Final Report for GNE13-053
Project Information
Declines of native bumble bee species have been recorded for over six decades in Europe and, more recently, are also being reported in North America. Bumble bees are a temperate species and are well-adapted to Maine’s cool spring climate, making them an important pollinator for the May to June bloom of lowbush blueberry. Bumble bee pollination is especially imperative in contemporary times as we see honey bee colony losses and the rising costs of honey bee hive rentals causing a burden on Maine’s growers. Although there is currently little consensus about the exact cause of bumble bee losses, there has been recent interest in the use of systemic neonicotinoid pesticides in 2North America’s crop systems and possible sublethal effects on bumble bee colonies.
This project seeks to investigate such effects using managed colonies of Bombus impatiens by feeding them a range of field-realistic doses of the neonicotinoid imidacloprid, placing them in one of six field sites in Midcoast Maine, and then taking measurements that serve as proxies for colony strength: the number of adults left in the colony at the end of the season; the area of the brood clump left in the colony; the weight of the brood clump; proportional weight change while out in the field; and average worker size.
Colonies that consumed more imidacloprid during the dosing period were found to have fewer bees at the end of the season (P = 0.10), gain significantly less weight in during the dosing period (P <0.0004*), and have a significantly smaller brood area at the end of the season (P = 0.0132*). Index (consumed) dose was found to have a marginally significant effect on brood weight at the end of the season (P =0.073), although field management type had a significant effect where colonies placed in fields with low-medium input had higher brood mass as compared to the organic-low input fields (P =0.036*).
In 2015, I compared these measurements to field components that included: field region; field management (organic – low input and low – medium input); bumble bee species richness; flower species richness; and the cumulative conopid incidence in each field measured by dissecting wild caught bumble bees.
I found differences in bumble bee species and flower diversity between the study fields and included these measures as field components that may affect the experimental Koppert colonies placed in those fields. The cumulative incidence of conopid infected bees over the season (from mid-July through September) across all fields was 15.8%, although there was a peak observed in mid-late August, after the Koppert colonies had been removed from the fields on 7/29/14. In late July, cumulative incidence across all fields was only 10.4%.
Introduction:
The purpose of this project is to investigate the effects of dietary imidacloprid on bumble bee colony growth and health in lowbush blueberry fields (Vacinnium angustifolium) in Maine in the face of recent population declines of bumble bees in North America. Bumble bees are the most important wild pollinator in many agroecosystems, where each individual pollinates as much as several honey bees. Native pollinators are adapted to local conditions and bumble bees are well-suited to temperate climates and are able to forage in cooler conditions and lower light levels than honey bees. Bumble bees also use sonication (“buzz-pollination”) while foraging, which is required for the adequate pollination of certain crops such as lowbush blueberry. Bombus species have been found to be the most effective pollinators in lowbush blueberry systems, with the ability to pollinate 6.5 flowers in the time it takes a honey bee to harvest just one. Beyond this, honey bee colony numbers are declining in the face of colony collapse disorder (CCD) and the prices of hives, which are a necessary purchase for many growers, have been climbing. Therefore, it is more important than ever to conserve our native pollinators to ensure adequate pollination of our commercial crops.
However, troubling evidence has surfaced concerning recent range reductions and declines in some North American bumble bee species. Various factors may be implicated in these observed declines. For example, North American bumble bees face habitat fragmentation, loss of forage resources and the introduction of novel pathogens through the widespread use of commercial bumble bees. Pesticides have also been widely blamed for the declines, especially the neonicotinoids (imidacloprid, clothianidin and thiamethoxam) that pervade treated plants and appear in the nectar and pollen of flowers, which are consumed by bees. Because there are multiple factors that have been proposed to be behind the declines, the scientific approach for investigating such factors must be multi-faceted.
