Progress report for FS23-352
Project Information
SSARE Project FS1-335 provided the opportunity for our research team to demonstrate that artificial insemination could be developed to work in the yak industry. During that project we had nine conceptions determined by ultrasound resulting in five healthy calves. The other four conceptions, we believe, aborted due to stress caused by a severe weather event during the second month of pregnancy. We also, at the beginning of this project, inseminated six angus cows owned by the University of Kentucky twice which has resulted in two hybrid calves and two more close to birth: A 66% conception rate with two inseminations per cow.
This basic purpose of this project is two fold: First; To improve our Artificial Insemination Process, from semen collection to actual insemination, to the point that it is a practical means to assist yak ranchers in improving their herd genetics, small acreage farmers who do not want to maintain a bull or desire to introduce yak genetics into their current operation, and larger beef producers who want to introduce yak genetics into their herd, and: Second; To perform a comparative growth study between yak, yak x angus and angus calves so that beef producers can make informed decisions about introducing yak genetics to their operation. Included in this will be a comparative meat analysis in terms of protein, fat content and taste of grass fed yak, grass fed angus and grass fed yak x angus sirloin to assist in meat production decision making.
The specific FS23-352 project objectives we worked on during this first year of the project are as follows:
- To successfully AI the angus at the University of Kentucky available for us use in this project as early as possible in the project so that we would be able to perform the comparative study described in the abstract.
- To review our semen collection protocol to determine where losses in sperm might be taking place before it is extended.
- Review our semen extending process to minimize the loss of motile sperm before freezing.
- To run side by side processing using several extenders to determine which extender yields the highest post thaw percent sperm motility.
- To determine if it is better fill straws before or after the initial cooling of the extended semen to 5C (41F) over 1.5 hours.
- To see where the freezing system used can be improved. This includes the actual freezing and going from having frozen straws in liquid nitrogen to having them in the LN storage tank.
- How to improve our actual insemination process to increase the conception rate.
The final objective during this first year was to participate in a SSARE sponsored Yak Conference, Project O522-157, help October 31, 2023.
The research/work done on each of these objectives follows. I have attempted to develop the sections addressing semen collection through insemination so they can serve as a "step by step" how to guide for persons interested in doing a yak AI program. This material will be uploaded to the SKAY project information website.
Cooperators
- (Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- (Researcher)
Research
A. Semen Collection
One of the key things we have learned in this project is, at least in terms of the twelve bulls we have worked with, that yak sperm is very ambient temperature sensitive. Like beef bulls, and as shown in the FS21-335 final report, yak semen quality in terms of motility and morphology is very dependent on the ambient temperature during the sixty days it was formed. Between the end of April and the at least mid October, while yak bulls can get yak cows pregnant in the field, the quality of their sperm is very low in terms of percent motility and percent with good morphology. Furthermore, if the bull was sick or had other sources of stress during sixty days of semen development, the quality of the semen can be negatively effected.
Also, and this is critically important relative to the entire AI process from semen collection through insemination, semen is very sensitive to the ambient temperature and extended semen is very sensitive to increases in the ambient temperature (which will be addressed in the sections on extending semen, cooling and freezing semen).
Over the past thirty plus years the development of quality beef bulls has included scrotal size, which is a heritable trait, as a key EPD. This is because the larger the scrotal size the greater the quantity and quality of the ejaculate as well as impacting the reproductive maturation of the heifers sired. This has not been done with yaks in the United States. There has been no emphasis in breeding programs on increasing scrotal size - equivalently, the quantity and quality of the semen. This "difference in quality" means that "Just because you can do it with beef semen does not mean you can do it with yak semen."
Consider how beef semen is typically handled during collection. The person doing the actual collection keeps their hand around the collection tube when catching the ejaculate. The collection tube is then either kept in their hand or placed in a pocket and taken to a microscope at the facility so a drop can be withdrawn and evaluated for motility. After that is done the collection tube will be placed in a water bath at approximately 99F.
This works very well for beef semen and as such this is how we had been collecting the yak semen. The problem is that the yak ejaculate is 101F and being caught in an a hand held test tube which has been exposed to ambient temperature, Since we must collect yak semen during the months of November through April (at the latest) the ambient air temperature will be cool and the hand held test tube will be at a lower temperature than the yak semen. Once it is collected the semen will be exposed to a lower temperature until it is put in the water bath which then at a higher temperature of 99F. In our situation, the semen was in the collection tube for close to five minutes in the outside ambient air during the motility measurement and transporting it to the heated room (only in the 70s's) where it was then placed in a 99F water bath.
I belabor this because "in doing what worked for beef semen collection" we unknowingly were killing sperm in our collection tube.
The solution was quite simple.
First: Once collected, the semen had to go to the water bath as fast as possible. A pipette was used to draw off about two drops of semen right after collection: One used to determine motility under a microscope and the second to be later stained to determine the percent of sperm with good morphology.
Second: A common chemical reaction toe warmer will produce an average temperature of 102F and last several hours. The toe warmer can be wrapped around the collection tube and held in the collector's to keep the semen much closer to 101F from collection to placement in the water bath.
