Progress report for FW22-399
Project Information
This project aims to develop assessment protocols that guide biostimulant use practices to improve crop and soil health or remediate crops damaged by herbicide drift. Experiments will be conducted on a seedling fruit orchard that was contaminated by drift in 2021.
Questions to be answered by investigation include:
- How closely do rapid and affordable, site-based assessments of leaf color patterns, microscopically detected bacterial:fungal ratios and changes in refractometer based Brix readings correlate with laboratory assessments of plant, soil, or compost nutritional status and microbial diversity?
- Can biostimulant additions (including bioactive composts) improve plant and soil function, as measured by seedling germination bioassays and substrate induced respiration (SIR), in herbicide-treated plants and soils?
- How long will herbicide contamination and detectable plant symptoms persist in plants and soils following biostimulant treatments?
Results will provide farmers, gardeners, and crop advisors with empirical data demonstrating use of biostimulants for improving plant and soil health in a seedling fruit orchard recovering from herbicide damage due to drift.
More broadly, the project will demonstrate the effectiveness of popular on-site and laboratory assessments for guiding decisions that effectively address routine or triage-based plant and soil health demands.
Findings will be disseminated in underserved rural, urban, and indigenous communities through a combination of podcasting and personal communication with growers at farmer's markets, community meetings, and presentations to nonprofit organizations that support grower education.
Research
- Evaluate utility of site-based and laboratory assessments of plant and soil biological health in fruit orchards for distinguishing between healthy (ie: nutritionally complete and biologically functional) and less healthy soils, leaves, and composts.
- Evaluate effectiveness of biostimulants (compost, compost teas, and fulvic acid for improving detectable plant and soil health responses.
- Evaluate plant and soil responses to various biostimulant treatments in the presence of herbicides.
Education
- Use a combination of live presentations, workshops, and blog articles to:
- describe effective visual, site-based, and laboratory monitoring techniques that support sustainable use of composts and other biostimulants or biological practices.
- illustrate mitigation strategies that can reduce losses associated with herbicide drift or other factors that damage plant and soil health.
- help growers, crop advisors, and general audiences gain appreciation for biological approaches to crop production.
- Help growers in underserved rural, urban, and indigenous communities optimize use of composts and affordable, on-site testing strategies to increase local, sustainable food production.
Cooperators
- - Technical Advisor
- - Producer
- - Producer (Educator and Researcher)
Research
Research Objective 1: Evaluating the correspondence between site-based plant and soil health indicators and lab-based soil, compost, and plant health tests.
Plant Leaf Analysis
Leaf samples for refractometer analysis were collected from 3 varieties of apple and 3 varieties of peach in mid-morning on Sep. 12, 2022. Attempts to extract sap with either a handheld garlic press, vice grips, or a constructed screw press were each unsuccessful. Sap sufficient for refractometer analysis was readily collected from clover, alfalfa, and pasture grass species in the surrounding cover crop.
Leaf and soil samples were collected in July, 2023 from mixed varieties of apple trees located on a seedling orchard at J.A.L. Farms in Fort Sumner, NM. Varieties included equal numbers of Honeycrisp, Gibson Golden, Aztec Fuji, and Arkansas Black.
Leaf samples for tissue analysis consisted of fully developed leaves collected from south-facing branches of apple trees that were located > 6 m away from the edge of each row. Leaves were collected and stored in a cold ice chest without directly contacting the ice. Trees on the southeast portion of the orchard were visibly chlorotic, and made up the bulk of the symptomatic leaf samples described below.
Leaf samples for sap analysis were carried out as above, except that old leaves located on the same branch as each sampled new leaf were also sampled. New and old leaves were placed in separate sample bags.
Harvested leaves were divided into categories based on visual symptoms as follows:
- Asymptomatic- Dark green, leathery textured leaves free of mechanical damage or other deformities.
- Light Green and soft- lighter green leaves which may or may not have a soft texture, free from mechanical damage or deformities
- Chlorotic-very light green or yellow leaves with varied patterns of chlorosis.
- Mechanical damage-includes insect damage, breaks from wind and rain, etc.
Symptomatic leaves were visualized with stereomicroscopy and photographed, before pooling all symptomatic leaves so that asymptomatic and symptomatic samples were sent to laboratories for analysis.
