Final report for GNC19-288
Project Information
With the goal of sustainably and holistically reducing producer expenditures towards labor, chemical, and equipment resources for the economic management of grapevine canopies and diseases, this proposal seeks to pursue a novel genetic avenue of canopy management. Towards alternatively alleviating the substantial chemical demands associated with grapevine fruit and canopy protection, unique grape germplasm will be explored. Specifically, lacelike leaf shape, a foliar trait under genetic control, will be characterized within segregating populations constructed from crosses between cold-hardy genotypes with simple, wild type leaves, and a mutant, cold-tender, Vitis vinifera L. cultivar with a highly dissected, nearly compound, lacinate leaf trait, ‘Chasselas Ciotat’. The unique leaves of ‘Chasselas Ciotat’ provide the opportunity for increased light and wind penetration within the canopy, potentially reducing overhead costs of both cultural maintenance and pesticide applications for producers.
On-going work within our lab has discovered the lacinate leaf trait is heritable in ‘Chasselas Ciotat’ derived self-progeny, but it is not dominant to wild-type leaves in F1 crosses. This indicates the necessity of both further exploration of its inheritance in segregating progeny and development of molecular markers for incorporation of the trait into diverse genotypic backgrounds. Following the creation of segregating populations through self and test-crosses with ‘Chasselas Ciotat’, leaf traits will be analyzed using chi-square tests to determine inheritance of qualitative traits. Qualitative analysis will be combined with quantitative leaf trait evaluation, measured through digital image processing techniques. These quantitative and qualitative data will be integrated with molecular characterization of the populations, thus enabling the identification of valuable Quantitative Trait Loci (QTL) to enhance marker assisted selection in future populations. The leaf trait will be beneficially combined with the North Dakota State University Grape Germplasm Enhancement Project’s program goals of integrating resistance traits against fungal pests such as powdery mildew and black rot into promising cold-hardy, interspecific Vitis spp. selections. Through dissemination of results in the form of germplasm for the North Central U.S. grape growing community, and through the dispersal of plant material and relevant molecular marker and linkage associations via referred publication, this project has the potential for substantial economic outcomes for growers across the Midwest and throughout the greater North American grape growing community in which canopy management for disease reduction and fruit quality improvement is a chief, annual concern. Simultaneously, extension activities such as notes, grower talks, and field-days strive to further expose regional grape-growers to results and progress.
Learning Outcomes: 4.6 Awareness of new alternative agricultural practices. [Development of genetic alternatives for canopy management]
- Focusing on reaching regional grape growers and educating them on new alternative agricultural practices, this project seeks to demonstrate breeding and cultivar development as an alternative method for grapevine canopy management and chemical mitigation.
- The tasks of extending educational resources will be accomplished through developing New Information via Output 3.2 to be distributed to growers through Output 3.3 and 9 (Educational Programs and Extension Notes).
Action Outcomes: 5.3 New research that builds on previous SARE funded R&E projects. [Development of future projects building on the successful completion of this project]
- Both the student and the advisor will strive to leverage this funded projects’ populations, genetic data, and germplasm towards pursuing future research projects on both the intra- and inter-university level following completion of this project.
Further learning and action outcomes will be accomplished through output 3.7 scientific journal articles and germplasm resources distributed to the research and grape breeding community.
- New understandings of the heritability of qualitative and quantitative leaf traits in grapevines with a focus on dissecting the inheritance of a novel, nearly compound lacinate leaf trait will enable breeding efforts to incorporate germplasm and lessons learned.
- Characterization of important Quantitative Trait Loci highly associated with foliar phenotypes to enable implementation of marker-assisted selection for precise decision-making, saving time, labor, and resources in cultivar development.
- Distribution of useful germplasm with the lacinate leaf trait, to enable broader impact outside of the NDSU GGEP.
Research
Grapevine S0/F1 Progeny
Seedling Production
A total of four grapevine populations were developed. Dried pollen, collected from ‘Chasselas Cioutat’, was applied to pistillate Vitis accessions (E.S. 5-8-17 and B.27) and to emasculated ‘Frontenac gris’ flowers. Additionally, the fruit from ‘Chasselas Cioutat’ flowers used for pollen collection were retained as a source of open-pollinated, presumed selfed seeds. The timing of the ‘Chasselas Cioutat’ flowering under greenhouse conditions did not intersect with the flowering of any other Vitis accessions, thus the seeds are assumed to be self-pollinated in their origin.
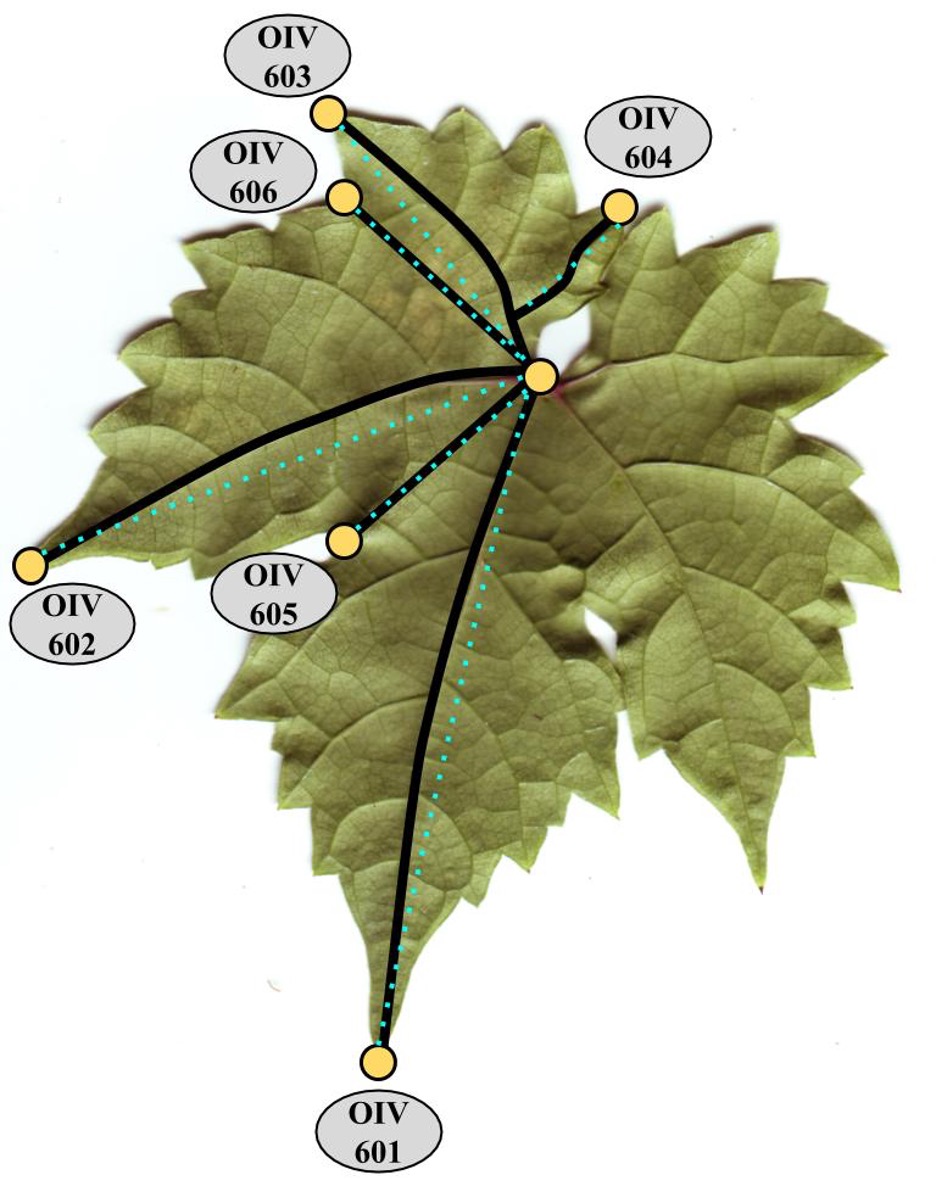
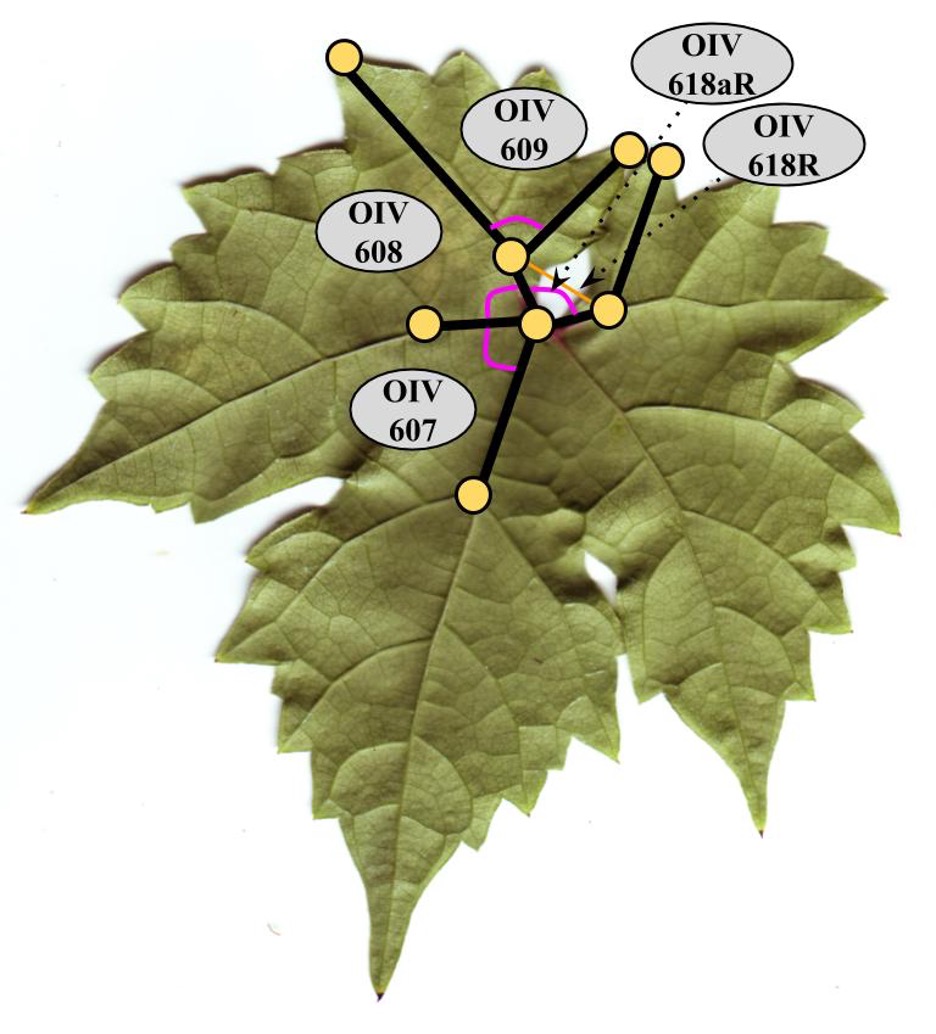
OIV Descriptors and Lengths
A total of 17 landmarks were placed on leaves using GRALED v2.04 (Bodor et al., 2012). This allowed for calculation of 11 OIV (International Organisation of Vine and Wine) and modified OIV descriptors (Table 2) and 15 additional landmark lengths (Table 3) (OIV 2009; Bodor et al., 2012).