Yaks sperm will die with cooling caused by the ambient air and will die from suddenly going from being cooled to suddenly heated in the water bath.
So the key steps in semen collection which we have come to use are:
- Yak bull is weighed (looking for changes which would be a sign of a health issue that could impact sperm quality.
- The yak's temperature is taken - again if out of the normal range could indicate a problem with the sperm,
- The yak's scrotal circumference is measured. In the bulls we have worked with a bull with a scrotal circumference less than 27cm never had semen with motility and morphology good enough for AI.
- The bull's Cowper's and Prostrate glands and the Seminal vesicle were very gently manually massaged for approximately two minutes.
- During this time the toe warmer was applied to the collection tube and given time to bring it up to temperature (in the hand of the collector).
- A small bovine electro-ejaculator was lubricated and inserted and ran on automatic intensity advancement. [This is like an internal muscle stimulator TENS unit for pain management.]
- Once the electro-ejaculator had advanced through its cycle, the collection tube was held in place to collect any final ejaculate and then take to the microscope where a pipette was inserted in the warmed collection tube to withdraw drops of semen for motility and morphology evaluation. NOTE: The collection tube had the toe warmer around it and the collector's hand around it to keep it warm from before the electro-ejaculator cycle was started to when the semen was put in the water bath. The time from the collection to the water bath must be kept at a minimum.
- The drop of semen used for determining motility is quickly placed on a slide that was on a slide warmer along with a prewarmed cover. A cold slide or cover will kill sperm resulting in a low motility reading. The motility reading is critical as our experience with yak has been IF THE MOTILITY IS LESS THAN 70% THEN THE SEMEN WILL NOT WITHSTAND THE EXTENDING, COOLING AND FREEZING. THE SAME IS GTRUE FOR MORPHOLOGY.
KEY SEMEN COLLECTION GUIDELINES:
1. KEEP SEMEN AS CLOSE TO 100F AS POSSIBLE DURING COLLECTION AND UNTIL IT IS PUT IN THE WATER BATH.
2. IF MOTILITY OR MORPHOLOGY ARE LESS THAN 70%, THE SEMEN IS NOT GOING TO EXTEND WELL. Note, if the motility or morphology are low it does not mean the bull is "bad". It could be the result of the bull not having ejaculated for quite a while. We have learned to let the bulls we intend to use for AI in with several cows in estrus so they can have done some breeding prior to collection. A low motility and/or morphology can also indicate the bull was under some kind of stress during the prior sixty days while the semen collected was being formed or that that there is a health/nutritional issue.
Also: AI requires high motility and morphology. As a herd bull the entire ejaculate (100's of millions of sperm) will be used to breed one cow. In AI it is more like ten to twenty thousand sperm - and not all of these are alive to start with as will be explained in the semen extending research section.
B. Extending Yak Semen
In this project we have used a number of different extenders. Some we purchased and others were TRIS extenders made by Dr, Lehmkhuler using formulas that had been used for years with beef cattle. In our experience the egg based extenders (ones where egg yolk was added or that contained a powdered yolk in them) worked the best.
With respect to adding egg yolk, the Researchers at the ICAR - National Research Centre on Yak in India were very clear about using fresh eggs. Eggs purchased at a grocery while fresh enough for consumption may not be fresh enough for making a high quality extender. Using the simple "how an egg sinks" method on eggs purchased at a grocery revealed than many were far from fresh. During this project we switched to farm eggs layed within twenty-four hours of making the extender verses store bought organic eggs used in the previous project FS21-335. The extender we have made under this project has been very good in terms of post thaw motility which, in part could be partially due to egg "freshness".
In using a fresh egg yolk we first make sure the shell is clean using distilled water and then break the egg shell keeping the yolk inside it and drain off as much of egg white as possible. Once this is done we roll the yolk out of the shell on paper towels and roll it around on the towels so they can capture the fluid on the exterior of the yolk leaving a clean "dry" yolk. We then use a pipette to pierce the yolk and withdraw the amount needed from within.
We use a warmer with a magnetic stirrer to mix the extender and added egg yolk. It is important that the prepared extender be a homogenous solution
Once prepared the extender(s) should be kept in the water bath before the semen arrives from collection. It is important to note that some extenders will go bad within a certain amount of time. One we use must have the semen added within ten minutes of making it, so timing the semen collection and having the extender ready at water bath temperature is critical.
Once the collected semen has been put in the water bath a sample is taken so that we can determine the sperm concentration per ml using an Accu-cell Bovine Photometer. Note: This device gives a sperm count per ml but does not differentiate between live and dead sperm. So if the concentration is 400 Million sperm/ml and the motility is 75%, one would assume a live sperm count of 300 Million sperm/ml.
Once the concentration of sperm is known the amount of extender needed to protect the semen can be calculated. During the past project FS21-335 we extended to achieve a sperm concentration of 100 Million total (alive and dead) sperm per ml in order to try to account for motility and morphology so that the living sperm concentration in the extended semen would be at least 60 Million sperm/ml. To achieve this concentration required we often had to use the minimal amount of extender allowed: a 1:1 semen to extender mix. But recall, this is for beef bulls, not yaks. Using this small amount of extender may have not been enough to protect the yak semen which is so temperature sensitive during freezing and thawing possibly contributed to the lower post thaw motility numbers we had been achieving during the FS21-335 project. [Again, what one must always keep in mind is that yak are not beef cattle: because a product or process works a certain way for beef cattle it does not mean it will work thew same way for yaks.]