Triplicate samples of new, mature leaves from the southeast, and remaining orchard were submitted to Ward Laboratories in Kearny, Nebraska for leaf tissue nutrient analysis.
Pairs of old and new leaf samples were sent to New Age Laboratories in South Haven, MI for sap nutrient analysis. Due to the precise selection of south facing, newly mature leaves and the small size of the trees that is required for the sap analysis protocol, it was not possible to collect enough leaves for repeat samples.
Site Based Microscopic Analysis of Fungal and Bacterial Abundance
Four soil samples representing the top 4 inches of soil were collected from the orchard, garden, and pasture on JAL Farms, in Fort Sumner, NM and from the garden at Spirit Farm, in Vanderwagon, NM. Each sample represented a composite of 12 cores collected from randomly distributed locations within the sample area.
Two compost samples were also collected. One sample came from Spirit Farm and one from JAL Farms. Both composts were made using Johnson Su Bioreactors(1). However, the compost at Spirit Farms, which operates on harvested rainwater, is irrigated irregularly by hand. The compost at JAL Farms was immature at the time of sampling. Each sample represented a composite of 12 small spades filled with compost which was removed from different areas within the bioreactor.
Soil and compost samples were each strained through a 3 mm sieve and blended to ensure uniformity. Samples were stored at 4oC and analyzed within one week of sampling.
Each soil or compost sample was analyzed by diluting 0.5 g of soil or compost in 50 mL of carbon filtered tap water. The diluted sample was rocked gently for 60 seconds. Aliquots of suspension were removed from the unsettled suspension using a transfer pipette, and placed in the counting chamber of a Neubauer hemocytometer (Figure 2) and examined at 400X magnification on a Motic type 102M compound microscope.
Bacteria cells present in each of the 5 diagonal squares in the center of the hemocytometer were counted. Fungal cells present in each of the four corner squares (one is outlined in blue), and in the center square of the same size were counted. If more than 300 bacteria were present in a single 0.2 mm grid, the sample was diluted and reanalyzed.
Three replicates of each sample were analyzed, providing a total of 15 bacterial, and 15 fungal values for each sample.
To convert the number of cells/ml of solution to cells per gram of dry soil or compost, soil and compost moisture content was determined gravimetrically. Fresh weights of 40 to 50 grams soil or compost were dried at 85oC to a constant weight (36 hours). Soil moisture (M) was recorded as (fresh weight – dry weight) / dry weight.
The number of cells/ml was converted to cells/gram dry soil by multiplying counted cells/mL in each square by the dilution factor (df) of 100 to get the total number of cells in the solution. This value was then divided by the dry weight (dw) of soil, which was calculated as dw = fw/(1+M) where fw=the fresh weight of soil (1 g).
Site Based SIR using the MicroRespTM system.
Substrate induced respiration via the MicroRespTM system offers tremendous flexibility to the end user, since respiration can be measured in the presence of almost any carbon source (ie: "substrate"). Typical applications select a variety of amino acids, carbohydrates, and carboxylic acids. Environmental contaminants of interest are also evaluated. To assist in identification of microbial responses indicative of soil health and response to pesticides, research articles in which MicroRespTM was utilized were reviewed to identify substrates that have already proven useful as universal respiration indicators, were involved in auxin metabolism, or have other potential as soil health restoration indicators. Substrates that reported the highest potential to inform soil responses relevant to herbicide remediation were selected. In cases where substrate availability or ease of handling were problematic, analog substances were substituted. The compounds used are listed in Table 1 by compound class and biological function.