Table 2. OIV and modified OIV descriptors used in monitoring grapevine leaf shape adapted from OIV 2009 and Bodor et al., 2012.
|
OIV Descriptor |
Description |
Unit |
|
601 |
length of vein N1 |
mm |
|
602 (L/R) |
length of vein N2 |
mm |
|
603 (L/R) |
length of vein N3 |
mm |
|
604 (L/R) |
length of vein N4 |
mm |
|
605 (L/R) |
length from petiole sinus to upper lateral leaf sinus |
mm |
|
606 (L/R) |
length from petiole sinus to lower lateral leaf sinus |
mm |
|
607 (L/R) |
angle between N1 andN2 |
angle (°) |
|
608 (L/R) |
angle between N2 andN3 |
angle (°) |
|
609 (L/R) |
angle between N3 and N4 |
angle (°) |
|
618aR |
angle of petiole sinus |
angle (°) |
|
618R |
length of petiole sinus |
mm |
Table 3. Landmark lengths used in monitoring grapevine leaf shape adapted from Bodor et al., 2012.
|
Landmark distances |
Description |
Unit |
|
01 to 02 |
length between landmark 01 and 02 |
mm |
|
01 to 03 |
length between landmark 01 and 03 |
mm |
|
01 to 04 |
length between landmark 01 and 04 |
mm |
|
01 to 05 |
length between landmark 01 and 05 |
mm |
|
01 to 06 |
length between landmark 01 and 06 |
mm |
|
01 to 07 |
length between landmark 01 and 07 |
mm |
|
01 to 17 |
length between landmark 01 and 17 |
mm |
|
07 to 08 |
length between landmark 07 and 08 |
mm |
|
08 to 10 |
length between landmark 08 and 10 |
mm |
|
08 to 16 |
length between landmark 08 and 16 |
mm |
|
10 to 12 |
length between landmark 10 and 12 |
mm |
|
10 to 14 |
length between landmark 10 and 14 |
mm |
|
12 to 14 |
length between landmark 12 and 14 |
mm |
|
14 to 16 |
length between landmark 14 and 16 |
mm |
|
16 to 17 |
length between landmark 16 and 17 |
mm |
Figure 1. Example landmark locations placed on grapevine leaves.
Figure 2. Output of leaf scan landmarking evaluation where (A) is an incorrectly landmarked leaf and (B) is a correctly landmarked leaf.
Figure 3. OIV descriptors 601-606 measured along each leaf sample.
Figure OIV descriptors 607, 608, 609 and modified OIV descriptors 618R and 618aR measured for each leaf.
Landmark and Morphometric Analysis
In addition to the mean values of individual OIV descriptors and lengths, overall principal component analysis was conducted on the collected traits using prcomp in R (R Core Team, 2021). Visualization of PCA results were conducted using the the factoextra package (Kassambara and Mundt, 2017).