In this project we have experimented with using extended total (alive and dead) sperm concentrations of 30 Million, 45 million and 60 million per ml. Over the past four times we collected and extended semen the 30 Million and 45 Million total sperm per ml post thaw numbers have been excellent and better than the 60 Million total sperm /ml concentration post thaw results.
The following are the post thaw results of three freezings of extended sememnfrom three bulls using two different extenders: BullXcell and Optidyl. The BullXcell uses a fresh egg yolk and the Optidyl comes with the egg yolk premixed (powder).
Bull & total sperm concentration Freeze # BullXcell post thaw motility Optidyl post thaw motility
Bull #47 30 million/ml 1 45-50% 35%
Sad Eyes 45 million/ml 1 55 % 40%
Phantom 30 million/ml 1 35% mid straw 40% straw end 30%
Phantom 45 million/ml 1 45%
Phantom 60 million/ml 66 minutes post thaw 30%
Bull & total sperm concentration Freeze # BullXcell post thaw motility Optidyl post thaw motility
Bull #47 45 million/ml 2 30% 25%
Sad Eyes 45 million/ml 2 50 % 45%
Phantom 45 million/ml 2 45% 35%
Bull & total sperm concentration Freeze # BullXcell post thaw motility Optidyl post thaw motility
Bull #47 30 million/ml 3 40%
Bull #47 45 million/ml 3 35%
Sad Eyes 30 million/ml 3 45%
Sad eyes 45 million/ml 3 45%
Phantom 30 million/ml 3 40% 45%
Note: If we take the lowest post thaw number which is Phantom, concentration of 30 million/ml and a post thaw motility of 30%. The motility of the semen collected from Phantom was 70% so at most 70% of the 30 million sperm/ml were alive at the time of freezing which is 21 million/ml. The post thaw motility of the 30 million sperm was 30% 0r 9 million sperm which means that 9 million of the 21 million sperm lived = 42% - which is very good since the rule of thumb for beef is that half the sperm will die during cryopreservation.
Again, as in the case of collecting semen, part of what made these results possible is that until the semen was extended it was kept at water bath temperature - 99F as was the extender. Once we have calculated the amount of extender required to make the desired sperm concentration/ml extended semen for insemination, the addition of the extender to the semen should be done as quickly as possible with the extended semen retuned to the water bath. Ideally you do not want the semen temperature to cool off while adding extender and then warm up when returned to the water bath. You want to minimize stress on the sperm.
C. Cooling the Extended Semen
Once the semen has been extended the next step is to cool it down to 5C=41F at a rate of approximately 0.25 degrees per minute. The total change in temperature will be the water bath temperature of 37C (99F) - 5C = 32C. An average rate of colling of 0.25C/minute will take approximately 32/0.25 = 128 minutes or just over two hours. we use a two cubic foot laboratory refrigerator which has a digitally controlled temperature. There is no way to set a cooling rate so what we had to do was to experiment setting different volumes of water in the refrigerator at 37C and using a digital thermometer with a wire probe watch how the interior temperature dropped over time if the refrigerator was at 5C before the warmer fluid was put in it. We did not have to wait the entire time for the refrigerator to cool to 5C but only long enough to see how many degrees it was cooling per minute once the a constant cooling rate developed. Once we had a volume of water at 37C that settled in at cooling at about 0.25C per minute we were done. In our case it was 400 ml. Knowing this if we were going to cool a total of 40 ml of extended semen we would put its container in a beaker of 360ml of water. If we were cooling 100 0.5ml filled semen straws we would deduct 50 ml for the semen and an additional amount for the rack or container that held them. The goal is to cool at a rate about 0.25C per minute. Taking a bit longer is better than cooling too quickly.
Note: The cooling needs to be done in a cold room: A room whose temperature is 5C. This is because once the extended semen has been cooled it cannot be warmed up without damage. The following will take place in the cold room:
Fill straws with the semen - done easily with a semen straw filling nozzle which allows you to fill up to 15 straws at a time.
Place an air pocket at the open end of each straw using a semen straw comb
Seal the end open end of the straw
Load the straws on a rack for additional cooling and then freezing or in a tray for the additional cooling . The cooling is for three hours at 5C in the refrigerator.
After the 3 hour cooling the straws are frozen and then loaded in a Liquid Nitrogen tank for semen storage.
Note: once the semen has been immersed in Liquid Nitrogen and is frozen at a temperature of -196C (-320F), it must be loaded in the Liquid Nitrogen tank in a way that minimizes its warming - which it will do in the cool room which is at 5C.
D. Loading straws after extending before cooling verses loading after cooling in the cold room.
Semen straws can be filled after extending rather than in the cold room. The filling, making an air bubble at the open end and sealing are done the same. After filling the straws they will be at the temperature of the room you filled them in. The extender will have protected the sperm from the change in temperature from the warming bath 99F to the ambient room temperature.