Conventional lowbush blueberry growers in Maine currently use imidacloprid in their fields in order to combat blueberry maggots and thrips. Although research has shown little direct effect on bee survival at the level of the colony, recent literature has suggested that imidacloprid, which is a neurotoxin, may affect colony development and reproductive output of bumble bee colonies. Recent research has demonstrated that dosed colonies have less success in producing reproductives (particularly queens) at the end of the season, which can have detrimental impacts on populations in the following year, even if the pesticide does not directly kill the bees. Therefore, we used commercially-raised sentinel colonies of Bombus impatiens that had been orally dosed for two weeks on a range of field-realistic doses of imidacloprid to monitor their growth and progress after the dosing has stopped. The colonies were placed in six different fields with varying management strategies, ranging from certified organic to conventional, in an effort to also investigate possible field effects.
It is the goal of the study to ensure that the current Insect Control Guide for Wild Blueberries in Maine is up to date and fully takes into account the health and sustainability of native pollinators in the cultural techniques to reduce insect infestation of pests.
Objective 1. To dose colonies of commercial B. impatiens with a range of field-realistic doses of imidacloprid and track their progress through the season:
24 small (~30 individuals per colony) colonies were ordered from Koppert Biological Systems (Romulus, MI) and delivered to Maine on 15 May 2014. The colonies were divided into four groups of six and each group was given a range of imidacloprid treatments as added into their only food source (a bag of Koppert “Bee Happy” food). The doses ranged from the control (0 ppb) up to 125ppb of added imidacloprid in the form of AdmirePro®. The bees were allowed to feed on the dosed food ad libitum for two weeks in the lab and colonies and their food bags were weighed daily to monitor growth and track food consumption. Bees were kept at growth chamber conditions (15:9 LD, 20°C, 55% rH) during the dosing period.
After this two-week period, samples from each food bag and five individuals from each colony were collected and frozen at -20°C for chemical analysis to analyze actual dosage. Each group was placed into one of six blueberry fields around Waldo and Hancock Counties (Figure 1), the same sites where wild bumble bee collections took place. These fields had management practices that ranged from small and organic-low input (three fields) to low-medium/conventional input (three fields). Colonies were placed > 10m apart to mitigate bees switching between colonies and were weighed once a week to monitor their growth.
Colonies were picked up from the fields on 29 July and 30 July 2014 and placed into a 5.5°C cold room overnight. Final colony weights were taken and then colonies were moved to a -20°C freezer to freeze-kill. Final counts of workers, drones, and queens along with estimated of brood area were made in the following weeks. The intertegular spaces (ITS) of all individuals from each colony were measured to estimate average worker size of each colony.
Objective 1 was reached with minor changes as described in Materials and Methods.

Objective 2. To monitor levels of conopid fly parasitism in the commercially-raised dosed colonies as well as in wild caught native bumble bees collected from blueberry fields around the state:
From 14 July 2014 to 24 September 2014, the six field sites where the colonies were placed were visited approximately every two weeks in the Midcoast region of Maine (Figure 1). Two to three researchers spent either an hour or collected 20 wild bumble bees at each site (whichever came first) as a measure of sample effort. Obvious queens were not captured. Researchers split up at field sites to minimize the possibility that collected bumble bees were all from the same colony. Specimens were marked with the date, field site, and the common name of the flower on which they were collected (if known) then brought back to the lab and placed in a -20º C freezer to freeze-kill.
Each bee was identified to the species level and dissected using the method described above.
Objective 2 was reached with the change that the commercially-raised Bombus impatiens colonies were not dissected for conopid presence due to initial dissections showing no presence of conopids and the time consuming nature of dissections. Therefore, levels of conopid parasitism in the wild bumble bees were used for field measurements.
Objective 3. To establish an effective rearing method for the native bumble bee species B. ternarius in order to use true native colonies in similar experiments as in Objective 1.