Using the shapes package in R, generalized Procrustes analysis was performed on landmarks with procGPA (Dryden, 2018). This allowed for principal component analysis of global shape which was then graphically rendered using ggplot2 (Wickham, 2009).
Grapevine S1/F2, BC1, and Intercrossed S0/F1 Progeny
Seedling Production
To date, over 2,000 seedlings have been generated through controlled crosses and self pollination to achieve a segregating generation. This work has been conducted in the greenhouse, using crop forcing techniques following seedling exit from juvenility stage.
Visual Phenotyping
Leaves of individuals are being monitored to the fifteenth true leaf stage, at which point plants are classified as 'Chasselas Cioutat' like mutants, or wild-type, simple leaves. For selected, genotyped individuals (~384), landmark morphometric analysis is on-going for a subset of 4-to-12 leaves per individual using a total of 34 landmarks.
Genotyping
Leaf tissue samples were submitted for genotyping via two unique technologies: genotyping-by-sequencing (GBS) and RNase H2 enzyme-dependent amplicon sequencing (rhAmpSeq). This has given rise to dense molecular maps which are undergoing construction via LepMap3 software, after which marker-trait associations will be further investigated.
Grapevine S0/F1 Progeny
OIV Descriptors and Lengths
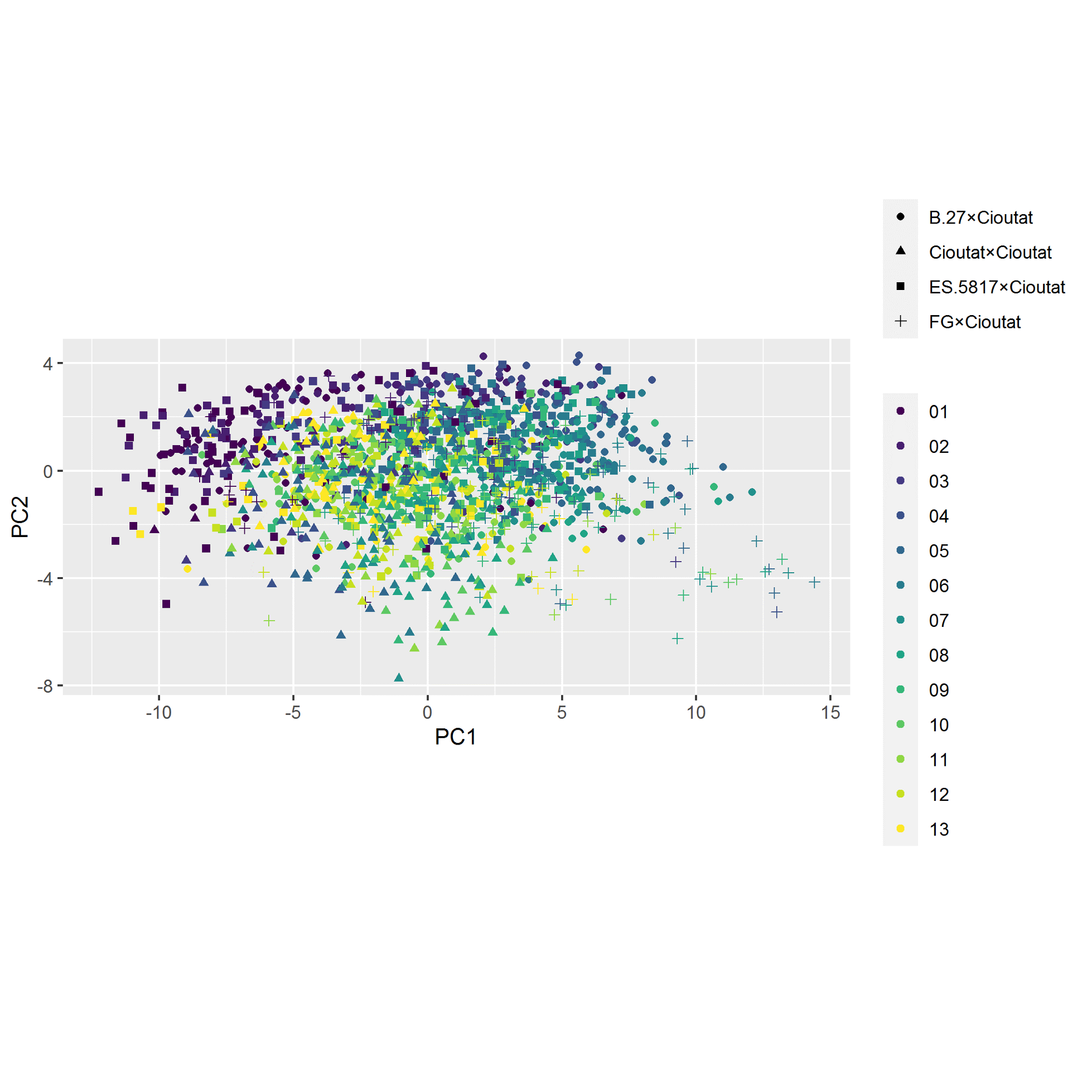
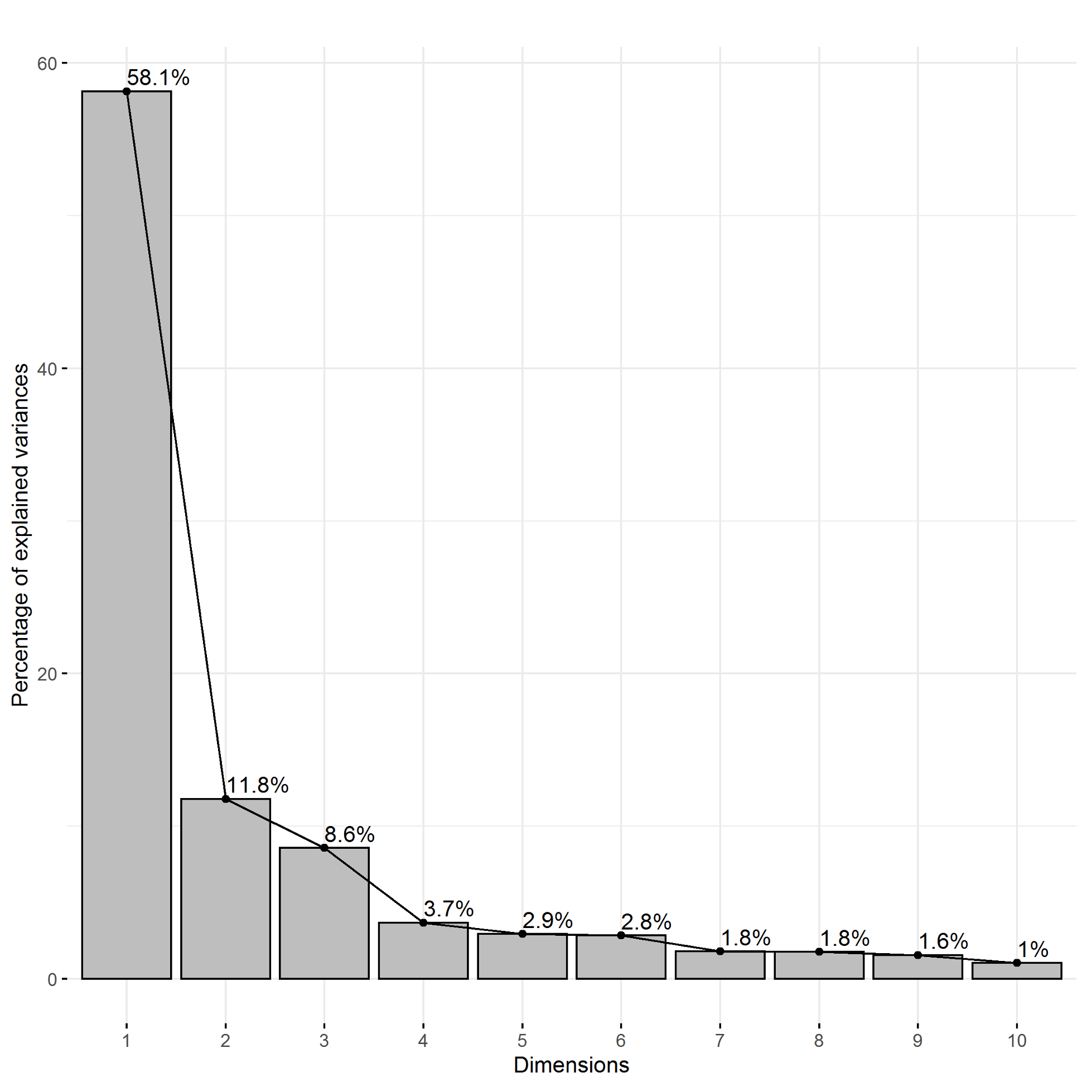
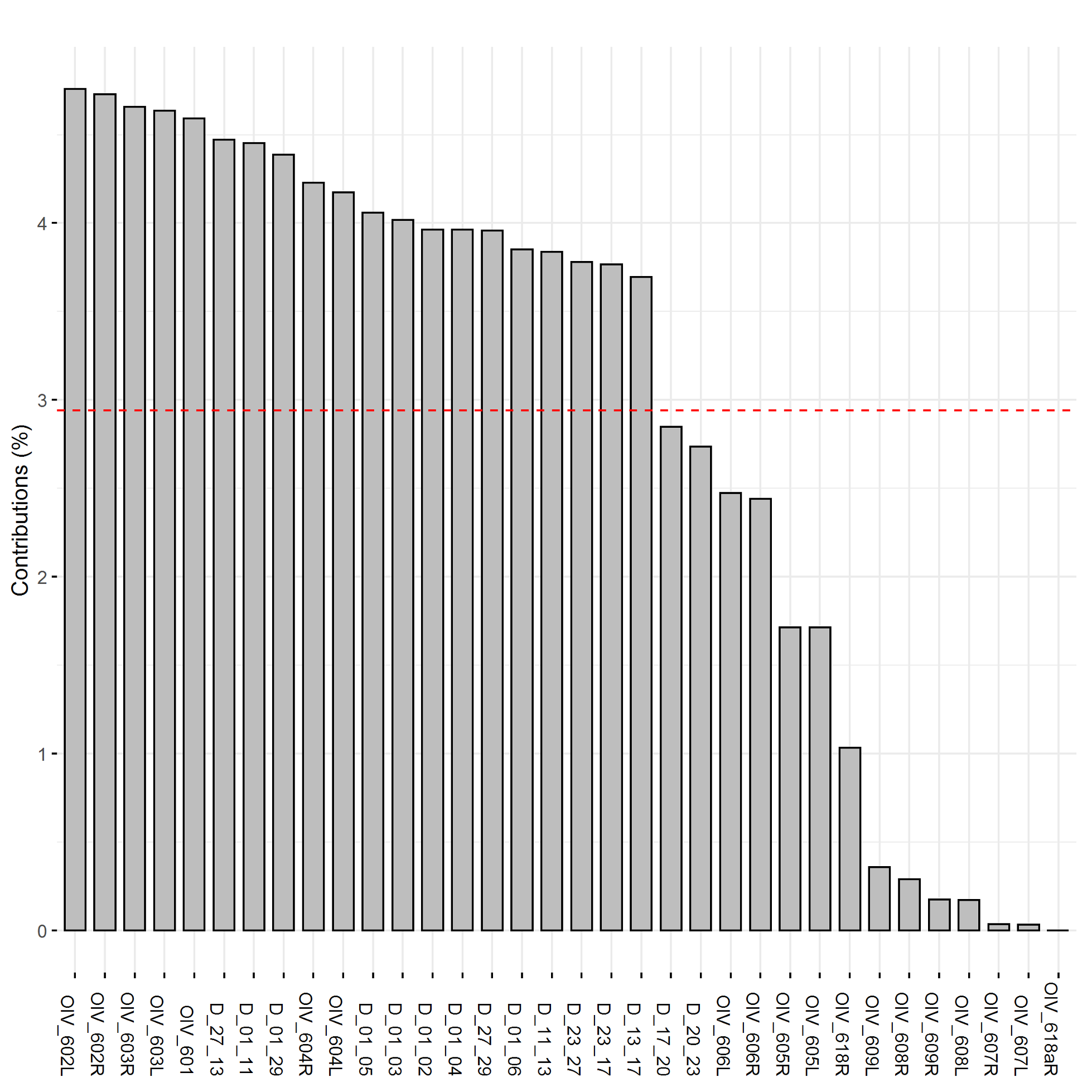
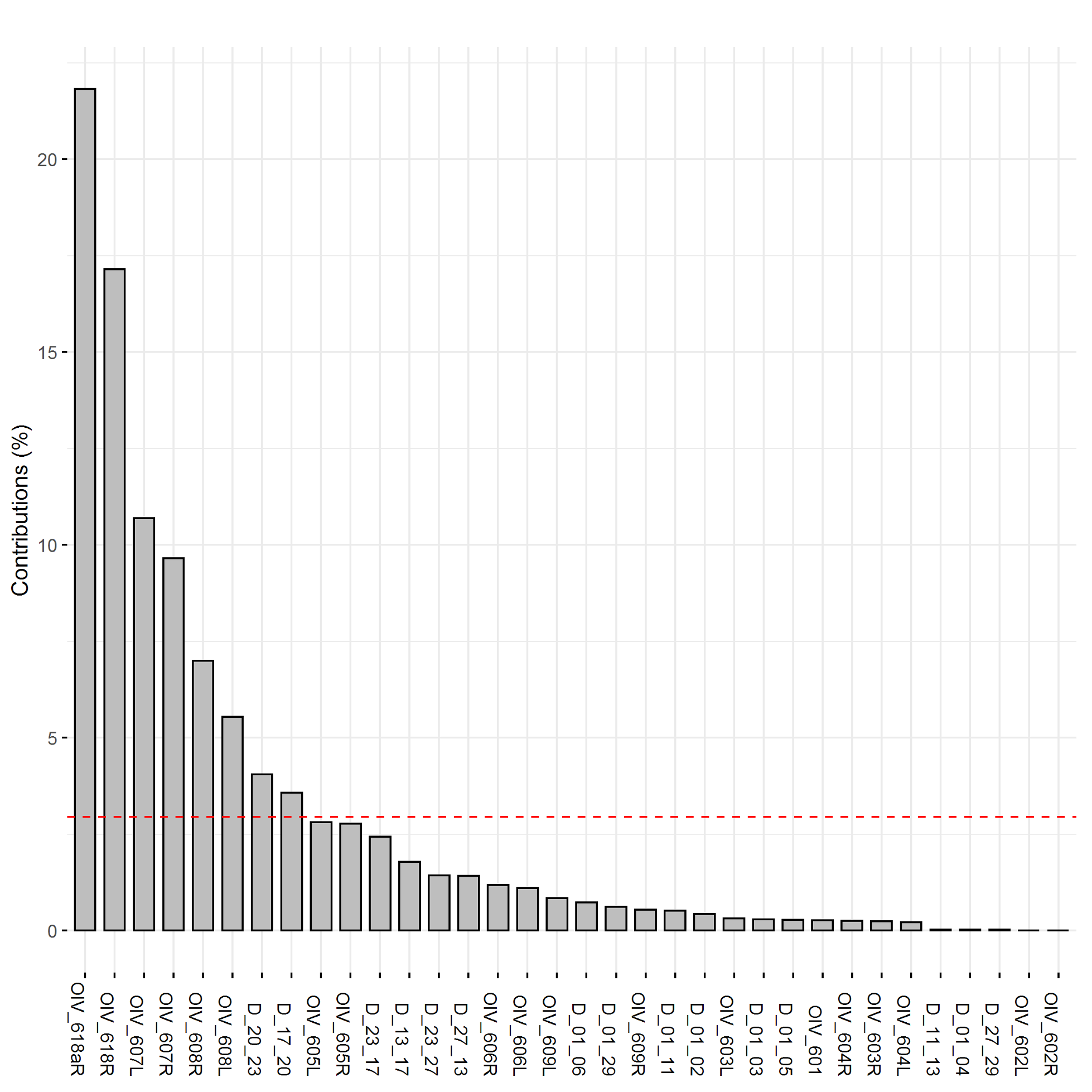
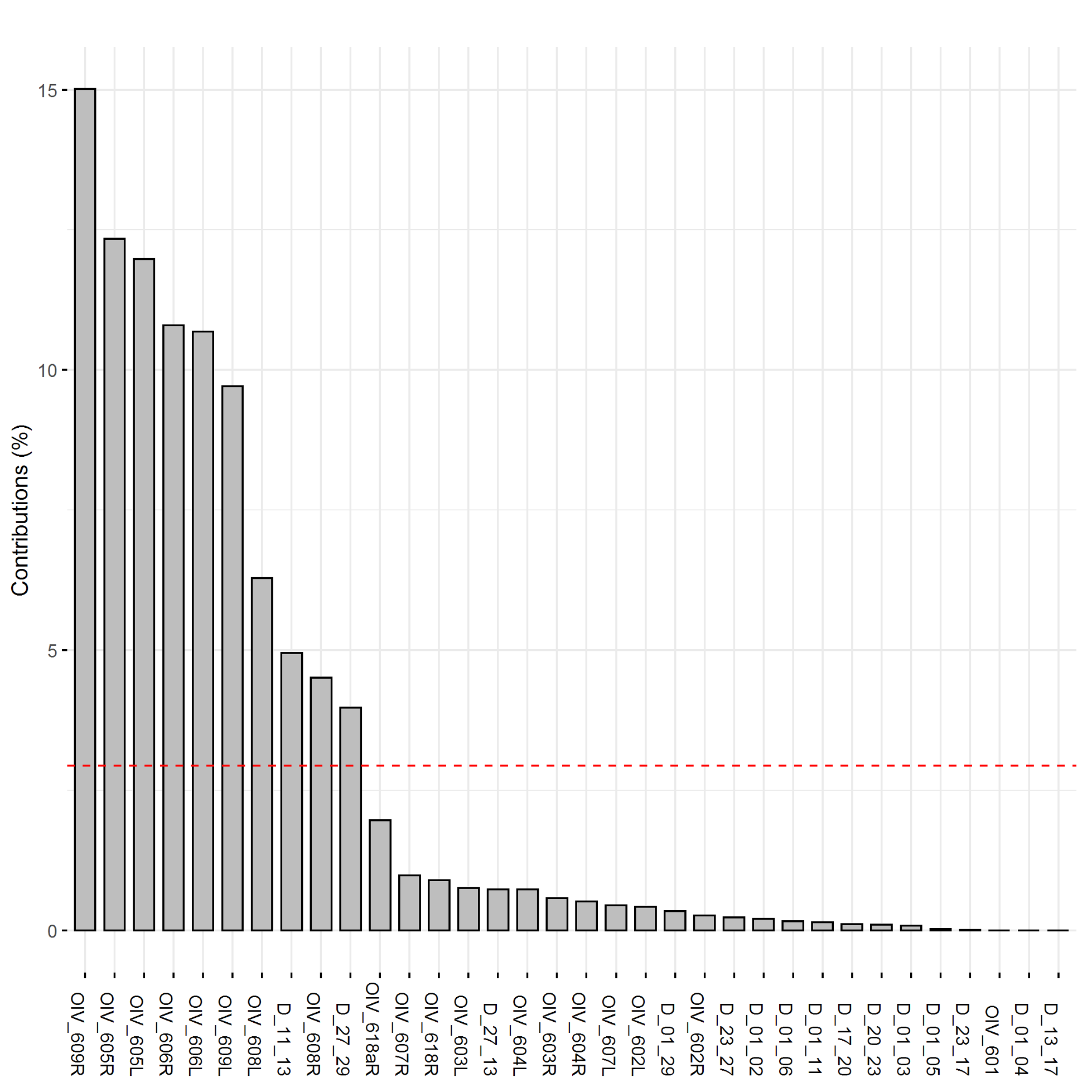
The plotting of principal component traits, based on OIV descriptors and specific lengths, along PC1 and PC2 depicts how PC1 was driven by length measurements, and can be viewed as a function of leaf age and developmental stage relative to sampling date (Fig. 5). The developmental landscape of leaf shape was further visualized by plotting the individual leaf PC values (Fig. 6). The eigenvalues of the top five PCs ranged from 61.8% to 2.7%, only the top three PCs accounted for more than 5% of variation individually (Fig. 7). PC1 was driven by OIV 601, OIV 602, OIV 603, OIV 604, and all non-OIV length measurements (Fig. 8). Most length measurements contributed nearly equal variance from each of the left and right version of the metric. PC2 variance is composed of OIV 607, OIV 608, and OIV 609 (angles between main veins) and the petiole sinus OIV 618aR and OIV 618 (Fig. 9). PC3 variation was also derived from OIV 608 and OIV 609, as well as OIV 605 and OIV 606, which are measurements of the petiole sinus to lateral leaf sinuses (Fig. 10).
