Final report for GNC20-300
Project Information
Maximizing Nitrogen Fixation in Cold-Hardy Hairy Vetch
Cool season cover crops used in organic systems have the capacity to improve soil health and nutrient contributions, if productivity and biomass can be optimized. Leguminous cover crops, in particular, provide nitrogen via biological nitrogen fixation (BNF), reducing the need for inputs such as manure, which can lead to overloading of bound phosphorus. The legume hairy vetch (Vicia villosa) is widely used as a winter annual cover crop in the Midwest because of its cold-tolerance and weed-suppressive effects. Although known to have among the highest BNF rates of common cover crop legumes, hairy vetch adoption has been limited in cold regions by management challenges, including weediness, termination difficulty, and inconsistent winter hardiness. Hairy vetch BNF also varies across landscapes, owing partially to the fact that recommended rhizobia inoculant strains commonly applied at planting may not be well-adapted to their environment. Cold growing conditions hinder BNF, but fortunately, in areas where hairy vetch was previously grown, there are indigenous populations of rhizobia that are well-adapted to their climatic conditions. This study will identify rhizobia strains best suited for symbiosis with two new cold-hardy hairy vetch ecotypes in the climatic conditions of the upper Midwest. Rhizobia adapted to severely cold climate conditions will be collected from the northernmost University of Minnesota field station. Two cold-hardy ecotypes of inoculated hairy vetch will be grown at 10°C, to reflect fall temperatures in the upper Midwest. Plants will be inoculated with rhizobia strains sourced from across a climate gradient, including the newly collected strains from northern Minnesota and existing strains from our extensive rhizobia collection. Plant BNF capacity and nodule number will be measured to determine efficiency of each rhizobia strain. I will disseminate my findings via a scientific publication and a talk at an organic-grower focused conference, with the potential action outcome of farmer inoculation strategy modifications.
The goal of this project is to produce inoculant information for hairy vetch that can be used in cover cropping recommendations for farmers in the upper Midwest, especially those in USDA plant hardiness zones 3 and 4, in order to meet prescribed organic practices outlined in the USDA National Organic Program.
Learning outcome 1: Organic researchers and extension agents will have access to information about legume host-rhizobia relationships, and optimal inoculation strategies for hairy vetch.
Learning outcome 2: Approximately 50 farmers will learn about the role of rhizobia in improving hairy vetch BNF during a field day. An additional 50 farmers will learn this information at an organic farming conference (MOSES or Minnesota Organic Conference).
Action outcome 1: Around 50% of growers from the field day and conference presentation will try using rhizobia inoculants to improve hairy vetch, or other legume, performance in the coming season.
Action outcome 2: The published peer reviewed article will provide a foundation for future research on hairy vetch cultivation in the upper Midwest.
Research
Pilot Experiment
A collection of 32 rhizobia strains isolated from Minnesota and North Carolina soils were tested as hairy vetch inoculants. Two cold-hardy ecotypes of hairy vetch (Albert Lea Cold-Hardy VNS and MSP 4045) were inoculated with each rhizobia strain, replicated 4 times. (32 rhizobia strains x 2 hairy vetch ecotypes x 4 replicates = 256 plants) Plants were grown in sterile growth pouches (see picture below), so that root systems were visible and nodule development could be tracked in real time. Plants were grown for 6 weeks in a growth chamber, set at 15C day/10C night to mimic fall planting conditions in the upper Midwest. Plants were watered daily with nitrogen-free nutrient solution, to ensure that all plant nitrogen came from biological nitrogen fixation. The number of days to onset of first nodule was tracked for each plant, over the course of 6 weeks. Because of the cold conditions, and potentially a lack of root insulation in growth pouches, plants and nodules were too small to collect mass data, or to determine total plant N content. Some rhizobia strains led to no nodulation after 6 weeks. As a result, this experiment served as a useful pilot to determine which rhizobia strains would be useful to study further.
Nitrogen Fixation Experiment
Challenges from the first experiment informed the design of the second experiment. First, due to challenges associated with the growth pouches (too much root exposure to cold, lack of structural support for vetch), modified magenta units (see picture below) were used to grow plants in the second experiment. Also, plants were grown for 10 weeks, rather than 6, to have plants and nodules big enough to weigh. Unlike growth pouches However, unlike in growth pouches, root systems are not visible in magenta units. Two destructive harvests were carried out to determine degree of nodulation at 6 weeks: harvest 1 at 6 weeks, and harvest 2 at 10 weeks. I hypothesized that nodule number at 6 weeks would predict biomass at 10 weeks. Six rhizobia strains were used as inoculant treatments based on their performance in experiment 1, with 3 strains from Minnesota and 3 from North Carolina. I also included two controls: a positive nitrogen control (plant given nitrogen-rich nutrient solution) and a positive nodulation control (plant inoculated with commercial inoculant). Once again, two hairy vetch ecotypes were used [2 hairy vetch ecotypes x 8 treatments (6 rhizobia, 2 controls) x 4 replicates x 2 time points = 128 plants].