Approximately 20 B. ternarius and B. vagans queens were captured during the month of May 2014 in blueberry fields in Downeast Maine and placed into plastic “rearing boxes” with a pollen ball and fed Koppert Bee Happy food ad libitum and kept in the growth chamber with the experimental Koppert colonies. Because no queens demonstrated nesting behavior and no eggs were laid within a 21 day period, all queens were released.
An attempt to use radio telemetry of captured and then released queens to locate nest sites for possible experimental use was not successful. One B. impatiens, one B. vagans, and two B. bimaculatus queens were fitted with 200 mg radio transmitters (Advanced Telemetry Systems, Isanti, MN) and released. We were able to locate three of the bees –all of which had died and were found on the ground.
Objective 3 was not reached, we were unable to establish an effective rearing protocol for Bombus ternarius or any other native Maine bee.
Objective 4. To measure the immunocompetence of the imidacloprid-challenged bees using measures of the enzyme phenoloxidase (PO) in bees collected from each colony before dosing, during dosing, directly after dosing, and at regular intervals throughout the season.
This work has not yet been completed, but I have prepared for this by learning the technique of how to measure PO levels using the frozen thorax of bumble bees. We have also compared the PO levels of different species of bumble bees (Koppert B. impatiens, B. ternarius, and B. vagans) and this data can be used in future comparisons.
Objective 4 has not been reached as those samples have not yet been processed.
Cooperators
Research
Objective 1. To dose colonies of commercial B. impatiens with a range of field-realistic doses of imidacloprid and track their progress through the season:
24 small research colonies (each containing ≈ 30 workers and queen) were ordered from Koppert Biological Systems (Romulus, MI) and delivered to Maine by refrigerated truck on 15 May 2014. From there, the colonies were transported to The University of Maine’s Blueberry Hill Farm in Jonesboro, ME. All hives included one quad box per colony in order to protect each cardboard research colony from the elements once they are placed at the field sites.
Colonies were left undisturbed once set up in the growth room (15:9 LD . 55% rH, 20°C) for 24h to allow bees to calm and acclimate. The experiment began the following day when the sugar water bags that come with colonies were removed, emptied, and filled with 1 liter of Koppert Bee Happy Food that were either only the syrup (for the controls), or dosed with one of five doses. Originally we had planned to use Pro-Sweet Liquid Feed ® from Mann Lake, but the viscous nature of that food made the mixing with the Admire® Pro difficult and we were uncertain if the chemical was able to be evenly distributed through the food bag.
The doses were initially supposed to encompass a range of field realistic doses that were used in Laycock et al. (2012): 0.08 mg/L, 0.51 mg/L, 3.2 mg/L, 20 mg/L, and 125 mg/L of imidacloprid. However, due to the results of a previous experiment in our lab (2013) that demonstrated that significant differences were only seen between in extremely high doses, we opted to streamline the experiment and only investigate four different doses: control (0 mg/L), 0.08 mg/L, 3.2 mg/L, and 125 mg/L. Therefore, only 24 colonies were purchased instead of the originally proposed 40 colonies.
The doses were created by diluting Admire® Pro Systemic Protectant (42.80% average % by weight imidacloprid, Bayer CropScience) with the Koppert Bee Happy Food. Admire® Pro was chosen due to its use in lowbush blueberry fields. There were six colonies per dose, resulting in a total of 18 colonies being dosed with imidacloprid and 6 colonies as controls. Each colony was also be given a pollen ball that was prepared by mixing ground pollen (Betterbee, Inc.) with Koppert Bee Happy Food to form a paste. All feed bags and pollen balls were be weighed before placing them inside the colony. During dosing, both colonies and bags were weighed daily to monitor growth and general health of the colony, as well as the ingestion of the Koppert Bee Happy Food by the colonies.
This period of dosing continued for a period of 13 days, whereupon the colonies were switched to 1 L undosed Koppert Bee Happy Food. The following day, 2 June 2014, colonies were placed at their field sites in groups of six, with each group containing one of each dose as well a control. Please see Figure 1 for field site locations.