You still need to slowly cool the semen in the straws to 5C at a rate averaging about 0.25C/minute.
The way you do this is to determine how much water in a beaker at room temperature for the refrigerator to cool it at approximately 0.25C/minute in the same way discussed previously. Once you have determined that volume what you need to do, depending on the number of straws you are cooling, is to sandwich the straws in a sealed baggie between two baggies partially filled with room temperature water (one below the straws and the other on top). The straws are in a sealed empty baggy to prevent them from getting wet if one of the water-filled baggies leaks. The total amount of water you can use to fill the all baggies is the volume of water you determined was needed to be in the refrigerator it would cool at 0.25C/minute minus the total volume of semen in the straws.
As an example, suppose it took a beaker with 350 ml of water at room temperature placed in the refrigerator so that it would cool at approximately 0.25C/min when s precooled to 5C. Suppose you have 100 0.5cc straws. Then you would need a total of 300 ml in your baggies as the total extended semen in the straws was 50ml. If you could put the fifty straws between two baggies with water then each baggie would have 150ml water at room temp. If you have to divide the straws with 50 straws between two sets of baggies then each baggie would have 75ml water. You need to be careful that there is sufficient water in the baggies so that the straws are surrounded by water and not essentially lying on empty plastic which will cause them to cool too quickly.
We have experimented with both methods if filling warm and then cooling or cooling and then filling. The post thaw motility results were very close with cooling before filling post thaw motility being 1-2% better than filling warm and then cooling.
Either way, the refrigerator needs to be in a cold room so that the cooled semen will not start to b=warm before it is frozen when removed from the refrigerator and that the straws frozen in LN can be stored with minimal temperature change.
Once the straws have been cooled to 5C they need to continue to cool at 5C for an addition all three hours to prepare the extended semen for freezing.
F. Freezing the cooled straws.
Once the straws have cooled for the additional three hours at 5C the extended semen is ready to be frozen in liquid nitrogen. in this project we have used two freezing techniques. The first is the same technique we used during the FS21-335 project. The system we used was one we built to control the descent of the freezing rack holding the semen straws into a Styrofoam container 21x13x10.5 inches (inside LxWxH) with 1.5m inch thick walls and a removable friction fit cover into which we had poured enough liquid nitrogen into which we could completely submerge the freezing rack with the semen straws. The system was a hand crank operate pulley which lowered what looked like the lift system on the front of a forklift made of wood with two right angle brackets large enough for the freezing rack to securely sit on.

A digital thermometer that could read below -140 C and had a wire temperature probe which we could attach to the rack without the exposed probe wire touching the rack was used to monitor the temperature of the straws as it was lowered according to the following protocol:
Starting temperature 5C => the brackets holding the freezing rack need to be able to raise approximately five inches above the walls of the Styrofoam box to keep the rack above the LN vapor in the box in order to start the process at 5C.
- Five minutes to go "smoothly" from 5C to -10C
- Four minutes from -10C to -40C
- Two minutes from -40C to -100C
- Three minutes from -100C to -140C
- We held the racket at -140C for one minute and then plunged it into the liquid nitrogen.
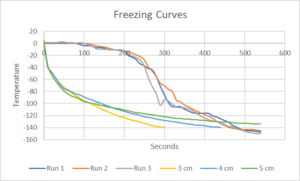
The closer the rack is to the liquid nitrogen the more a slight lowering would decrease the temperature. The Temp vs. Time graph shown is of three times we froze semen using this protocol. What is important is that even though the descents were not smooth and had large differences, all of the resulting post thaw motilities were very close to one another.
To test the necessity of a controlled temperature descent we used a second system to freeze the semen, This system consisted of a rectangular metal container 10x10x8 inches which was filled two-thirds full of liquid nitrogen. A small rack that could hold about twenty semen straws was mounted on a rectangular metal frame mounted on two Styrofoam pontoon that floated on the liquid nitrogen. The frame was designed to set how high the straws would be above the liquid nitrogen. The adjustment is from 2 to 5 centimeters. The temperature probe of the digital thermometer was attached to the rack holding the straws in order to monitor the temperature of the air surrounding the straws.
Once the frame is adjusted to the desired height the straws should be above the liquid nitrogen, the frame with the straws is placed on the liquid nitrogen and the temperature is monitored. When the temperature decreases to -140C the frame is manually turned over and the straws submerge in the liquid nitrogen.
The chart below shows the post thaw motility of straws frozen using both methods. In both cases the straws were taken directly from the liquid nitrogen and placed in a semen straw warmer set at 99F for one minute before being cut in the center and placing a drop of the thawed extended semen placed on a warmed slide with a warmed cover to be examined under a microscope for percent motility. Note: Whenever motility is being evaluated at least two individuals separately estimate the percentage of mobile sperm.