Table 1. Identification codes for aqueous control and eleven carbon substrates used in substrate reduced respiration.
| ID | Compound Class | Biological function |
| water | control | electron carrier |
| aa1 | amino acid | protein synthesis |
| aa2 | amino acid | protein synthesis and and cellular detox |
| aa3 | amino acid | protein synthesis and desiccation tolerance |
| aa4 | amino acid | protein synthesis, cellular stress tolerance |
| am | amide | chitin component |
| ca1 | carboxylic acid | TCA cycle, root exudate |
| ca2 | carboxylic acid | fungal secretions, metal chelation |
| fa | fatty acid analog | energy source, cell membrane component |
| phe | phenolic | component and degradation product of humus, lignin |
| s1 | simple sugar | universal energy source, simple sugar |
| s2 | disaccharide | cellular energy, dessication tolerance |
SIR Analysis of Orchard Soil and Compost
Composted manure was initiated in fall of 2022 by transferring manure and straw collected from cattle pens to one of three Johnson-Su style composting bioreactors. Samples of bioreactor compost were drawn in July of 2024. One sample, drawn from 12 random points within the interior regions of the bioreactor was gathered from each bioreactor. An additional bioreactor, established on Spirit Farm in Northwestern NM, also provided a sample of compost.
Soil samples were collected from the J.A.L farms orchard (3), or from the vegetable garden at Sprit Farm. Each sample represented soil from 12 four inch deep cores gathered from random points throughout the sample area.
Each compost and soil sample was manually homogenized and past through a 3 mm sieve. One 60 g aliquot of the homogenized sample was subjected to gravimetric moisture analysis to determine water content. The remaining sample was subjected to substrate induced respiration testing (SIR) using a modified MicroRespTM protocol and the substrates described above. Modification of the protocol was necessary due to supply chain failures. Flat bottom detection plates recommended for MicrorespTM could not be obtained at the time of analysis. It was necessary to use round bottom detection plates. However, corresponding round-bottom strip well plates could not be found for CO2 Calibration assays. Therefore, accurate calibration of CO2 emission rates was not possible. However, a calibration curve developed using flat bottom strip well plates confirmed the anticipated inverse relationship between absorbance readings at 570 nm and CO2 emission.
Microbial activity across substrates was calculated based on normalized absorbance at 570 nm as described in the MicroRespTM Technical Manual (2022).
Research Objective 2: Evaluate the effectiveness of biostimulants alone, and in the presence of added nutrients for improving plant and soil function, including function in the presence of herbicide contamination.
Herbicide Residue Testing
Leaf and soil samples were collected for herbicide residue testing according to laboratory recommendations, and submitted to South Dakota Agricultural Laboratories in Brookings, South Dakota for analysis.
SIR Analysis of Herbicide Remediation
A bulk sample of soil will be collected from JAL Farms orchard in April of 2024 by collecting 20 cores from each row of trees, mixing thoroughly, and sieving soil as described above. Subsamples of the bulk mix will be combined with biostimulant at nutrient blends at 1X and 2X, the recommended application amounts. Samples will then be subjected to MicroRespTM analysis as described above.
A linear mixed effects model with field as a random effect is being developed in R software (lme4 package) to compare microbial response across herbicide and biostimulant treatments. Microbial diversity will also be calculated using the Shannon-Weaver Index (H=- Σpi * ln(pi)), so that microbial diversity may be used as an indicator of soil remediation
Seed Germination Bioassays Herbicide Remediation
Seed germination bioassays will be carried out in summer 2024 as described in the proposal.
Research Objective 1: Evaluating the correspondence between site-based plant and soil health indicators and lab-based soil, compost, and plant health tests.
Leaf Sap Analysis by Refractometry
The failure to extract sufficient sap from apple and peach tree leaves prevented refractometry analysis. It could be that the thicker, more ligneous nature of tree leaves compared to softer tissued plants promotes immediate adsorption of sap by leaf carbohydrates, as it is extracted from the vascular tissue. It is possible that homogenizing tissue in an aqueous buffer would allow refractometry of the extract. However, the cost of a useful homogenizer and the time involved in preparing and clarifying reliable extracts and calculating dilution factors suggest such an approach would have minimal utility for routine monitoring of plant nutrients on orchard trees.
Visual Leaf Analysis.
Figure 1 provides examples of common (A., B., F, ) and occasional (B, C-E, G) symptoms observed on apple trees at JAL Farms in Spring of 2022. Asymptomatic leaves made up 57.5% of newly mature sampled leaves. Light, soft green leaves made up 22.1%. Chlorotic leaves made up 1.2%. Mechanically damaged leaves made up 19.09 %. Much of the mechanical damage was due to a spring hail storm.