The B. impatiens colonies were placed on the edge of each bearing field, sometimes on the non-bearing portion, and at least 10m apart form one another to mitigate the chance of workers migrating between colonies. Location of any honey bee hives in the field were taken into account and the research colonies placed as far away as possible to prevent honey robbing and aggression. Placement in the field coincided with the height of blueberry bloom, allowing the colonies to forage on blueberry blossoms.
Bees were allowed to forage in the fields for several weeks until the end of July. During this time, colonies were left mostly unperturbed except they were checked and weighed once a week to monitor growth. Partnering farmers were always contacted prior to researchers visiting the fields to ensure that no spraying had taken place in the area that would limit entry.
In the original methods, the bees were to be collected again and taken back to Blueberry Hill Farm for a second round of dosing in mid-July using the same methods as before, using the same dosage for the same time (13d) and then placed back into fields around in late July. The intention was to make this second dosing coincide with the rise of N. bombi that has been observed in Maine’s bumble bee populations during the late summer. However, we did not do this second round of dosing due to the deteriorating conditions of the colonies already in the field – males were plentiful and we believed the colonies to be in senescence and therefore decided to allow them the chance to produce reproductives in the field rather than back in the lab. Queen excluders were also included on the Koppert colonies with the intention of making the exit hole too small for newly emerged queens to disperse so that they could be counted at the end of the season.
Colonies were picked up from the fields on 29 July and 30 July 2014 and placed into a 5.5°C cold room overnight. This timing was designed to coincide with the period directly before the wild blueberry harvest. Final colony weights were taken and then colonies were moved to a -20°C freezer to freeze-kill. Final counts of workers, drones, and queens along with estimated of brood area were made in the following weeks. The intertegular widths of all individuals from each colony were measured to estimate average worker size of each colony.
The original intent was to do multiple measures of the colonies in the individuals in each colony. Samples of five workers from each colony were proposed to be collected each week that would be freeze-killed for later processing. We did not do this each week as we were already concerned about the number of workers left in the senescing colonies as it was and did not wish to impact worker numbers and colony reproductive output. Dissections were not performed on Koppert colony bees due to our shifting focus to using levels of conopids and Nosema bombi in wild bees as our proxy for levels in each field.
Laycock, I., K.M. Lenthall, A.T. Barratt, J.E. Cresswell. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology. 21:1937-1945.
Objective 2. The monitoring of conopid fly populations
From 14 July 2014 to 24 September 2014, the six field sites where the colonies were placed were visited approximately every two weeks in the Midcoast region of Maine (Figure 1). Two to three researchers spent either an hour or collected 20 wild bumble bees at each site (whichever came first) as a measure of sample effort. Obvious queens were not captured. Researchers split up at field sites to minimize the possibility that collected bumble bees were all from the same colony. Specimens were marked with the date, field site, and the common name of the flower on which they were collected (if known) then brought back to the lab and placed in a -20º C freezer to freeze-kill.
Each bee was identified to the species level, the intertegular space (ITS) measured, the age estimated on a modified four-point scale based on wing wear, and the abdomens dissected to look for macroparasites.
Objective 3. Establishing an effective rearing method for B. ternarius
20 wild B. ternarius and B. vagans queens were in May 2014 in blueberry fields in Downeast Maine. We had intended to only focus on B. ternarius for rearing, but because queens are not always easy to find, we branched out to include B. vagans in the rearing protocol. Small starter nest boxes were made out of plastic containers that contained a ventilation hole, a cut off portion of a foam Dixie cup (supplemental egg cup), and a piece of foam peanut to simulate a pupa. Liquid Koppert Bee Happy Food was mixed with ground pollen to create pollen balls that were be placed into the wax cup, and liquid feed was also be provided via a small plastic cup with a wick dipped in for easy feeding. Cotton batting was be placed inside for insulation.