Post Thaw Motilitiy - Comparison of Freezing Methods
Bull Sperm Conc. 3cm above LN 4cm above LN 5cm above LN Timed lowering into LN Freezing Method
Sad Eyes 30M BullXcell 50% 45% 40% 45%
Sad Eyes 30M Optidyl 45% 25% 50% 45%
Sad Eyes 45M Optidyl 40% 25% 30% 40%
#47 30M BullXcell 50% 45% 25% 45-50%
#47 30M Optidyl 25% 25% 25% 35%
#47 45M BullXcell 20% 40% 30%
#47 45M Optidyl 25% 25% 25%
Phantom 45M BullXcell 45% 35% 45%
The following figure shows the temperature of the rack the extended semen straws were placed on during the two methods of freezing. The gray, blue and red curves are for three freezing runs using the timed lowering of the rack through the LN vapor and into the LN and the yellow, light blie and green curves show the temperature change using the float and plunge method with the colors indicating how high above the LN the rack was floating until the temperature at that height decreased to -140C.
F. Loading Frozen Semen Straws into LN Storage Tank for Preservation
Under SSARE Project FS21-335 after the straws were submerged in the LN at the end of the freezing process we would use tweezers and pick up the straws, place them in a Goblet which was already attached to a Cane and lower it into the LN tank. Every time a new straw was added the Cane would have to be raise out of the LN tank high enough to place the straw in the Goblet and then lowered again. Even being done in the cold room at 38F the straws were going from -196C into 5C long enough to place them in a Goblet and for the Cane with the Goblet to be lowered in the LN tank which was at -196C, and then raised and lowered four times until the Goblet was filled with five straws. While this was done as quickly as possible, the straws were exposed to five temperature variations, the largest being from -196C to 5C and back to -196C for at least 8 seconds.
Under the current project what we have done is to fill the Styrofoam container used to lower the freezing rack to a height (1.5-2 inches) of liquid nitrogen. Once the straws are submerged on the rack in the LN at the end of freezing process we remove the rack leaving the straws in the LN. After several minutes we start sampling straws for post thaw results. Taking them one at a time from the LN and quickly move them to the warm room and place the straw in a semen straw warmer at 99F. [This does not harm the extended semen as all temperature changes [From -196C to the cold room temperature of 5C to the warm room at 22C and then into the warmer at 37C ] are all increasing temperatures. After one minute in the warmer the straw is cut in the middle and a drop of the extended semen is placed on a warm slide with a warm cover plate placed over it. The thawed extended semen is the examined by at least two people (experienced in estimating motility) who estimate the motility and who do not share their result until each person has examined it.
While frozen straws from each combination of bull, extender and sperm concentration are being examined for post thaw sperm motility, the straws lying in the LN in the bottom of the Styrofoam container are being loaded in Goblets which are then placed in Canes with everything submerged in the LN. This is done using 16 inch tweezers. Once a Cane, which holds two Goblets filled with five straws each is filled, the Cane is quickly moved from being submerged and with extra LN surrounding the straws in the Goblet is quickly moved in the LN straw storage tank. This method almost completely eliminates any temperature change exposure to the straws once frozen.
G. Insemination
The critical issue in the artificial insemination of yaks is knowing when to inseminate. During the FS21-335 Project and during the beginning of this project when we artificially inseminated the angus at the University of Kentucky with frozen yak semen we were following a slightly modified seven day CIDR protocol which is used for beef cattle. In this modified protocol we eliminated the injection of gnRh at the time of CIDR insertion, left the CIDRs in for seven days and upon removal injected the yaks with 5ml of Lutalyse. Approximately 72 hours after the removal of the CIRDs, the time used in the beef industry, we would inseminate the yaks. We would then do a second insemination at approximately 96 hours. What we did know at the time of insemination was if a yak's reproductive system was near estrus but we could not tell if the yaks were in actual standing heat. The fact that we did not know exactly when they were in estrus meant that breeding results have been inconsistent. Using unfrozen extended semen on a group of ten yak cows we had a conception rate of 40%. With frozen semen we have had conception rates between 0 and 25%using the same sequencing protocol. When we inseminated the angus we had a 66% conception rate with pour frozen semen which was excellent. The reason it worked so well was because they were breeding angus for which the seven day protocol works very well.
Dr. Anderson had researched different sequencing protocols being used at the yak research centers in China and India and, based on their insemination results, has recommended we use a five day protocol developed for Bos Indicus which consists of giving 5ml of Lutalyse at the time of CIDR insertion and a second 5ml injection of Lutalyse when the CIRD is removed. GnRh would be given after in insemination if the yak was not in standing heat. To test this system we set up four trials in which we sequenced three yak cows following this system in or corral in which we had positioned four game cameras. Twenty-four hours after removing the CIDRs we brought a bull into the corral with the cows and turned the cameras on letting them run until approximately 120 hours from the CIDR removal. In all four trials standing heat ranged from 60 hours to 110 hours. Within any one trial there was no clustering of estrus.
We still did not know if a cow was estrus within the time range. To solve this we took a friendly bull that was no longer consistently producing semen good enough for this project and Dr. Prater performs an epididymectomy, removing the epididymus which holds the sperm before entering the vas deferens. In a bull the epididymectomy is far easier on the bull and less invasive than a vasectomy. The purpose of this was to have a completely intact bull who simply coiu0ld not get the cows pregnant. This bull would serve two purposes. Having a bull present with cows helps stimulate and "cluster" estrus in beef cattle and the behavior and the bull (mounting) would signal which cows were in standing heat. We could tell this by placing patches made for this purpose just above the tails of the yaks. These patches will turn a bright red from the pressure and movement of being mounted by the bull.