Data was collected on shoots, roots, nodules, and plant nitrogen content. At 6 weeks, nodules were counted, and shoot/root biomass was dried for 48 hours and weighed. Nodules were too small to be removed from plants and weighed; there was also too little shoot biomass to determine plant nitrogen content. At 10 weeks, nodules were counted, and they were big enough to be dried and weighed. Shoot and root biomass was also dried and weighed. Combustion analysis was performed to determine nitrogen content on shoots, but the data is not ready yet at the time of this report’s submission.


Pilot Experiment:
This experiment tracked the number of days to nodule formation across a collection of 32 rhizobia strains. When comparing days to first nodule across the two hairy vetch ecotypes, Albert Lea plants developed nodules slightly earlier than MSP 4045 plants. However, a higher percentage of MSP 4045 plants ultimately developed nodules. Time to nodulation was higher for Albert Lea plants than MSP 4045 plants (p = 0.003).
To determine mean effect of individual rhizobia strains on nodulation rate, results were combined across hairy vetch ecotype, resulting in 8 inoculated plants for each individual rhizobia strain. Mean days to nodulation was then calculated for each strain, with values ranging from 23.33 days to 34 days. However, some strains only nodulated 2 of the 8 plant replicates, leading to artificially low standard deviations in those cases. To address this issue, I identified “consistent nodulator” strains, determined by selecting strains that nodulated seven or eight of the eight total plants originally inoculated by each strain. This resulted in selection of 6 of the total 32 strains for further study in experiment 2. Interestingly, of the 32 strains tested, 5 of the 6 consistent nodulators were Minnesota-origin strains. I then broadened the category to look at rhizobia strains that led to nodulation in over half of plant replicates. Nodulation speed was variable across these “successful” nodulators, with mean days to first nodule ranging from 27 to 33. The high standard deviations suggest that few of the strains have consistent nodulation speeds, at least in this experiment.
Table of Consistent Nodulators:
| Rhizobia Strain Number | Rhizobia Strain Origin | Mean Number of Days to First Nodule | Standard Deviation |
| MNC8 | Minnesota | 27.125 | 4.79 |
| NCW8 | North Carolina | 28 | 5.56 |
| MNC5 | Minnesota | 29 | 5.56 |
| NCC5 | North Carolina | 29 | 4.74 |
| MNC7 | Minnesota | 29.125 | 5.98 |
| NCW2 | North Carolina | 29.2 | 2.16 |
| NCW5 | North Carolina | 29.2 | 2.16 |
| NCC8 | North Carolina | 29.33 | 4.8 |
| MNW7 | Minnesota | 29.4 | 6.65 |
| NCC1 | North Carolina | 29.57 | 4.57 |
| NCC4 | North Carolina | 29.83 | 5.03 |
| NCC3 | North Carolina | 30.8 | 4.32 |
| MNC1 | Minnesota | 30.85 | 5.04 |
| MNC3 | Minnesota | 31 | 7.4 |
| NCC7 | North Carolina | 31.6 | 6.18 |
| MNC4 | Minnesota | 31.75 | 8.0 |
| NCW3 | North Carolina | 31.83 | 5.98 |
| MNC6 | Minnesota | 33.66 | 5.085 |
Nitrogen Fixation Experiment:
This experiment sought to address three main questions:
- How well does early nodule number predict biomass production?
At both 6 and 10 weeks, nodule number and plant biomass were highly correlated (R2 = 0.68 at 6 weeks, and 0.67 at 10 weeks). However, nodule number at 6 weeks was not correlated with biomass at 10 weeks (see figure 1). These results suggest that growth chamber experiments that run for 6 weeks or less may not give useful information on nitrogen fixation in hairy vetch, especially at low temperatures.
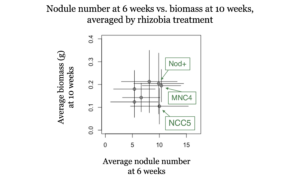
- Can cold-adapted rhizobia increase biomass production compared to a commercial inoculant?
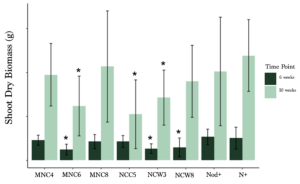
Rhizobia strain geographic origin did not have a consistent or reliable effect on hairy vetch biomass accumulation. However, the best-performing strain (MNC8) came from Minnesota, and the worst-performing strain (NCC5) came from North Carolina. This suggests the best rhizobia partner for hairy vetch might come from Minnesota soils, but the amount of screening required to find that strain would be extensive. No strain outperformed the commercial inoculant (Figure 2).