Queens were kept at growth chamber conditions (15:9 LD, 20ºC, 55% rH), left undisturbed for 48 h following placement in starter box, and then checked daily for eggs. No queen laid eggs after 21 days and so all queens were released.
Objective 4. Measuring the immunocompetence of imidacloprid-challenged workers
This work has not yet been completed, but I have prepared for this by learning the technique of how to measure PO levels using the frozen thorax of bumble bees. We have also compared the PO levels of different species of bumble bees (Koppert B. impatiens, B. ternarius, and B. vagans) and these data can be used in future comparisons.
Dosed Koppert Colonies
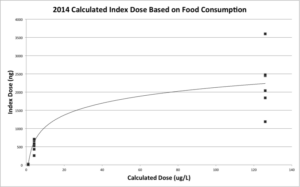
Due to the fact that feeding aversion was seen to occur wherein the colonies given the highest dose of imidacloprid were observed to eat significantly less (Figure 2), it was necessary to calculate an “index dose” of imidacloprid consumed per colony using the amount of food consumed and the dose to get a more accurate representation of the amount of imidacloprid consumed per colony (Figure 3). From this calculation, we replaced the treatment (ppb) with this index dose for each individual colony.

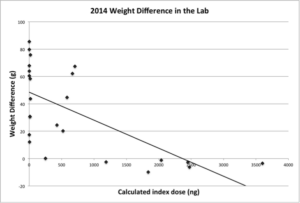
A significant effect of index dose on the weight difference of each colony from the start to the end of the two-week dosing period was observed (Figure 4).
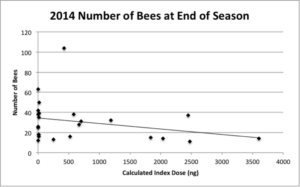
Measurements of the ITS of workers of each colony taken at the end of the season showed no significant effect of either field management or index dose on final worker size. Numbers of bees (drones and queens included) counted at the end of the season in each colony also display a downward linear trend with increasing index dose with a nearly significant effect of index dose on bee number (P = 0.10) (Figure 5).
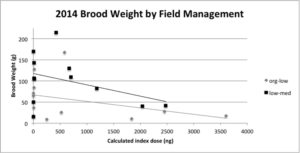
Index dose had a marginally significant effect (P = 0.073) on brood weight taken at the end of the season, but interestingly field management had a significant effect (P = 0.036*; Figure 6) where the low-med input fields produced colonies with more brood mass than the org-low input fields.
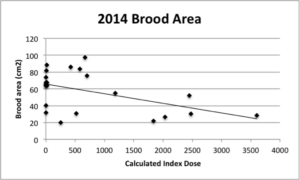
Calculated index dose was also found to have a significant effect on brood area at the end of the season (P = 0.0132; Figure 7).
Wild Caught Bees
In 2014 from 7/14/14 to 9/22/14, 304 wild bumble bees were collected from the six fields and all were identified to species and dissected to look for conopid larvae. 48 (15.8%) of those bees were found to contain a conopid larva. Using the wing wear as a proxy for age on an assigned 0-3 scale, we found that older bees were significantly more likely to contain a conopid larva (Wald’s X2 = 11.51, df = 1, P = 0.0008**) (Figure 8). However, it should be noted that conopid infection may cause a bee to act “clumsily”, which may also result in excessive wing wear. There was no significant relationship between conopid infection and size (ITS) (Wald’s X2 = 0.77, df = 1, P = 0.38).
There was also a difference in wild bumble bee diversity between fields. For instance, only three unique bumble bee species were caught at Bucksport low-organic input field in Region 3, but seven unique species were captured in Stockton Springs 2 low-organic in Region 1. A Shannon’s Diversity Index for each field was performed (Table 1).
|
FIELD |
REGION |
SHANNON’S BUMBLE BEE DIVERSITY |
|
Stockton Springs 1 (low-organic) |
1 |
1.34 |
|
Stockton Springs 2 (low-organic) |
1 |
1.22 |
|
Penobscot 1 (conventional) |
2 |
0.87 |
|
Orland (conventional) |
3 |
1.45 |
|
Bucksport (low-organic) |
3 |
0.98 |
|
Penobscot 2 (conventional) |
2 |
1.14 |
Table 1. Shannon’s Diversity Index of the bumble bee species in each field. The most diverse field was Stockton Springs 1 and the least was Bucksport.