Our first attempt to use the Bos Indicus sequencing system with the "teaser" bull and use of tail patches was done on March 13th, and 14th, 2024 after we had made the three successful semen collections with high post thaw motility results. Twenty-one yaks were sequenced along with a Jersey cow and half Jersey heifer.
Approximately 48 hours after CIRD removal one yak started showing signs of being near estrus as detected by her movements using the Cattle Manager System which will successfully predict estrus in beef cattle based on their movements.
By 60 hours after CIDR removal the bulls in an adjacent pasture were all along the fence-line. The teaser bull had mounted the one yak who showed signs of estrus earlier according to Cattle Manager. This was known by the patch having been rubbed red.
At 70 hours post CIDR removal 5 yaks we in estrus (red patches). All of the yaks were bred except a small 400 pound three year old female. We were going to wait to see if she would come into heat. The Jesey and half Jersey heifer were also bred but had exhibited no signs of estrus.
At 96 hours post CIDR removal twelve more yaks had red tail patches and were in estrus. All the yaks who had not been in estrus before were bred at this time EXCEPT the young small female which showed no sign of coming in heat and a twenty year old cow who also showed no sign of coming into heat. The Jersey and half Jersey also showed no sign of being in heat by internal examination which would be expected since this was a Bos Indicus protocol and they would respond to the seven day Bos Taurus protocol. Dr. Anderson, based on how the remaining two yak cows which had not yet come into estrus felt when inseminating them, believed they would come into estrus in at about 108 hours post CIDR removal.
Two straws were used at each insemination. All of the straws contained a minimum of 30M extended sperm and the lowest post thaw motility was 30%. In beef cattle the semen will survive approximately 12 hours once placed in or at the cervix. Also, once a cow comes into estrus the basic AI procedure is to breed her after twelve hours during ovulation. The breeding at 72 hours should have covered the yak that was showing signs of estrus early on and the two who were predicted to be in standing heat at approximately 108 hours assuming the viability of the sperm for twelve hours.
These animals will be pregnancy tested approximately the week of April 15th, a minimum of 28 days post insemination, using ultrasound, Those who are open will restart the Bos Indicus sequencing protocol with the exception of the twenty year old and three year old yaks. The Jersey cow and half Jersey will start the seven day protocol two days earlier so they can be rebred when the open yaks are. The seven day protocol does not include giving Lutalyse in the beginning so if they are pregnant inserting the CIDR before ultra-sounding them two days later will not harm a possible pregnancy.
Research on the yak hybrids has not started yet as we are awaiting the birth of the final hybrid involved in the study.
As stated in the abstract, research wise this project has two overall objectives:
To improve the entire artificial insemination process developed under FS21-335 to the point it will yield conception rates which will make it a reliable means for:
- Yak breeders to introduce new yak genetics into their breeding programs
- Small herd yak breeders to eliminate the expense and infrastructure required to maintain multiple bulls for their operation
- Breeders interested in importing yak semen
- Small acreage farmers who desire to introduce yak genetics into their operation. Note: In general most yak bulls will not breed non-yak bovine and as such AI is the only way to introduce yak genetics through cross species breeding. Because of the calf size birth limitations of yak cows, using Bos Taurus bulls to breed yak cows is not a safe option.
- Commercial beef producers to bring yak genetics into their production portfolio as a niche market: eg. grassfed yak x angus.
The second basic research objective was to produce yak-angus hybrids that we could use for a comparative study with yak and angus calves in terms of growth and forage consumption rates so we could determine if there is economic gain in yak x angus hybrids for meat production. This part of the project will include the purchase of grassfed yakxangus meat to compare with grassfed yak and grassfed angus meat in terms of market quality: Protein and fat content as well as a comparative taste and cooking evaluation.
First Basic Objective: Artificial Insemination Process
In the first year of this project we have analyzed each component of our process from semen collection to insemination in terms of producing extended yak semen with good post thaw motility and a system combining a Bos Indicus five day sequencing process, teaser bull and the use of patches that will change color when a yak is "ridden" by another yak indicating the animal is in standing heat.
The needed improvement in post thaw motility was through a combination of process changes which protected the collected and extended semen from sperm damaging/killing temperature changes, calibrating the extended semen cool down from 37C (99F) to 5C (41F) at as rate of average rate of 0.25C/minute, switching to actual fresh eggs rather than organic store purchased eggs, switching from high concentration to lower concentration (from 100M sperm/ml to 30Mand 45M sperm per ml which allowed us to use three and four times more extender which provided more protection to the semen, the use of a cold room at 5C to work with the semen once it was ready to be cooled to 5C, and, loading the frozen semen in Goblets and Canes for storage in liquid nitrogen with the straw not leaving being immersed in liquid nitrogen until the entire Cane was filled and was removed from the LN. and placed directly into the LN semen storage tank which was also at -196C with the straws in the Goblets surrounded by excess LN during transfer.
When inseminating the yaks, the semen straws were moved from the tank directly to the straw warmer (99F) for one minute and then placed inside the inseminator's clothing until withdrawn just before insertion. Again, protection of the extended semen in paramount in the process.