Considering that PC1 accounts for leaf size, the PC measurements may be driven by leaf emergence time relative to sampling. Sampling occurred when vines had between 16 and 20 unfolded leaves. Leaves 01 and 02 rarely expanded much and leaves 10 to 13 were generally still in the process of expanding, thus quite small. As a result, the highest PC1 values generally belonged to leaves 05 to 09, because they were largest at sampling. From negative to positive PC1 values there is a general transition from initial emerged leaves (leaves 01 to 03), to recently emerged leaves (10 to 13), followed by the most expanded leaves at time of sampling. PC2 gives rise to separation among sampled leaves, with the earliest emerging leaves having the greatest values and the most recently emerged leaves generally having the lowest values. However, the OIV PCs examined have a high amount of overlap between groups of leaves and are not crisply defined based purely on OIV and length metrics.
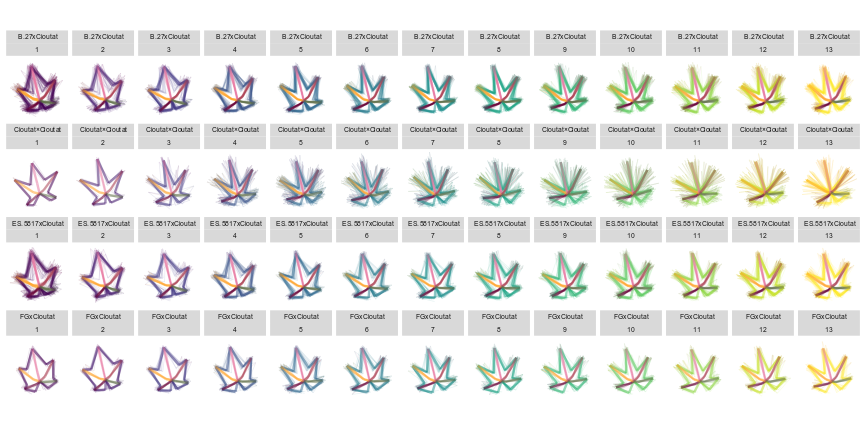
Figure 5. Plotting of calculated traits along the axis of PC1 and PC2 for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 6. Plotting of individual leaf PC1 and PC2 values for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 7. Plotting of eigenvalues for the first ten PC for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 8. Trait contribution to PC1 for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 9. Trait contribution to variation of PC2 for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 10. Trait contributions to variation of PC3 for OIV and distance measurements of leaves 01 to 13 of seedlings from four grapevine populations.
Landmark Morphometrics
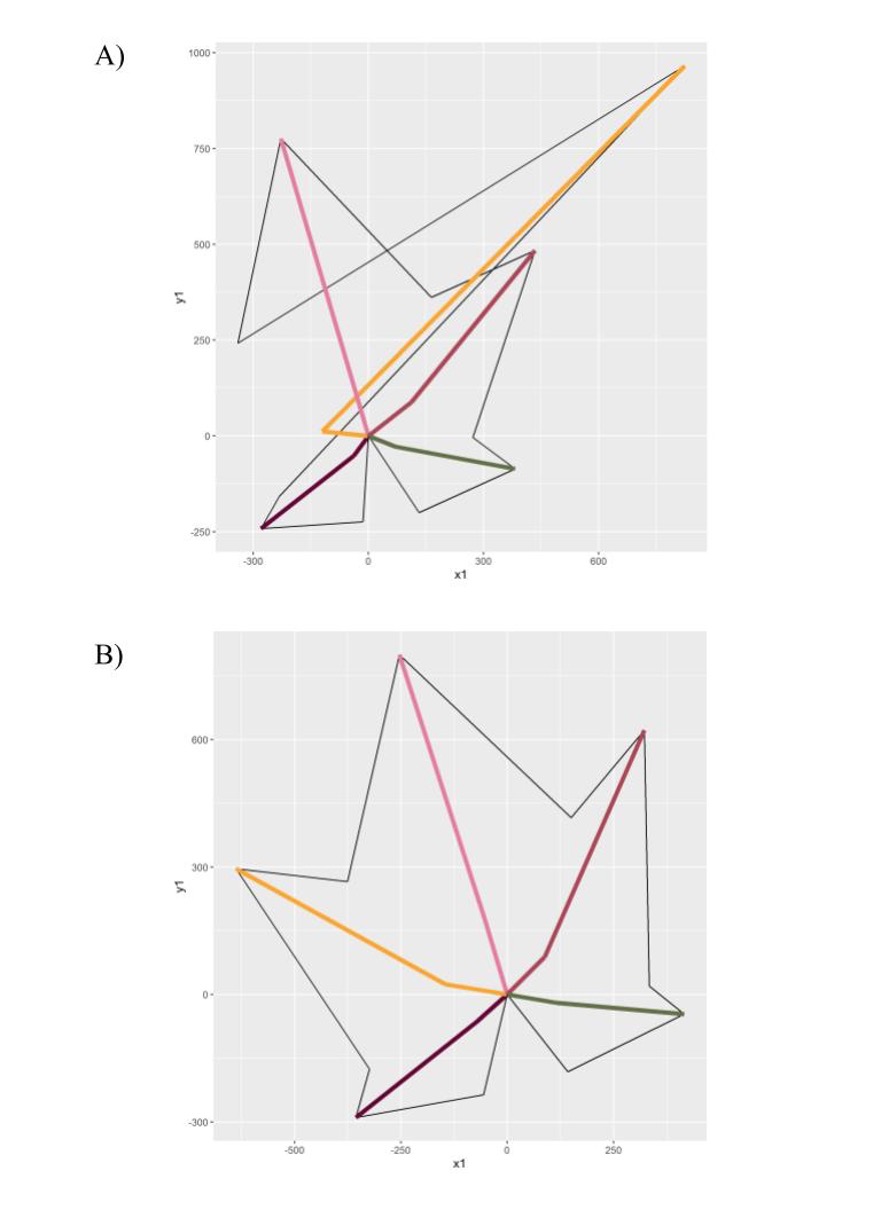
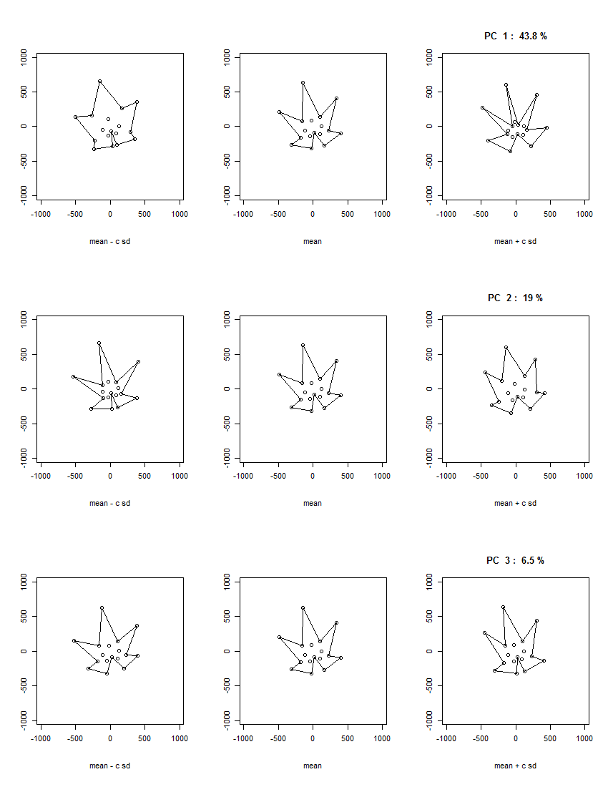
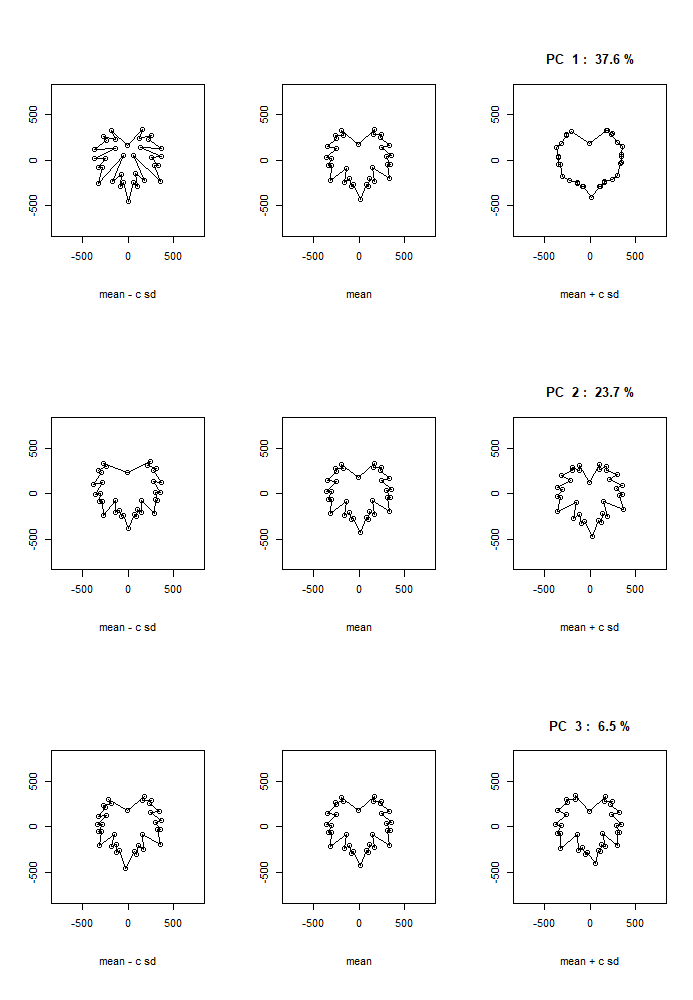
Generalized Procrustes analysis gave a visual interpretation of landmark morphometric approaches. The PC GPA1 accounted for 43.8% of variation, it was driven by sinus depth and opening of the petiole sinus (Fig. 11). The PC GPA2 accounted for 19% of variation and again was driven by sinus depth and relative opening, while PC GPA3 accounted for 6.5% of variation and appears to describe the opening of the petiole sinus.