Plant diversity was also calculated at each field by using the recorded plants the bees were foraging on when captured (Table 2).
|
FIELD |
REGION |
SHANNON’S FLOWER DIVERSITY |
|
Stockton Springs 1 (low-organic) |
1 |
2.40 |
|
Stockton Springs 2 (low-organic) |
1 |
1.77 |
|
Penobscot 1 (conventional) |
2 |
1.78 |
|
Orland (conventional) |
3 |
1.70 |
|
Bucksport (low-organic) |
3 |
0.94 |
|
Penobscot 2 (conventional) |
2 |
1.84 |
Table 2. Shannon’s Diversity Index of the flowers in each field. The most diverse field was Stockton Springs 1 and the least was Bucksport.
Four field characteristics were used as predictors of macroparasite (conopid) rates at each field. Bumble bee species richness + diversity and Flower species richness + diversity were both found to be highly correlated (bumble bee: 0.8326, plant: 0.8780), and so only richness was used in analysis.
- Bumble bee species richness (total number of species found at each field)
- Region (1, 2, or 3)
- Field management
- Flower species richness
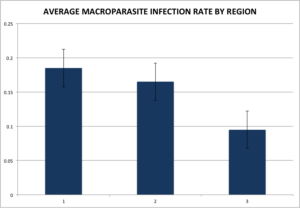
Stepwise linear regression was used to select models to examine relationships between the predictor variables and macroparasite rates. We used a mixed-procedure with the probability to leave and enter at a = 0.250. We found that there was a significant difference between regions, with region 3 having a significantly lower rate of macroparasite infection as compared to regions 1 and 2 (Figure 9).
Cumulative incidence through the 2014 season for each field (Table 3).
|
FIELD |
REGION |
CUMULATIVE INCIDENCE OF CONOPID INFECTION |
|
Stockton Springs 1 (low-organic) |
1 |
16% |
|
Stockton Springs 2 (low-organic) |
1 |
21% |
|
Penobscot 1 (conventional) |
2 |
13% |
|
Orland (conventional) |
3 |
11% |
|
Bucksport (low-organic) |
3 |
8% |
|
Penobscot 2 (conventional) |
2 |
20% |
Wild Caught Data + Dosed Koppert Colonies
I used the data from the wild caught bees for field variables and then used these in analyses to ascertain if any of these variables might have had effects on the Koppert boxes. Here, we look for significance of field effects on the health measurements of the Koppert boxes that go beyond the possible effects of the dietary imidacloprid.
AVERAGE WORKER SIZE:
Conopid incidence in each field and field region were found to be significant effects on average worker size in each of the Koppert boxes at the end of the season.
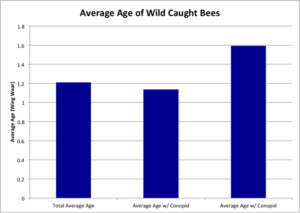
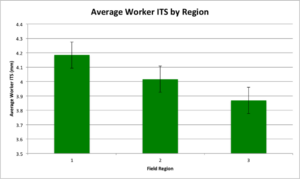
Conopid incidence: F(1, 20) = 8.1608; P = 0.0098**, the higher the conopid incidence at the end of the season, the larger the bees (Figure 10).
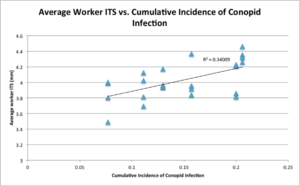
Field Region {3&2 -1}: F(1, 20) = 4.9518; P = 0.0377*, region 1 had a higher average worker ITS than regions 3 & 2 (Figure 11).