The post thaw results were excellent with the worst post thaw motility of the initially motile extended semen being 42%. Note we have collected and processed semen three times in a row and achieved similar high post thaw motility so we believe we basically have this part of the overall process finalized.
The key remaining question in the semen preservation process is whether to use the timed lowering of the straws into liquid nitrogen method or the float the straws over the liquid nitrogen at a set height until their temperature decrease=s to -140C and then plunge them into the liquid nitrogen. Answering this question will be made over the next several semen collections in which we will use the float and plunge method of freezing on more straws with BullXcell, Optidyl and at least one TRIS based extender. If we opt for the float and plunge method which is far more fool proof for others to use then we will need to either float the straws in a larger (LxW) container so the straws can be filled in the Goblets and Canes while in the liquid nitrogen or have a step in which the frozen straws are "dumped" with the liquid nitrogen they were frozen in into a larger (LxW) container that would allow us to fill the Goblets and Canes immersed in the LN with the straws.
The final question in finalizing the entire process is reliably synchronizing and detecting standing heat for insemination in a cost effective way that can be done on a farm. The process we just used for the first time (Bos Indicus synchronization protocol plus teaser bull and use of patches that will indicate if the animal has been mounted) showed two groupings of yaks in standing heat (5 at 70 hours post CIDR removal and 12 at 96 hours post CIDR removal with two remaining expected to be in estrus at 108 hours. We do expect good results since we were breeding at estrus in 17 or 19 yaks and with eight hours of the remaining two. The big question is how long the extended sperm will remain viable within the reproductive tract of the cows. The efficacy of this protocol will be answered in three planned inseminations this spring (including the one we did March 13 and 14, 2024). The second insemination will take place eight and nine days after we pregnancy test using ultra-sound the yaks bred March 13 and 14th after a minimum of 28 days. Those open will be sequenced again on that day using the same protocol. The third insemination will occur near the end of May after we are able to pregnancy test and sequence any that are open after the second the second insemination round. After May the weather the summer weather places stress on the yaks so conception rates decline as well as the quality of bull semen. We will not be able to further test an insemination protocol until 60 days after calving (March 2025).
Second Basic Research Objective: Yak X Angus
The University of Kentucky Martin-Gatton College of Agriculture, Food and Environment provided six angus cows at the C. Oran Research Farm for us to inseminate with yak semen prepared for the project. We first inseminated the angus with yak semen we had contracted a genetics company to prepare during the FS21-335 Project to compare with the extended semen we were preparing. A standard seven day beef synchronization was used but the inseminations yielded no conceptions. The angus cows were then synchronized a second time and this time they were bred with semen straws we had previously prepared. Four of the six conceived: A 66% conception rate. This high rate may have been due to the fact that with the angus, unlike the yaks, we knew the right time to breed them given the synchronization protocol.
At this point we have three hybrids with one more yet to be born. The birth weight of the hybrids were just over sixty pounds for the bull calf and between 45 and 50 pounds for the two heifer calves. For comparison, yak birth weights are typically between 25 and 35 pounds.
Angus baby first day of life Yak baby three days old
The remaining hybrid should be born by June 1. Weights will be recorded and they will be kept with their mothers on grass at the University of Kentucky C. Oran Research Farm. Once they have been weaned and weights recorded, they will be moved to the Zhi-ba Shing-ga Yak Farm where they will be placed in two 1.5 acre grazing study pens along with four yak calves born at the end of 2023 in two adjacent grazing study pens once they have been weaned. A comparative weight gain study will be conducted through the grazing season with weights recorded monthly. A forage analysis will be performed on the grazing pens.
When the grazing season ends they will be moved to a set of study pens used to track weight gain and forage consumption. They will be in these pens from November 1, 2024 to April 1, 2025 at which point they will return to the grazing study pens. Again, weights will be taken monthly and hay consumption will be measured based on the number of pounds of hay they have been fed in the covered hay feeders. The hay will also be analyzed.
The comparative weight gain study will be carried out until they are two years old.
During this final year of the study we will also purchase grass-fed yak x angus sirloin from a ranch in Kansas to do a comparative meat analysis at the University of Kentucky Martin-Gatton College of Agriculture, Food and Environment of grass fed yak, angus and yak x angus sirloin in terms of protein, fat and moisture content. We will run a blind comparative taste test of the three meats and also invite several local chefs to cook with the three meats and share their observations.
The information from the comparative weight gain study, meat analysis, blind taste test and chef's reviews will be written up and made available to small acreage farmers and larger beef producers through posting on the SKAY Research Group website on which the FS12-335 project results were posted as will this project's results. A one page summary will also be sent out through the Southern Region to Cooperative Extension Agents, to the National Black Farmers Organization and to the Black Farmers Network along with a notification of the full project results posting on the SKAY website. We will also submit an article with these results to state Beef Organizations in the Southern Region and publications serving small acreage and hobby farms. This information along with the entire project results will also me made available to yak breeders on the North American Yak Association website which is being developed.