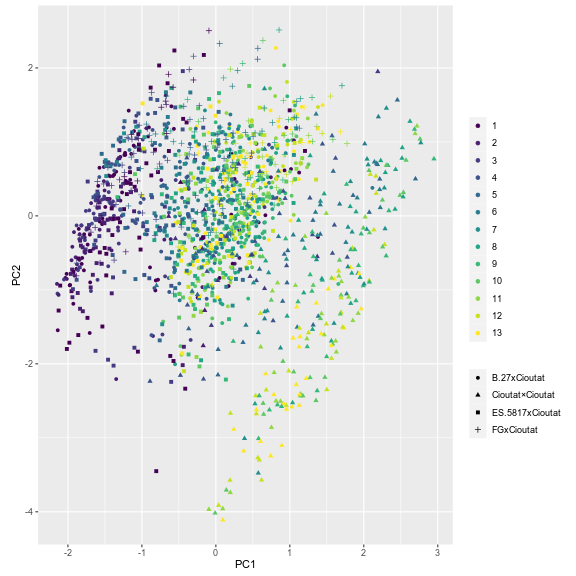
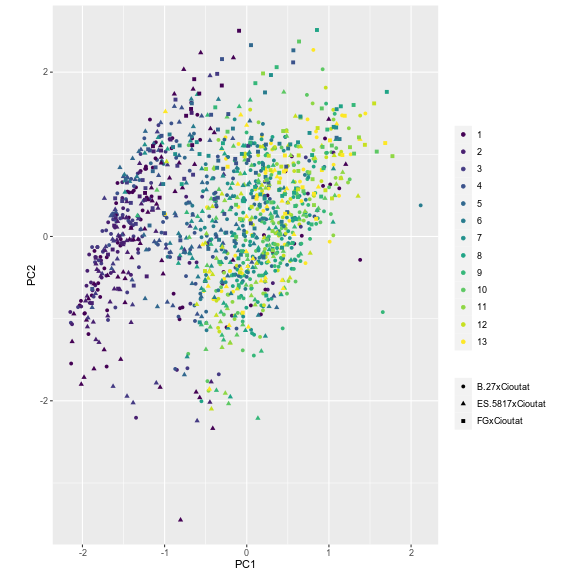
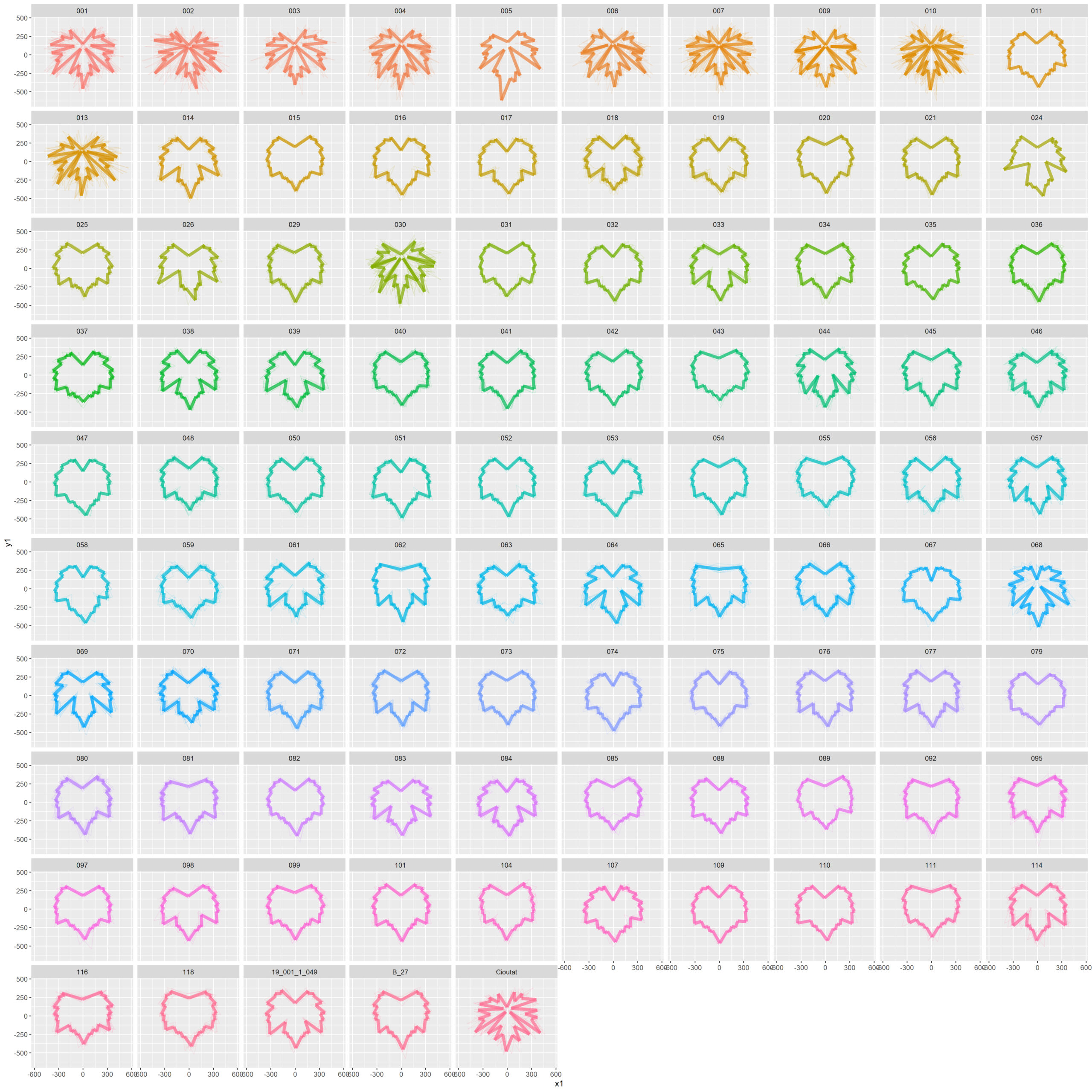
The distribution of individuals along the PC GPA plot yielded an evident and clear separation among leaf number (Fig. 12). The earliest to emerge leaves are clearly separated from the most recently emerged leaves with separation along PC GPA1 and PC GPA2; however, a mixture occurs around leaves 03-06 indicating a developmental transition. The separation of developing leaves became clearer when ‘Chasselas Cioutat’ selfed progeny were removed from plots (Fig. 13). This was attributed to their influence as an extreme case for leaf shape variation.
This developmental transition was best visualized in the mean leaves of each cross (Fig. 14). The increase in sinus depth was clearly progressing in mean leaves 04, 05, and 06. After this point the overall mean leaf shape shifts towards a more stable, mature leaf form.
Figure 11. Plotting mean leaves plus/minus one standard deviation for GPA PC1-3 based on landmark morphometric analysis of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 12. Plotting of individual leaf PC1 and PC2 values based on landmark morphometric analysis of leaves 01 to 13 of seedlings from four grapevine populations.
Figure 13. Plotting of individual leaf PC1 and PC2 values based on landmark morphometric analysis of leaves 01 to 13 of seedlings from four grapevine populations with ‘Chasselas Cioutat’ presumed-self progeny omitted.
Figure 14. Mean leaf shapes of leaves 01 to 13 of seedlings from four grapevine populations.
Conclusion
Leaf shape changes were examined along the developmental axis of grapevine seedlings starting with the first true leaf (leaf 01) after cotyledon emergence (leaf 00). Except for one individual, ND.19.001.1.012, no transition to ‘Chasselas Cioutat’, mutant, lacinate leaf shape was observed in S0/F1 hybrid progeny.
Most of the observed ‘Chasselas Cioutat’ presumed-self S1 progeny had leaves exhibiting the lacinate trait, and all scanned samples exhibited the lacinate, compound leaf trait. However, potentially greater variance for this leaf trait was observed in a larger population of inbred and presumed-inbred, direct seedlings from ‘Chasselas Cioutat’ (data not shown). Boyden (2005) and Snyder and Harmon (1939) also observed simple leafed progeny within ‘Chasselas Cioutat’ derived selfed progeny. Possible explanations for the observations found in this study and those of Boyden (2005) and Snyder and Harmon (1939) include potential inconsistency in defining the compound leaf trait as a binary trait or classification system, inadvertent open-pollination by non-intended parental genotypes, or compounded effects of heteroblasty and heterophylly on leaf shape expression. Within S1/F2 progeny, observed variation in lacinate leaf shape expression occurred within a single plant (data not shown). This pre-existing potential for variation within a plant when combined with variable growth conditions (shade, heat, age, fertilizer, humidity, watering status, etc.) may obscure visual phenotyping of specific characteristics prone to fluctuate within sample plants (Jones, 1995; Jones et al., 2013; Chitwood et al., 2016a; Spriggs et al., 2018; Baumgartner et al., 2020; Bryson et al., 2020).
Leaf shape changed drastically with newly emerging leaves as monitored by generalized Procrustes analysis of landmark morphometrics and traditional leaf variation metrics (Fig. A1-A34). Leaf size and shape and plant morphology are fluid and environmentally, genetically, and heteroblastically dependent (Wolf et al., 1986; Kaplan, 2001; Tsukaya, 2003; Chitwood et al., 2016a; Baumgartner et al., 2020; Bryson et al., 2020; Li et al., 2021).
Additionally, another unexamined facet was the effect of leaf growth and deformation. Due to the constraints of leaf measurement, which requires entire leaf removal for scanning, our sampling scheme was destructive for the first 13 leaves sampled along a plant with a single collection timepoint. Non-destructive measurement techniques in future research may enable researchers to monitor leaf development, growth, and the effect of growth on morphological characteristics (Kuchen et al., 2012; Rolland-Lagan et al., 2014; Wolf et al., 1986).
An example of environmental driven leaf shape responses was found in the aquatic species Rorippa aquatica, with drastically different leaf shapes within different environmental contexts (Nakayama et a., 2014). Lower temperatures or submergence in water drives leaf complexity while warmer temperatures and lack of submergence lead to simple leaf shapes. Leaf size and leaf shape are dependent on independent genetic and environmental factors (Baker et al., 2015). Like temperature and moisture availability, light also plays a role in leaf characteristics (Chitwood et al., 2015).