Laboratory leaf tissue and sap analysis
Leaf samples analyzed for nutrient content in leaf tissue were divided into asymptomatic and symptomatic leaf samples, with symptomatic samples representing the range of symptoms described in the previous section. Figure 2a shows that nitrogen (N), phosphorus (P) and potassium (K) levels were higher in asymtomatic leaf tissues, while calcium levels were higher in symptomatic leaves. Surprisingly, only calcium levels fell below recommended levels of nutrients for apple, even in the symptomatic tissues.
| Figure 2. Nutrient levels detected in leaf tissue of asymptomatic and symptomatic leaves. |
 |
Results of the leaf sap analysis conducted at New Age Laboratories are shown in figure 3. In sap analysis, differences between nutrients measured in old and new leaves. Deficiencies in potassium, magnesium, and total N are indicated with red highlights. These results were easier to explain, based on visual symptoms, than the results obtained from the tissue analysis. Application of foliar fertilizer containing potassium, magnesium, and nitrogen also removed the chlorosis.
| Figure 3. Results from leaf sap analysis of old and new apple leaves. |
 |
Reliability Assessment of Microbial Direct Counts and Fungal:Bacterial Ratios
A reliability analysis of the direct counts and fungal bacterial ratios revealed that the direct counts were 98% reliable for the log transformed averages of the bacterial counts for a given sample, and 92% reliable for log transformed averages of the fungal counts for a given sample. The log transformed averages of the fungal:bacterial ratios were only 72% reliable.
A subsequent analysis of unrelated soil and compost samples (not shown) revealed that the fungal counts can reach 98% reliability when only spores are counted. Decayed plant material can sometimes resemble certain fungal fruiting bodies viewed under the microscope. It is thought that eliminating fruiting bodies, hyphae, and other non-spore structures improves reliability by reducing the opportunity for false positives during examination.
The low reliability of the fungal:bacterial ratios was surprising, since fungal:bacterial ratios are widely regarded as a useful soil health indicator. It is worth noting, however that a reliability assessment simply refers to the consistency of the measure. While these results suggest we can be confident that the same sample will give the same result when the same method is applied, it tells us nothing about the value of the measurement for predicting soil health. The correlations between microbial counts and fungal to bacterial ratios should become more clear when the values obtained from field samples are compared against soil chemical and nutritional parameters.
SIR of Orchard Soil and Compost
The boxplot in figure 4 shows the normalized absorbance (Ai) at A570 nm for each of the twelve substrates in the presence of orchard soil, garden soil, or compost from four different bioreactors. Quantitative analysis will be carried out in spring of 2024. In general, a low Ai at A570 nm indicates a high microbial response. As might be expected, the four composts produced higher microbial responses (lower Ai's at 570 nm) than the orchard soils. It is of interest to note that the phenolic (phe) substrate was heavily metabolized by Compost 3, which also appears to be the most active compost overall. Microbes that excel at degrading phenolic compounds are thought to be better at degrading lignins and cellulose. If the difference proves significant in future analyses, this may be a good quality indicator for revealing when a compost is mature. The garden soil came from a garden at Spirit Farm that has been heavily amended with organic matter over a period of several years, so it is not surprising that the overall microbial response is similar to that of compost.
Additional analyses are underway quantify responses by substrate and by microbial source, to identify substrates among the twelve that act as indicators of each sample type, and to calculate the diversity indices associated with each sample.
| Figure 4. Microbial functional analysis via substrate induced respiration. |
 |
Research Objective 2: Evaluate the effectiveness of biostimulants alone, and in the presence of added nutrients for improving plant and soil function, including function in the presence of herbicide contamination.
Herbicide residue analysis
Herbicide residue testing screened for a panel of 17 class-4 herbicides, including the picloram, triclopyr, clopyralid, and 2, 4-D that was detected on trees from JAL Farms as a result of pesticide drift in 2021. Soil testing revealed no signs of pesticide contamination. However, leaf tissues revealed traces of 2, 4-D (3.88 ppb) and dicamba (1.22 ppb). Due to the fact that dicamba was not detected in 2021, and 2,4-D in 2021 was only present in leaf tissue at 5.61 ppb, and was not detected in the soil, it is likely that the 2,4-D and dicamba detected in 2023 represent separate drift incidents. Both chemicals are used by external entities. While the levels of herbicide detected in 2023 were insufficient to cause the classic symptoms attributed to class-4 herbicide damage, the presence of dicamba and 2, 4-D may explain the chlorotic symptoms observed in the absence of clear tissue nutrient deficiencies.