Educational & Outreach Activities
Participation Summary:
The outreach plan proposed for this grant has not been implemented since we have just developed the final protocol for artificial insemination (semen collection through insemination). We have very good repeatable (3x) results from collection through semen post thaw motility but will not know how successful our in semination protocol based on the use of the Bos Indicus sequencing protocol, the presence of a teaser bull and the use of patches that indicate when a cow has been ridden to know when the yak cows are in standing heat and ready for insemination. We will have our first results from using this insemination protocol in mid-April (2024) and results from a second and third test of this protocol at the end of May and late June/early July (2024). If the sequencing protocol is successful based on the percentage of pregnancies we obtain per test, and we believe it will be based on the reproductive condition of the yak cows in our first breeding test, then we will write up the entire protocol and results and make them available as stated in the proposal.
They will also be presented at the Yak Conference to be held in late October/November 2024 at Morehead State University. At this point in time we think that a "two fold" conference would be more helpful: An on-line Yak Conference consisting of all the presentations and a "Hands On Working Conference: for training people on basic yak husbandry, AI and sequencing for insemination and hybridization.
As noted previously (Results Section) we plan to submit the artificial insemination (collection through insemination) and the comparative information from the hybridization for publication.
If the sequencing protocol we are testing does not yield high enough pregnancy percentages then:
- We will release all the information, procedures and results, in our process from collection through semen freezing with post thaw results. This will include the results of at least two more collections and processing which will be done April 4, 2024 and at least one more time before mid May at which point the quality of the bull semen will degrade due to the increasing ambient temperature.
- We will test the actual insemination process for sperm motility losses - thawing the semen, cutting the straw, inserting it in the insemination gun, "keeping it warm until physical insemination - which can all be done in the cold room at different temperature settings - and then quickly do a motility test to see if there were significant motility losses. If losses occurred and explain the lower than desired conception rates then we will determine how we can reduce semen motility loss in the process and test it in the cold room until we have have good results. The test of the modified process to get actual conception rate will not be able to be done until late fall when six yaks will be open for breeding and then again in February-March when thirteen more yaks will be available for breeding,
- If the problem was not in losses in sperm motility occurring between removing the semen straw from the storage tank to just before insertion of the insemination gun in the yak, then we will test other sequencing protocols using the teaser bull (without insemination since conception will decrease with increasing ambient temperature) to see if we can achieve better clustering of the yaks in standing heat. If this is the case we will not be able to test the new sequencing with insemination until late fall when six yaks will be open for breeding and then again in February-March when thirteen more yaks will be available for breeding,
- Once we have resolved the sequencing-insemination issue we will attach the detailed process and data to the previously released information so the entire process and results are available to yak breeders, small acreage farmers, Black Farm Organizations and larger meat producers.
In terms of the second basic part of the study which focuses on developing data for informed decision making by small acreage farmers and meat producers on the potential of first cross (F1) yak-beef hybrids:
As stated above two of the four hybrids have been born with the remaining two due within the next two months. The comparative growth rate and hay consumption study up to eighteen months of age will not be completed until January 2025 at which point the results will be written up and made available.
The comparison of F1 (first generation) yak x angus will be started as soon as we are able to purchase grass-fed yak x angus sirloin and ground from a ranch in Kansas. As soon as the comparative meat evaluations (grass-fed yak, grass-fed yak x angus and grass-fed angus) are done: protein, fat and moisture by the University of Kentucky and the blind taste test and chef comparison are done, the results will be written up and distributed to yak breeders, small acreage farmers, Black Farm Organizations and larger meat producers using the methods described in the proposal. We hope to have this completed by the time of the Yak Conference to be held as partn of this project (late October/early November, 2024).
The project information will be disseminated as follows:
- Through the previously described SKAY website which we will also ask the Cooperative Extension Agent in the Southern region to advertise;
- Through direct mailings of the final reports/fact sheets etc. to the National Black Farmers Association, Black Farmers Network and the Black Farmers and Agriculturalists;
- Through presentations at the two Yak Conferences;
- Through video recordings of presentations and demonstrations which will be available on the SKAY website and YouTube; and,
- Articles will be written summarizing each of the basic research area results (the entire AI Process (semen collection through insemination) and the comparative yak, yak x angus hybrid and angus study to provide data for informed decision making. These will be submitted to State and regional Beef publications/journals and to small and hobby farmer magazines for publication.
Learning Outcomes
One yak meat producer with over 80 yak requested information on sequencing yak cows for their breeding program this spring. The farm will be implementing the Bos Indicus protocol we have started to use in this grant.
A second yak breeder has requested information on the AI program development and wants to implement in their herd to develop a possible yak x beef hybrid meat herd.
A third yak breeder wanted information on cross breeding yaks with Highland cattle using artificial insemination.
The fourth yak breeder is myself.
The USYAKS Yak Association Science Committee is following the progress being made as they want to be able to have a successful AI procedure available to address the high coefficient of inbreeding in the US due to the small gene pool the herd is built on.
The one yak breeder in New Zealand has requested information on the AI process we are developing due to the high coefficient of inbreeding in his herd which is impacting fertility, growth rates and health in his herd. He specifically wants to be able to successfully inseminate his cows if he is able to secure yak semen from abroad.