Limited work has focused on the inheritance of leaf shape traits in grapevines (Bešlić et al., 2005; Boyden, 2005; Welter et al., 2007; Nikolić, 2015; Demmings et al., 2019). However, leaf shape, physiological function, and other morphological characteristics of grapevines remain an important point of future research focus. Altering grapevine physiology and morphology through selective, informed breeding provides an opportunity for more sustainable grapevine cultivar development in the face of new pests, pathogens, and climatic challenges (Cousins and Prins, 2008). Within tomatoes, leaf shape has been linked to fruit quality and crop suitability (Rowland et al., 2020). Within grapevines, more work is necessary to identify any genetic or physiological correlations between fruit composition, plant productivity, and overall plant health with leaf shape traits.
Future Work
This work focused on three crosses with a unique pollen parent, ‘Chasselas Cioutat’. The leaf shape transitions observed followed a similar pattern and may be driven by ‘Chasselas Cioutat’ and the heavy V. riparia background of the seed parents; thus, they may not be entirely representative of grapevine developmental leaf shape changes. To understand more about the morphological development of foliar anatomy in seedlings of Vitis spp., similar work could be conducted within more diverse populations using different parents within a quantitative genetic approach to further elucidate dominant, additive, and epistatic effects.
The ultimate research focus should strive towards the goal of understanding the inheritance of ‘Chasselas Cioutat’ lacinate leaf anatomy in grapevines. Towards this goal, a subset of the phenotyped seedlings from this experiment were maintained under greenhouse conditions. Following sexual maturation and flower production, these individuals were either selfed (when hermaphroditic, perfect flowered), backcrossed to ‘Chasselas Cioutat’ (when female, pistillate flowered), or crossed with half-sibs from other S0/F1 populations. On-going segregation analysis of leaf morphology is anticipated to yield insight into the inheritance of the unique, lacinate leaf trait.
Within the examination of heteroblastic and heterophyllic leaf shape variation in Vitis spp. it is important for researchers to combine genetic maps and expression analysis to identify genes involved in the process of grapevine phase change transition from juvenility to maturity, and to better understand leaf shape maintenance and expression. When examining future progeny for effect of alleles, major genes likely involved in leaf shape expression based on their relevance in other plant species include PHANTASTICA (PHAN), LEAFY (LFY), FLORICAULA (FLO), Class I KNOTTED1-LIKE HOMEOBOX (KNOX1), CUP-SHAPED COTYLEDON (CUC), and JAGGED (JAG) (Kim et al., 2003a; Tsukaya, 2004; Champagne et al., 2007; Kimura et al., 2008; Koenig and Sinha, 2010). Regulation of adaxial identity is conducted by PHAN, with overexpression driving formation of simple leaf blades (Kim et al., 2003a; Kim et al., 2003b). Additional overexpression leads to ectopic blade formation. Additionally, PHAN dictates two different forms of palmately compound leaves (peltately and non-peltately palmate), and plays a role in determining placement of leaflets; this is combined with CUC and JAGGED regulation of leaf dissection (Kim et al., 2003b; Barkoulas et al., 2007; Koenig and Sinha, 2010; Bar and Ori, 2015).
Formation of compound leaf primordia requires a more intricate developmental differentiation process than simple leaves, and the presence of germplasm with compound leaves has implications to evolutionary adaptation and applied breeding (Blein et al., 2008; Blein et al., 2010; Nicotra et al., 2011; Ogden and Lacroix, 2017). A blastozone forms at primordium margins before yielding the primordia of leaflets (Hageman and Gleissberg, 1996). Harevan et al. (1996) examined the expression and phenotypic effect of a maize homeobox-containing Knotted-1 (Kn1) gene in tomato, with observed intensification of compound leaf phenotypes. Consequences of transgenic tomato plants expressing the Kn1 gene included super compound phenotypes, bushy growth, and loss of apical dominance. Similar results with Kn1, a KNOX gene, observed increasingly lobed leaves with overexpression; this was combined with abnormal leaf morphology (Lincoln et al., 1994). From the perspective of leaf serrations and lobiness, these traits also warrant extended investigation, as they also contribute to the variation in leaf shape observed.
Lobed leaf expression is triggered by KNAT1 in arabidopsis, Arabidopsis thaliana (L.) Heynh. (Chuck et al., 1996). Similarly, KNOX protein expression leads to dissected leaves in hairy bittercress (Cardamine hirsuta L.), a close relative of arabidopsis (Hay and Tsiantis, 2006). Further work refined understanding of developmental cues and leaflet formation in hairy bittercress focused on SIMPLE LEAF3 and KNOX1 (Barkoulas et al. 2008; Kougiousmoutzi et al., 2013; Vlad et al., 2014). Leaf developmental processes in Brassicaceae species have supported the evidence of KNOXI proteins in expression of complex leaves (Bharathan et al., 2002). Additional work with a recessive mutant allele at the BLADE-ON-PETIOLE1 locus (bop1-1) is proposed to determine regulation of class I KNOX genes, with mutants producing ectopic blades along the sides of petioles in Arabidopsis (Ha et al., 2003).
Within Fabaceae, the KNOX1 genes, FLO, and LFY genes have been shown to influence leaflet number and compound expression of leaves in soybean (Glycine max) and alfalfa (Medicago sativa), with FLO and LFY playing major roles (Champagne et a., 2007). Specifically, within the pea clade, LFY is a major regulator of foliar complexity. The expression of UNIFOLIATA (UNI) has also been implicated with control of leaf and flower formation in pea, Pisum sativum L. (Hofer et al., 1997).
Mungbean (Vigna radiata L.) is another example of a plant which transitions from simple, juvenile leaves to compound, adult leaves (Jiao et al., 2019a; Jiao et al., 2019b). Jiao et al. (2019b) identified epistatic interactions between heptafoliate leaflets1 (hel1-1) and small-pentafoliate leaflets1(smp1-1) allele mutants leading to increased leaflet number with reduced size relative to hel1-1 and smpl1-1 mutants alone. Again, these traits do not express in juvenile leaves, similar to the WT trifoliate compound leaf expression.
Hormonal regulation is an important point to investigate further in progeny. Within compound leaf primordia, auxin has been shown to determine site of developing leaflets (Koenig et al., 2009). Expression of LYRATE genes occurs in the auxin determining sites, helping with early events at the shoot apex (David-Schwartz et al., 2009). Auxin has been further explored in tomato leaf shape dynamics recently by Wu et al., (2018). Cytokinin has also been implicated in developmental processes for tomato compound leaf development (Shani et al., 2010).
In tomato, sampling leaves of varying ages showed that as leaves become increasingly complex there is an increased expression of CUC genes and decreased expression of TCP genes (Chitwood et al., 2012). Simultaneously, this demonstrated that KNOX was not involved in increasing heteroblastic complexity of tomato leaves. The CUC genes have an important role in both heteroblasty and heterophylly by converging, but different means (Chitwood and Sinha, 2016).
Despite the lack of KNOX genes’ role in heteroblastic expression of tomato leaf shape, the KNOX genes are critical in evolutionary leaf shape variation in tomato and remain important to heterophyllic responses such as leaflet production for shade avoidance (Chitwood et al., 2015).
MicroRNA expression analysis will be useful to understand more about the transition from juvenile to sexually mature tissue in grapes as it relates to heteroblastic form (Poethig, 2013; Lawrence et al., 2020). Multiple microRNAs are involved in the regulation of phase change transitions from juvenile to adult shoot characteristics (Huijser and Schmied, 2011; Poethig, 2013). In addition to miR156, important protein-protein interactions are involved in increased leaf complexity associated with plant maturation (Rubio-Somoza et al., 2014).
Leaf number and plant carbohydrate status may work to regulate miR156 levels (Yang et al. 2011). This may contribute to the phenomena of leaf shape discrepancies between spur- and cane-produced leaves in Vitis spp. compared to the leaves of suckers; these are often morphologically different based on visual assesment. Along with relative moisture available due to proximity to soil and timing of growth in relation to annual heat cycles, the level of miR156 may also play a role in grapevine sucker growth characteristics compared to cane- and spur-pruned shoots from established trunks considering the importance of miR156in shade avoidance (Xie et al., 2017).
In tree species, Wang et al. (2011) demonstrated the importance of miRNA for vegetative phase changes, indicating the importance of miR156 as a conserved regulator pathway in dicotyledonous plants for maintenance of juvenility. As trees from diverse woody species transitioned out of their juvenile phase miR156 abundance decreased while miR172 increased. In Passiflora edulis, miR156 and miR172 follow a similar pattern with miR156 correlated with juvenile leaf traits and miR172 correlated with adult leaf traits (Silva et al., 2019).
Along with microRNA expression, SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factors, NAC, and YUCCA genes are also likely involved in the developmental transition of some woody species based on investigations in Gevuina avellana (Ostria-Gallardo et al., 2016). NAC transcription factors are among the largest families of plant transcription factors encompassing (NAM, ATAF, and CUC) (Wang and Dane, 2013). They have demonstrated involvement in stress responses including light, drought, and salt (Wang et al., 2014a; Wang et al., 2014b, Wang et al., 2014c, Wang et al., 2017). The understanding of relationships between leaf morphology and stress responses is expanding, and the overlap of responsible genes and transcription factors likely reduces the ease of discernibility for certain heteroblastic and heterophyllic responses (Hwang et al., 2010; Song et al., 2012; Zhang et al., 2012; Fambrini and Pugliesi, 2013; Belluau and Shipley, 2018).
Monitoring developmental physiology transitions in grapevine seedlings may also yield insight into heteroblasty as has been investigated in Passiflora edulis using hyperspectral techniques, moisture stress index (MSI), normalized difference vegetation index (NDVI), and photochemical reflectance index (PRI) (Fernandes et al., 2020).
Okra leaf upland cotton (Gossypium hirsutum) is an example of a similar mutant type in which a traditionally simple leaf is now complex (Andres et al., 2014; Kaur et al., 2016; Andres et al., 2016; Andres et al., 2017). In the case of okra leaf upland cotton, a HD-Zip transcription factor has been demonstrated as the causal gene of the okra allele (Andres et al., 2016). This gene is homologous to the LATE MERISTEM IDENTITY1 (LMI1) gene of Arabidopsis and its mutation has a 133-bp tandem duplication within the promoter that is correlated with the phenotype of okra leaf shape in upland cotton. Andres et al. (2016) further identified increased transcription of photosynthesis related regulators; this up-regulation may be useful for breeding applications, and it is similar to observed trends within tomato (Chitwood et al., 2013). Further akin to upland cotton, Zhou et al. (2019) showed that patterns of compound leaves in Medicago truncatula are altered by mutations to a HD-ZIPIII gene, termed REVOLUTA (MtREV1). Mutations in similar homeodomain-leucine zipper gene families may be important to the compound leaf expression in ‘Chasselas Cioutat’, and their expression may be mediated by miRNA.