References
- J. DC, S.-J. HC. (2010), https://youtu.be/DxUGk161Ly8 -.
- V. Saul-Tcherkas, Y. Steinberger, Substrate utilization patterns of desert soil microbial communities in response to xeric and mesic conditions. Soil Biology and Biochemistry 41, 1882-1893 (2009).
- G. Bongiorno et al., Soil management intensity shifts microbial catabolic profiles across a range of European long-term field experiments. Applied soil ecology : a section of Agriculture, ecosystems & environment 154, (2020).
- M. B. Sassi, J. Dollinger, P. Renault, A. Tlili, A. Bérard, The FungiResp method: An application of the MicroResp™ method to assess fungi in microbial communities as soil biological indicators. Ecological indicators 23, 482-490 (2012).
- P. G. Dennis, T. Kukulies, C. Forstner, T. G. Orton, A. B. Pattison, The effects of glyphosate, glufosinate, paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci Rep 8, 2119 (2018).
- A. N. Stepanova, J. M. Alonso, Auxin catabolism unplugged: Role of IAA oxidation in auxin homeostasis. Proc Natl Acad Sci U S A 113, 10742-10744 (2016).
- T. Mohammad Emad, K. Sa’eb, L. Mary, S. Jesus, U. Adrian, Soil community catabolic profiles for a semiarid reclaimed surface coalmine. International Journal of Mining, Reclamation and Environment, 1-17 (2023).
- C. D. Campbell, S. J. Chapman, C. M. Cameron, M. S. Davidson, J. M. Potts, A Rapid Microtiter Plate Method To Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 69, 3593-3599 (2003).
- M. J. Fernández-Gómez, R. Nogales, H. Insam, E. Romero, M. Goberna, Role of vermicompost chemical composition, microbial functional diversity, and fungal community structure in their microbial respiratory response to three pesticides. Bioresour Technol 102, 9638-9645 (2011).
- O. Gazdag et al., Density and Diversity of Microbial Symbionts under Organic and Conventional Agricultural Management. Microbes and environments 34, 234-243 (2019).
Research Outcomes
Leaf Sap Extraction
Although Brix values taken from leaf sap of most annuals and many forage crops can provide valuable indicators of general plant health, extracting leaf sap from fruit trees is less feasible for routine monitoring on orchards. This is because the leaf sap is simply too difficult to extract. Conceivably, leaf extracts could measured by preparing an aqueous suspension of the minced leaves, filtering off suspended solids, and measuring the Brix values of the remaining solution. However, obtaining meaningful values in this manner would require numerous additional steps, including an accompanying leaf moisture analysis in order to correct for varying moisture concentrations. The added analysis time would eliminate the convenience that makes leaf Brix monitoring attractive.
It should be noted that Brix monitoring of fresh fruit is a well proven technique for assessing sugars. As such, it remains a valuable tool for monitoring fruit quality.
Laboratory Analysis of Leaf Tissues and Sap
In the data presented here, laboratory analysis of leaf sap was more effective at identifying deficiencies that informed remediation of interveinal chlorosis than leaf tissue analysis. However, it should be noted that the small number of samples utilized for this study prevents drawing strong conclusions in the absence of additional research. Leaf collection for sap analysis was more time consuming, and the number of leaves required was impractical for routine sampling of fruit trees with trunk diameters < 2 inches in orchards with less than 500 trees because not enough leaves are likely to meet the sampling criteria. Sap analysis was also more expensive, and had a longer turnaround time than tissue analysis.
Microbial Direct Counts and Assessment of Fungal:Bacterial Ratios
Direct counts of soil microorganisms can provide a highly reliable and cost effective method to monitor soil microbial populations. However, the method does require some initial investment, including purchase of a suitable compound microscope and hemocytometer. It also takes a commitment to learning how to distinguish fungi from bacteria under the microscope.