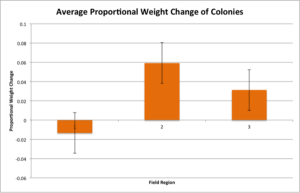
AVERAGE PROPORTIONAL WEIGHT CHANGE OF THE COLONIES FROM END OF LAB TO END OF FIELD:
Here, there was only a possible correlation with Field region{1 vs. 3&2} (Figure 12). Colonies placed in region 1 lost significantly more weight while out in the field as compared to regions 2 and 3 where the colonies gained weight.
Discussion
The result that the Koppert bees that were fed the higher doses of imidacloprid consumed less could be feeding aversion, the result of imidacloprid toxicity (Laycock et al. 2012), or a combination of the two. If it is the former, then it may be that bees may be able to “sense” neonicotinoids in their food and therefore avoid them when given the opportunity to forage on other resources (Godfray et al. 2014). However, the opposite has been found in a recent study that demonstrated that the European Bombus species B. terrestris preferred food solutions containing imidacloprid, even though they ended up consuming less food overall (Kessler et al. 2015).
Although unsurprising that the higher dosed colonies gained less weight through the dosing period, it was interesting to see that they were unable to fully recover once out in the field and the dosing stopped. Although in the field sites for eight weeks, the higher dosed colonies contained fewer bees at the end of the season and weighed significantly less than lower doses and created smaller brood clumps. In a previous study using microcolonies, B. terrestris worker fecundity was observed to decline with increasing imidacloprid dosage (Laycock et al. 2012).
As for why fields that were low-medium/conventional input had higher brood mass than organic fields, this could be more of an individual field effect or regional effect than management. Two of the three organic fields were in Stockton Springs, ME and in close proximity (~3 km as the crow flies), meaning that a local weather pattern might have had a significant effect on the growth of those colonies.
The total cumulative conopid prevalence of 15.8% at all six sites is within the range found in other in previous regions: 0.02% in western Oregon (Maxfield-Taylor 2014); 60% and 22% in western Massachusetts (Gillespie 2010); 20%-30% in Europe (Schmid-Hempel & Schmid-Hempel 1988; Schmid-Hempel et al. 1990; Shykoff & Schmid-Hempel 1991); and 25% in Virginia (Malfi & Roulston 2013). However, it is important to note in such studies that timing of collections is important. Although 15.8% was the cumulative prevalence through the season, we found levels as low as 0.05% in late September and as high as 27.3% in mid-August. We also found that bees that scored higher on the wing wear point system (a proxy for age) were significantly more likely to contain a conopid larva. This may be due to several factors, although it is thought that the presence of a conopid larvae stresses the host and can alter foraging behavior (Schmid-Hempel & Schmid-Hempel 1990). This stress could lead to additional wing wear.
Although there did seem to be a significant relationship between conopid prevalence and the number of bees in the commercial colonies remaining at the end of July, it is important to note that the period of relatively highwe abundance of conopids is after the time when the dosed Koppert colonies were at the field sites. Therefore, it is unlikely that conopid prevalence had a direct effect on the number of bees in the commercial colonies.
Gillespie, S. 2010. Factors affecting parasite prevalence among wild bumblebees. Ecological Entomology 35:737–747.
Godfray, H. C. J., T. Blacquière, L. M. Field, R. S. Hails, G. Petrokofsky, S. G. Potts, N. E. Raine, a. J. Vanbergen, and a. R. McLean. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proceedings of the Royal Society B: Biological Sciences 281:20140558.
Kessler, S. C., E. J. Tiedeken, K. L. Simcock, S. Derveau, J. Mitchell, S. Softley, J. C. Stout, and G. a. Wright. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature. Available from http://www.nature.com/doifinder/10.1038/nature14414
Laycock, I., K. M. Lenthall, A. T. Barratt, and J. E. Cresswell. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21:1937–45.