Ultimately, aggressive investigation of leaf shape combined with genetic maps and expression data will enable elucidation of genetic factors controlling compound leaf characteristics in Vitis spp. The compound, lacinate leaf shape observed in ‘Chasselas Cioutat’ is technically compound, yet the traits genetic regulation may differ from the demonstrated control of compound leaf expression in other dicotyledonous plant. While ‘Chasselas Cioutat’ leaves are technically compound, this is visibly due to an intensification of dissection rather than distinct leaflet formation. Thus, the developmental control may vary relative to the complex control of compound leaves observed elsewhere (Jones et al., 2013; Du et al., 2020; Israeli et al., 2020). It is likely that more than one gene is responsible for control of lacinate, compound leaf expression, as proposed by Boyden (2005). Further work with mapping populations derived from the individuals described here are anticipated to enable identification of critical genetic components of leaf shape in Vitis spp. and the transition from simple to complex.
2. Grapevine S1/F2 Progeny
Efforts to understand the complex, compound leaf shape within 'Chasselas Cioutat' derived progeny are on-going. Landmark morphometrics and visual classification of leaf-type have been applied. Additional landmarks were placed on each individual leaf for morphometric screening for a total of 34 landmarks around each leaf to capture leaf shape variation to a greater extent.
Initial classification of leaf shape into a two-class system enabled chi-square goodness of fit evaluation of progeny. Table 2.1 takes a single recessive loci approaching accounting for only one loci, 'Chasselas Cioutat' derived, homozygous recessive in state. Two of the four main S1/F2 progeny approached, but did not reach acceptance at (p<0.05). The remaining progeny sets did not approach acceptance for a single recessive loci.
Table 2.1.Compound leaf shape one-loci hypothesis, single recessive (aa) allele control (df=1).
|
Family |
Wild Type |
Mutant |
Chi‐square (χ2) |
|
(E.S. 5.8.17 × Chasselas Cioutat) Self ⦻ |
171 |
38 |
5.182 |
|
(Frontenac gris × Chasselas Cioutat) Self ⦻ |
1287 |
373 |
5.667 |
|
(North Dakota V. riparia × Chasselas Cioutat) Self ⦻ |
1317 |
295 |
38.591 |
|
(Minnesota V. riparia × Chasselas Cioutat) Self ⦻ |
219 |
33 |
19.048 |
Re-examined as a two-loci hypothesis, with one loci segregating and exhibited under recessive control while a second segregating loci requiring dominant allele control for complete expression approached a closer level of acceptance in three of the four progeny examined (Table 2.2). Heteroblastic, environmental, and developmental stage influences on leaf shape all may contribute to variable classification of leaf shape across individual plants. Heterogeneity of leaf form and function may have lead to greater or lesser expression under the greenhouse grown conditions evaluated. For these reasons, landmark morphometrics were further applied to leaf shape variation evaluation.
Table 2.2.Compound leaf shape two-loci hypothesis, (aa) recessive + (B_) dominant allele control (df=1).
|
Family |
Wild Type |
Mutant |
Chi‐square (χ2) |
|
(E.S. 5.8.17 × Chasselas Cioutat) Self ⦻ |
171 |
38 |
0.044 |
|
(Frontenac gris × Chasselas Cioutat) Self ⦻ |
1287 |
373 |
0.344 |
|
(North Dakota V. riparia × Chasselas Cioutat) Self ⦻ |
1317 |
295 |
0.214 |
|
(Minnesota V. riparia × Chasselas Cioutat) Self ⦻ |
219 |
33 |
5.289 |
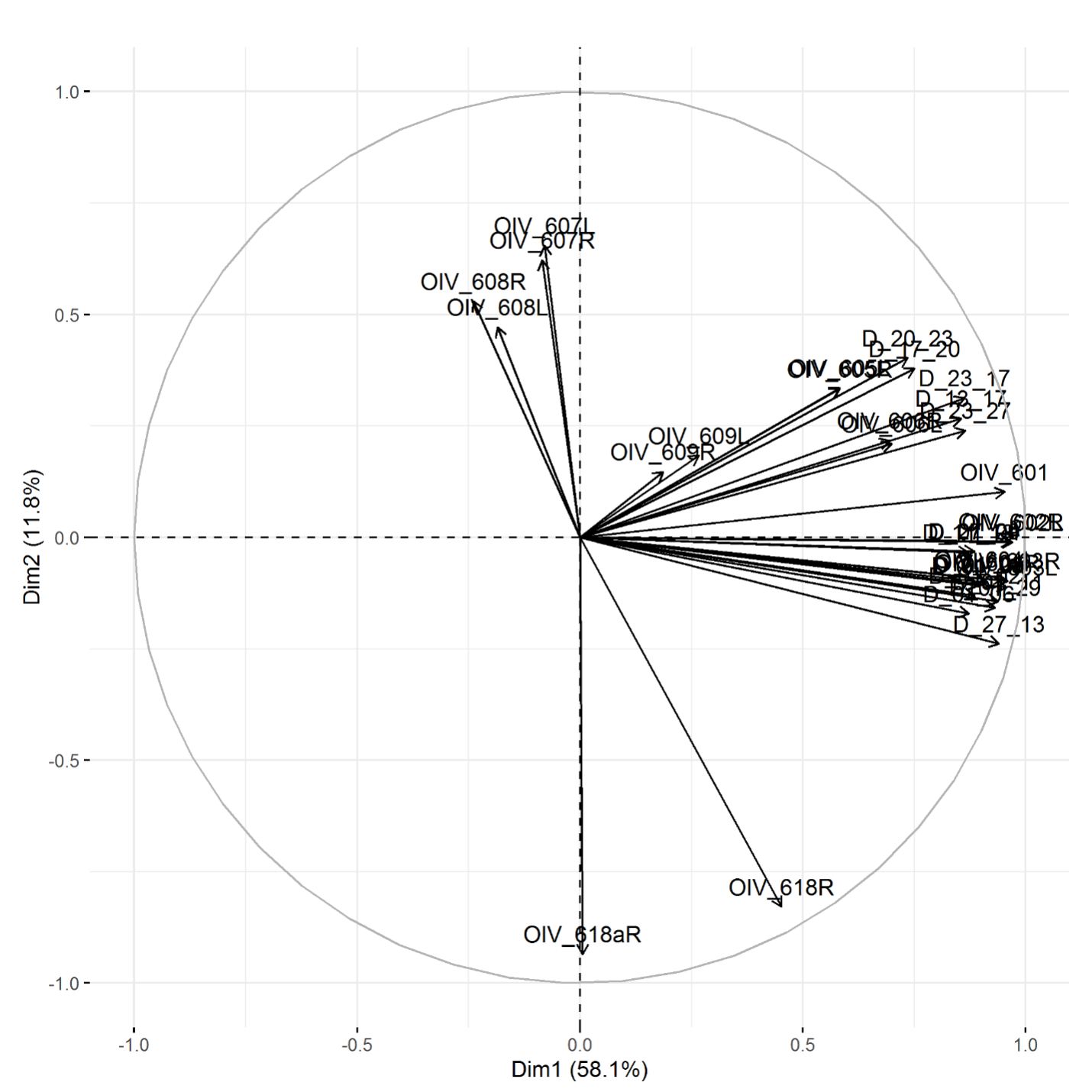
Principal component analysis was run on both the overall samples and on the individual’s calculated. For the variation in overall leaf samples, PC1 captures lobiness of leaves and accounts for almost 38% of variation among all leaf samples (Fig 2.1). For the inbred population examined in-depth here, 20_032 ([North Dakota V. riparia × 'Chasselas Cioutat'] Self ⦻), individual mean leaf shapes based on landmark morphometrics are depicted in Fig. 2.2. PC2 captures variation in the branching angle of petiole adjacent veins. PC3 appears to capture angle of main vein branching to left or right. Overall, individual leaf samples grouped cleanly based on PC1 for their classification as mutant, ‘Chasselas Cioutat’ like leaves, with minor exceptions overlapping. This mid-range, intermediary PC1 phenotypes likely stems from 1. inconsistency of several plants to uniformly exhibit mutant leaf forms and 2. High rate of lobiness without full, dissected, mutant leaf form of some simple leafed individuals classified as wild-type. The need for quantitative classification led to multiple attempts to compensate including 1. assessment of multiple leaves leading to a “mean” score for wild-type to mutant leaf form and 2. the landmark morphometric approaches discussed here-in.
The GPA of individual mean leaf shapes indicates the two primary PC account for over 75% of variation when examining foliar variation in this specific family (Fig. 2.1). PC1 is composed of lobiness with negative values associated with a leaf shape more similar to the paternal grandparent (‘Chasselas Cioutat’) and positive values associated with a wild-type, V. riparia-like leaf shape. Progeny ranged in their overall leaf shape, with many F1/S0 progeny having intermediate leaf forms as depicted in Figs. 2.3-2.4.
Figure 2.1. Plotting mean leaves plus/minus one standard deviation for GPA PC1-3 based on landmark morphometric analysis of leaves from a single S1/F2 progeny family ([North Dakota V. riparia × Chasselas Cioutat] Self ⦻).
Figure 2.2. Mean leaves of selected individuals within a single S1/F2 progeny family ([North Dakota V. riparia × Chasselas Cioutat] Self ⦻).