For the best quantitative results, samples should be diluted to a concentration of < 300 bacterial cells per 0.2 mm grid, and fungal cell counts should only include spores. Tap water used to suspend microbes should be free of chlorine (carbon filtration is sufficient).
Microbial Functional Analysis
Substrate induced respiration implemented with a variety of different substrates offers a powerful technique for exploring differences in microbial community function across samples. More research is needed to determine how these functional variations support soil remediation.
Education and Outreach
Participation Summary:
Educational outreach objectives were modified in year 1 included two invited presentations. On March 3, 2023, Mary Lucero delivered a presentation entitled Cover Crops and Low Till Management at the 2023 NM Fruit Growers Workshop in Abiquiu, NM. Approximately 50 growers and agriculture professionals were present. On March 9, she presented Maintaining Healthy Soils in an Unhealthy Ecosystem to the New Mexico Farmers' Marketing Association in Santa Fe, NM. Approximately 45 of the enrolled participants attended this section. Both presentations were delivered in communities with diverse and historically underserved populations.
Outreach in Year 2 included:
- A soil sampling workshop presented to biofertilizer consultants and community gardeners in cooperation with Texas Earth in Idalou, Texas in March of 2023
- A presentation entitled Achieving Sustainable Local Agriculture by Leveraging Microbiomes for Soil Restoration, delivered at the Western SARE Building Partnerships for Agricultural Sustainability Summit in Phoenix, AZ, December 2023
- A field day at JAL Farms that demonstrated soil sampling techniques, microbial analysis, and use of a refractometer for crop nutrient monitoring.
The initial objective of recording 20 podcast episodes was modified due to technical difficulties associated with recording quality and with making the recordings and transcriptions ADA compliant. After consulting with SARE staff, it was decided to drop the podcasts altogether and look for alternative outreach opportunities. Outreach has since been carried out through the two live presentations discussed above. A third presentation has been scheduled for August of 2023.
In both completed presentations, the benefits of biologically based agricultural approaches, laboratory testing, and on-site monitoring were highlighted. For Cover Crops and No Till Management, the audience consisted of experienced fruit growers, so the presentation used soil test data to illustrate the loss in soil quality following deep tillage and laser leveling, followed by the restoration which occurred over the course of two years without tillage, when using cover crops and biostimulants in place of synthetic fertilzers. Participant survey data from this presentation was gathered by the Rio Arriba County Cooperative Extension Service, and will be made available at a later date.
In Maintaining Healthy Soils in an Unhealthy Ecosystem, the audience consisted of less experienced farmers market gardeners, including less
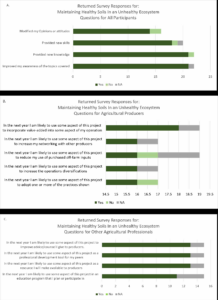
traditional urban and first time growers. This time, the topic centered on basic soil health principles, the benefits of biological practices, and elementary monitoring steps relevant to small operations. Examples of environmental and anthropogenic factors that can interfere with soil health were discussed. Program feedback was assessed using the Western Region Sustainable Agriculture Research and Education Program Outreach Survey. Survey results are illustrated in Figure 1, to the right. A smaller group was anticipated, so only 30 surveys were available to distribute. Twenty-two of these surveys were returned.
The four questions addressed to all respondents (Figure 1A) indicated the presentation was effective in providing new awareness (100% of respondents), new knowledge (95% of respondents), and skills (81% of respondents) that impacted opinions or attitudes.
Responses to questions presented to producers (Figure 1B) revealed unanimous willingness to adopt new practices and diversify production. Curiously, all responses also indicated willingness to use the project to increase networking with other producers. Ninety-four percent of respondents planned to use some aspect of the presented information to reduce purchased off-farm inputs. An additional 94% were willing to adopt one or more of the practices shown.
Fifteen agricultural professionals responded to the survey. Professional responses were overwhelmingly positive, as all answers were either yes (83%), or not applicable to their situation (17%).
Education and Outreach Outcomes
Projects for federal grants that include podcasting or other audio-visual media production need to include budget for transcription and other ADA compliance factors.
Information about sustainable agriculture that illustrates, not only the long term global benefits, but the immediate benefits to the producer has been well received in live presentations.



