not protect the sperm as time passed post thaw for all of the bulls. Sample 1 may have been from a straw that had problems during freezing.
Based on the March-April, 2024 and the March 2025 insemination using the bull with known good extended semen, the Bos Indicus Protocol with the use of Estrotech patches and a teaser bull appear to provide a reliable way to know if a yak is in estrus. The fact that the CASA data revealed there is only a three hour window in which the percentage of inseminated semen with progressive motility is acceptable by artificial insemination standards makes the timing critical.
Beef cattle insemination follows the AM-PM rule: Estrus in the morning, breed in the evening-Estrus in the evening breed the next morning.
We bred the yaks close to estrus: If the Estrotech patch was red in the morning we bred the yak by noon. At that time we didn’t know abuot the three hour window and assumed a window similar to beef cattle: That the progressive semen would last roughly twelve hours during which ovulation would occur.
=> What we don’t know is how long after estrus does ovulation occur in yaks. This needs to be researched. Once known the insemination would be done the number of hours between estrus and ovulation after estrus so as to get the three hour progressive motility window as close to ovulation as possible.
If yak are similar to beef cattle with ovulation occurring 12-24 after the beginning of estrus, then our insemination protocol work out like this:
_____EEEEEEEEEEEE___________ OOOOOOOOOOOO_______> time
SSS SSS
1ST insemination 2nd insemination
Where each E represents an hour starting with coming into estrus – standing heat at the 6th E.
Each O represents each hour of ovulation.
And each S represents an hour of sperm being progressively motile.
=>Note: We are getting conception during the first insemination which indicates that yaks are beginning estrus sooner than the Estrotech patches and teaser bull are indicating. Understanding how many hours estrus started in yaks before Estrotech patches or the teaser bull begin to show that they are nearing standing heat needs to be studied so we can better time the first insemination.
Even so, using the current insemination protocol with extended semen with good three hour progressive motility has yielded good results.
The key is to use semen from bulls which have good three hour progressive motility of their post thawed Bullxcell extended semen.
Second Research Objective: Yak X Angus - Research Results
The hybridization component of this project had three questions:
- Could we hybridize beef cattle easily with the yak semen we produced?;
- How the hybrids grew compared to yak bulls; and,
- How the meat from the hybrids compared to that of yak – especially in terms of leanness and protein.
We saw these as the basic questions small acreage farmers and beef producers would want to know the answers to before seriously considering yak – beef cross.
- In terms of the first question, as noted previously, six University of Kentucky angus cows were inseminated using frozen extended semen we had produced following the traditional seven-day beef cattle sequencing – insemination protocol. The result was two-thirds of the angus conceived and had calves. Dt. Les Andersen, Reproduction Specialist at the University of Kentucky College of Food, Agriculture and Environment noted the only issue will be the quality of the yak semen available: The seven-day protocol will work well and is familiar to AI Inseminators.
- Four yak bulls born in November of 2023 were compared with the four hybrids born in April and early May of 2024. The following chart shows the averages in weight and daily gain rate. Note, the hybrid group consisted of two females and two males.
Yak – YakxAngus Weight/Weight-Gain Data
Yak YakxAngus
- Birth weights: 30-35 lbs 45-62 lbs
- Average Weight at 12 months: ---- 560 lbs
- Average Weight at 15 months: ---- 710 lbs
- Average weight at 18 months: 440 lbs -----
- Average weight at 21 months: 506 lbs -----
- Average increase in body weight
- during three-month weight gain study: 15% 27.75
- Average daily gain during the
- three-month study: 0.6 lb/day 1.36 lb/day
=>Note: The average daily weight gain for yearling angus on grass ranges from 1.5 to 2.5 pounds daily depending on the quality of the forage. On lower-quality native or warm -season grasses this may decrease to daily gains of 1-1.5 lbs.
Clearly, in terms of growth, the hybrids outperformed the yak bulls weighing forty percent more than the yaks at six months younger age. If one was to assume that the hybrids maintained a 1.36 lb/day weight gain into the future, the hybrids would reach a thousand pounds at 21 months: Twice the weight of the yak bulls in this study at 21 months.
So, as far as achieving a process-ready weight, the hybrid is far superior than the yak in terms of time.
- Comparison or yak meat to the yakxangus meat. Due to the weight/age of the yakxangus hybrids we will not be able to process one of them to evaluate its meat for another five to six months. When we have had the hybrid meat evaluated we will attach the results as an addendum to this report.
A comparison of grassfed premium and choice angus, bison and grassfed yak sirloin by the University of Kentucky showed:
- All three were very close in protein at 23-24%,
- Yak had more moisture,
- And the yak sirloin had 1% fat, bison had 2% and the premium and choice angus were at 5-6% fat.
The reason this data is included is because of the similarity of yak and bison meat in terms of protein and leanness, one would expect that the protein and leanness of yakxangus would be close to that of beefalo which is a bison – beef cross that is at least 3/8 bison.