- Does one hairy vetch ecotypes tend to produce more biomass at low temperatures?
The two hairy vetch ecotypes, Albert Lea cold-hardy and MSP 4045, performed equally well at 15C day/10C night temperatures. There was no observable difference in their biomass accumulation. Ultimately, rhizobia treatment had a greater effect on biomass accumulation.
Educational & Outreach Activities
Participation Summary:
Results from this project were shared at poster sessions at three conferences: American Society for Horticultural Science Annual Conference, Tri Societies International Organic Meeting, and the MOSES Organic Farming Conference. Across the three conferences, I engaged with approximately 50 agricultural professionals who visited my poster to learn about my results. At the MOSES organic farming conference, approximately 20 farmers came to my poster, many of whom were curious about hairy vetch as a winter annual cover crop.
Several outreach publications are in progress or planned for the future. Because we received feedback that farmers are over-surveyed, we shifted our attention from surveying farmers about legume use to publishing extension articles and having multiple outreach opportunities. I have published two extension articles related to this project. First, I wrote an article entitled "Rhizobia Inoculation for Organic Farming Systems" for the University of Minnesota Extension newsletter "Fruit and Vegetable News." The article covers both broad ideas related to biological nitrogen fixation and some information specific to my project. Then I wrote an article for a broader audience entitled "Inoculating Garden Legumes" for University of Minnesota Yard and Garden News. I have also hosted two outreach events at the Minnesota State Fair, in August 2021 and 2022, in collaboration with the Forever Green Project at University of Minnesota. I discussed winter annual cover crop strategies with over 200 State Fair attendees who visited our booth, about half of whom were farmers. Finally, I am preparing a manuscript describing the results of my experiments with plans to publish in Plant and Soil.
Project Outcomes
At the MOSES conference, I had many conversations with farmers about management challenges associate with vetch. I gave advice based on my experience testing vetch in experimental conditions and brought their concerns back to my advisor to lay groundwork for future grants. At three outreach opportunities (MOSES, Tri Societies Annual meeting, and the Minnesota State Fair), I raised awareness of hairy vetch as a promising cover crop not just for organic systems, but for annual grain systems across the Midwest. With greater hairy vetch adoption across the region in concert with this research on improved nitrogen fixation, farmers can rely more on biologically fixed nitrogen rather than manure or chemical fertilizers (in the case of conventional farmers). These changes have economic benefits (less money on fertilizer) and social benefits (less labor to spread manure, fewer complaints about odors related to manure fertilizers).
I also forged a connection to an inoculant company which works frequently with organic farmers. I tested rhizobia strains which are of interest to the inoculant company, could go to market, and become a more useful management tool for hairy vetch for farmers than existing commercial strains. This would allow farmers to get more "bang for their buck" from a commercial inoculant. Also, building a connection with the inoculant company will generally create more synergy in bringing products to market that are most needed by farmers.
Knowledge: We found several rhizobia strains that are good candidates for inoculant development in the upper Midwest, as they can contribute as much nitrogen to a vetch plant as N-enriched nutrient solution. We learned that hairy vetch ecotype, while possibly important for establishment and winter survival, is not a critical factor in nitrogen accumulation. We learned that nodulation speed is not a critical trait for developing cold-hardy inoculants, which lays the foundation for future experiments to explore other rhizobia traits which may play a more critical role.
Attitude/Awareness: By forging a partnership with a small inoculant company that works with organic farmers, we learned more about the process of developing promising strains into inoculants which are easy and inexpensive for farmers to use. During two outreach opportunities at the state fair, I learned that there is a desire among conventional growers in Minnesota, not just organic growers, to adopt winter annual cover crops like hairy vetch. Through interacting with organic growers at MOSES and in writing extension articles, I learned more about the management challenges associated with vetch, especially termination challenges. These lessons will inform future grant applications and research efforts related to hairy vetch.
Skills: The grant gave me the opportunity to present to a mostly scientist audience at Tri Societies, and a mostly farmer audience at MOSES, so I developed my science communication skills, especially in conveying the importance of my work and results to different audiences. From a technical standpoint, I learned a lot about growing vetch at low temperatures in growth chambers (of which there was not much knowledge when I started), which will be useful to share with other researchers focusing on this critical winter cover crop. I learned how to facilitate a public/private partnership with an inoculant company. We learned how IP protection works in the development of rhizobia inoculants, paving the way to develop an inoculant which can be used by organic producers growing hairy vetch.