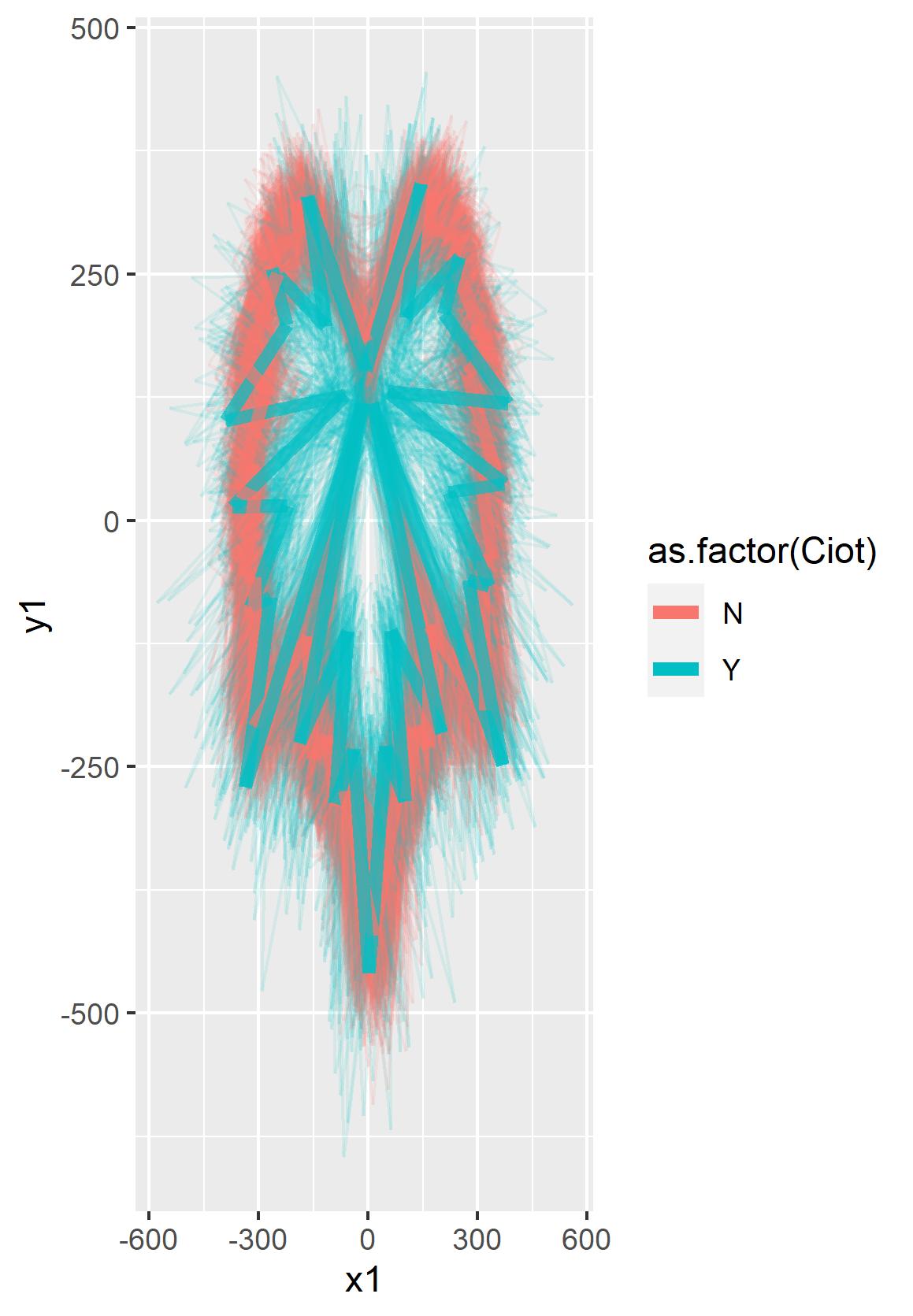
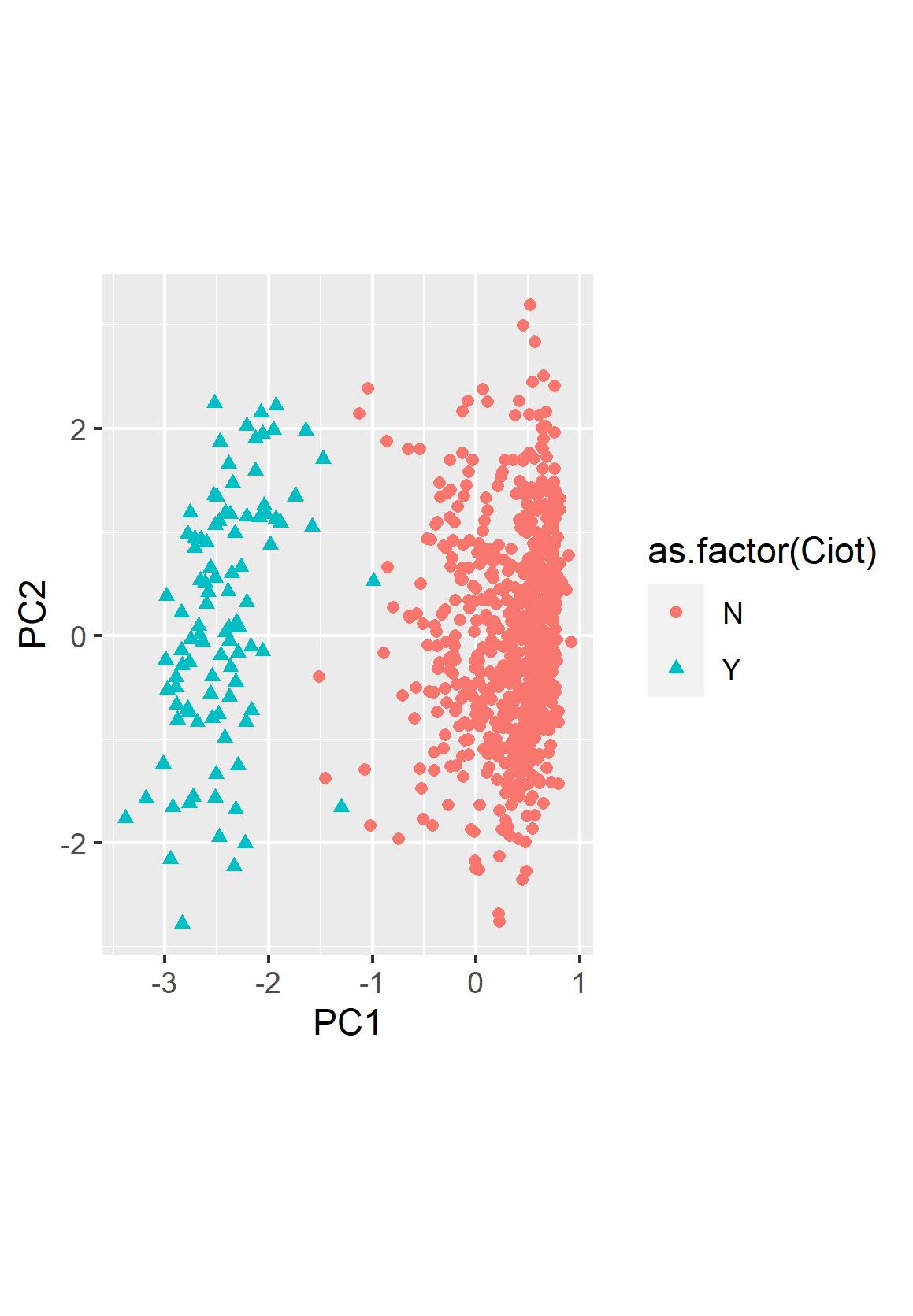
Figure 2.3. Overall leaves traced as visually defined as wild-type (as.factor(Ciot)=N) or mutant/'Chasselas Cioutat' (as.factor(Ciot)=Y) laceleaf type for a single S1/F2 progeny family ([North Dakota V. riparia × Chasselas Cioutat] Self ⦻).
Figure 2.4. Plotting of individual leaf PC1 and PC2 values based on landmark morphometric analysis of leaves as visually defined as wild-type (as.factor(Ciot)=N) or mutant/'Chasselas Cioutat' (as.factor(Ciot)=Y) laceleaf type for a single S1/F2 progeny family ([North Dakota V. riparia × Chasselas Cioutat] Self ⦻).
Conclusions
Current issues stemming from the COVID-19 pandemic have led to delays in sequencing acquisition; thus, marker-trait associations remain incomplete. NCR-SARE funded sequences from samples submitted for GBS in 2020 have not yet been received in full. Sequencing has been acquired to-date in the through collaboration in an ongoing SCRI project, VitisGen2. However, due to incomplete population DNA extraction in 2020, additional individuals were re-submitted in 2021 for which sequencing results are pending. This has led to incomplete genetic coverage of populations through both sequencing technologies, and thus, at this time genetic map construction is incomplete.
Stemming from the fact that, to this point, genetic map construction has failed due to lack of individuals fully sequenced, tentative genome wide association analysis of marker-trait relationships have been investigated. These have given promising, but incomplete indications of potential quantitative loci of interest.
The populations developed remain a part of the NDSU-GGEP and are transitioning to re-utilization for further genetic screening and phenotyping to aid in the program's goal of developing climate adaptive grapevines capable of surviving harsh winters of the Upper-Midwest.
Educational & Outreach Activities
Participation summary:
Presentations:
- Research was highlighted as part of the Winter 2020 North Dakota Grape and Wine Association Annual Conference (Mandan, ND).
- Research was highlighted during the Winter 2021 Montana Grape and Wine Association Annual Conference (Virtual).
Field Days
- Research was highlighted as part of the Summer 2020 North Dakota Grape and Winery Association Summer Farm Tour: Grapevine Breeding Research Update held at the NDSU Agriculture Experiment Station (Fargo, ND), 4e Winery (Casselton, ND), and Red Trail Vineyards (Buffalo, ND).
Other
- Website launched in Fall 2020. [https://www.cropbreeding.org/home/grape-populations/chasselas-cioutat-progeny].
- Based on web analytics:
- Website engaged sessions in 2020: 89 (average engagement time: 4m 06s; users from 2 countries)
- Website engaged sessions as of 02/24/2021: 165 (average engagement time: 3m 13s; users from 4 countries)
- Website engaged sessions in 2021: 128 users (average engagement time: 2m 11s); users from 8 countries.
- Total website engagement:
- Users: 147
- Users from 19 US states: North Dakota, California, Oklahoma, Kansas, Ohio, Alabama, Georgia, Kentucky, Minnesota, Montana, Washington, Arkansas, Idaho, Illinois, Louisiana, Missouri, New York, Virginia, Wyoming
- Users from 8 countries: USA, Finland, Turkey, Canada, Germany, Iran, China, and Netherlands
Project Outcomes
Learning Outcomes: 4.6 Awareness of new alternative agricultural practices. [Development of genetic alternatives for canopy management]
- We reached farmers at multiple events in 2020-2021, despite COVID-19 restrictions.
- The project was presented digitally as part of the American Society for Enology and Viticulture Student Presentations in 2020 (https://www.youtube.com/watch?v=pxnr6d0SurI&feature=emb_logo).
Action Outcomes: 5.3 New research that builds on previous SARE funded R&E projects. [Development of future projects building on the successful completion of this project]
- The NDSU GGEP submitted two new grant proposals in 2021 to to leverage this funded projects’ populations, genetic data, and germplasm towards pursuing future research projects on both the intra- and inter-university level following completion of this project; both of which were funded.
- The student researcher now serves as faculty at Montana State University with anticipated project proposals for the 2022 grant cycle (and beyond) to build on knowledge gained.
Output 3.7: scientific journal articles and germplasm resources distributed to the research and grape breeding community.
- Manuscripts are in preparation for dissemination of results, with expected submission in 2022-2023, pending genetic data receipt in full.
- Valuable germplasm remains in the propagation pipeline of the NDSU-GGEP (field and greenhouse) to establish distribution for researchers and breeders.
Collectively, through the project we have observed outstanding variation in progeny of Vitis spp. The unique leaf shape of 'Chasselas Cioutat' segregates with unexpected consequences to seration, depth of sinuses, and variation within plants. Marker-trait-associations remain inconclusive and in development due to the lack of fully sequenced population date; however, these results are anticipated to change how we apply selection within the North Dakota State University Grape Germplasm Enhancement Project for this leaf shape trait. Furthermore, the populations developed are serving as a foundation for future work within the program as a learning tool for graduate students.
A new collaboration was established with the University of Minnesota grapevine breeding program where seedlings from a similar cross were developed and shared for phenotyping.
Two additional graduate students within the NDSU-GGEP program (one MS, one new PhD) have been integrated into the populations from this NC SARE project. One student (MS) assisted with leaf shape scanning, and is subsequently undertaking efforts for population retention following the graduation of student- Andrej Svyantek. An additional student (PhD), is now maintaining the populations generated for this project towards completion of his PhD work mapping subsequent climate-adaptive traits such as ripening time, dormancy acclimation, and cold-hardiness.