The following is from an article entitled “The Other Red Meats: Michigan Bison, Beefalo and Yaks” [12/12/2023: Taste the Local Difference https://www.localdifference.org]
“According to the American Beefalo Association, beefalo is a mix of ⅝ domesticated cattle and ⅜ native American bison, and now a USDA-recognized breed. Any higher percentage of bison and the animal is referred to as a cattalo.
Ground Beef Nutrition Vs Ground Bison, Beefalo, and Yak
Based on testing from the USDA, beefalo demonstrated higher levels of vitamins, more protein, ⅓ less cholesterol, 79% less fat, and 66% fewer calories than conventional beef. It’s even won the “Best Steak” award at the American Royal Steak Competition.
According to the International Yak Association, yak meat is quite lean. It does not marble like conventional beef, rather the fat forms on an exterior layer that is easy to cut away. The flavor is mild, clean, and juicy due to its higher moisture content.
Similar to yak meat, bison also has no marbling, and therefore, a richer red color than beef. Its flavor is slightly sweeter than beef while containing fewer calories.
Explore the following nutrition information for a more detailed comparison between the different meats:
- Beef (90/10) (100g): 185 cal; 13g fat; 18g protein
- Beef (80/20) (100g): 243 cal; 19g fat; 18g protein
- Bison (100g): 179 cal; 9g fat; 25g protein
- Yak (100g): 150 cal; 7g fat; 20g protein
- Beefalo (100g): 143 cal; 5g fat; 23g protein.”
Our expectation concerning the yakxangus meat is that it will be very similar to that of beefalo: Possibly leaner due to the fact that it will be 50% bison and not as low as 37.5% bison. Aso, the protein should be slightly higher based on the meat samples we have had tested.
If this is the case, then yakxangus, or yakxbeef in general will be a naturally leaner, high protein red meat that has a much faster growth rate than yak. It may also serve as a “gateway” to people moving to yak meat out of desire for an even leaner meat.
Educational & Outreach Activities
Created a power point which designed to introduce yaks and basic care issues to farmers in the Southern Region: Sent out through the Cooperative Extension system: Sent out with link and QR Code, Also to Historical Black Universities with agricultural programs and Black farmers organizations Black Farmers Network, National Black Farmers Association, Southeastern African American Farmers Organic Network and to the Kentucky Black Farmers Association.
Created a booklet on Artificial Insemination In Yaks from collection to insemination for people wanting to get involve in semen collection, processing, and insemination.
Participation summary:
Education/Outreach Description
Participated in the Yak Conference held at Morehead State University on October 31, 2023 which was part of SSARE Project 0522-157. Our participation focused on familiarizing the yak breeders in attendance with the AI process from collection to insemination and included demonstrations
Over these two projects FS1-335 and FS23-352 it has been hard to interest beef producers and farmers enough to take the time to attend a presentation/conference. An article in a beef producer magazine which has a readership of over 10,000 farmers and an article in Mother Earth News resulted in no inquiries.
What we decided to do was focus on “Introducing Yaks” and the basics involved in raising them: Not to sell the person on yaks but to create enough interest in some that they would reach out for more information. We also needed to do this in a way which would not “interrupt” their work schedules and allow them to learn about yaks at their convenience without having to sit through a long presentation.
What we have done is to put together a power point presentation which can be downloaded on one's computer or phone and watched piecemeal as a person had time. The power point is:
From the Himalayan Mountains to Eastern Kentucky
The Domestic Yak
An Introduction to Yaks
and Yak Care.
A link and QR code to access the power point has been sent to the Cooperative Extension Agents in the Southern Region; To the Black Farmers Network, the National Black Farmers Association, Southeastern African American Farmers Organic Network, and the Kentucky Black Farmers Association; and, To the following Southeastern HBCU Agricultural Programs:
- Florida A&M University
- Tuskegee University
- North Carolina A&T State University
- Alcorn State University
- Southern University and A&M College
- Fort Valley State University
- South Carolina University
- Alabama A&M University
- University of Tennessee at Martin
- and, Kentucky State University.
The link and QR Code are being posted on The North American Yak Association website and on the Face Book social media. The pamphlet we have put together on yak artificial insemination is being posted on The North American Association website as a public resource.
Learning Outcomes
A small acreage farmer in North Carolina with a small number of yaks and beef cattle requested semen to inseminate their beef cattle want to produce yak x beef for meat.
A beef producer in Brazil contacted us about obtaining yak semen to produce hybrid beef.
One yak meat producer with over 80 yak requested information on sequencing yak cows for their breeding program this spring. The farm will be implementing the Bos Indicus protocol we have started to use in this grant.
A second yak breeder has requested information on the AI program development and wants to implement in their herd to develop a possible yak x beef hybrid meat herd.
A third yak breeder wanted information on cross breeding yaks with Highland cattle using artificial insemination.
The fourth yak breeder is myself.
The USYAKS Yak Association Science Committee is following the progress being made as they want to be able to have a successful AI procedure available to address the high coefficient of inbreeding in the US due to the small gene pool the herd is built on.
The one yak breeder in New Zealand has requested information on the AI process we are developing due to the high coefficient of inbreeding in his herd which is impacting fertility, growth rates and health in his herd. He specifically wants to be able to successfully inseminate his cows if he is able to secure yak semen from abroad.