Malfi, R. L., and T. H. Roulston. 2013. Patterns of parasite infection in bumble bees ( Bombus spp.) of Northern Virginia. Ecological Entomology:n/a–n/a. Available from http://doi.wiley.com/10.1111/een.12069
Maxfield-Taylor, S. A. 2014. Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon. Oregon State University.
Schmid-Hempel, P., C. Muller, R. Schmid-Hempel, and J. A. Shykoff. 1990. Frequency and ecological correlates of parasitism by conopid flies (Conopidae, Diptera) in populations of bumblebees. Insectes Sociaux 37:14–30.
Schmid-Hempel, P., and R. Schmid-Hempel. 1988. Parasitic flies (Conopidae, Diptera) may be important stress factors for the ergonomics of their bumblebee hosts. Ecological Entomology 13:469–472.
Schmid-Hempel, P., and R. Schmid-Hempel. 1990. Endoparasitic Larvae of Conopid Flies Alter Pollination Behavior of Bumblebees. Naturwissenschaften 77:450–452.
Shykoff, J. A., and P. Schmid-Hempel. 1991. Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 22:117–125.
I expect that this project will be a unique contribution to the study of the sublethal effects of neonicotonoids on bumble bees. Currently, similar work has been done but in a lab setting only and using the European managed bumble bee, B. terrestris. Therefore, this work will be the first using North American bee species that not only follows the colonies through the lab-dosing period, but also their progress throughout the season when the dosing period ends and the colonies are placed out in field sites. This will be key in determining susceptibility of exposed bees to parasites in pathogens in a natural setting.
It is expected that these findings will generate a publication or publications in a peer-reviewed journal for the focus on North American bumble bees. Although predicting possible effects on agricultural management practices is difficult, the results of this research will be informative in determining sublethal effects of imidacloprid on bumble bees, which will be useful in the future for making suggestions for best growing strategies in the lowbush blueberry system. Beyond this, the results will help shape future investigations into the effects of neonicotinoids on native bees and potentially lend clues as to why some bumble bee species are becoming more prevalent while others are in decline.
In addition, my cooperation with blueberry growers in Maine will enable me to not only relate my research findings to them directly, but also through an annual presentation at the Maine Wild Blueberry Field Day at the University of Maine’s research farm in Jonesboro, ME and an annual report submitted to the University on research in blueberry fields. As I am also on Specialty Crop Research Initiative (SCRI) grant, I will continue to work toward the goals outlined there in which I contribute to a “Pollinator Toolbox” to aid stakeholder growers in assessing pollinator efficacy and monitor the native bee populations in their fields.
An additional component to my dissertation is citizen science and science communication, specifically through working with the Maine Department of Inland Fisheries and Wildlife on the Maine Bumble Bee Atlas (MBBA). The MBBA is a five-year project using a cadre of citizen scientists to gather data on Maine’s bumble bee species, including information on relative abundance and distribution. The project is currently in its second year and data from this project has been used in presentations given to MBBA volunteers during training workshops. Additionally, I am occasionally invited to give presentations to the general public and organizations and data from this project is often included in these talks. Please see the Publications and Outreach section for more information.
Electronic access to information about the SCRI pollinator security project will be available to stakeholders.
Education & Outreach Activities and Participation Summary
Participation Summary:
Web Content
Information about the Pollination Security for Fruit and Vegetable Crops in the Northeast can be found here: http://articles.extension.org/pages/70119/pollination-security-for-fruit-and-vegetable-crops-in-the-northeast
My personal biography on the website: http://articles.extension.org/pages/70104/kalyn-bickerman-the-university-of-maine#.Uo4q2qzAEcI
A video about research topics in lowbush blueberry can be found at the below link. My research is highlighted beginning at 6:50:
https://www.youtube.com/watch?v=hj3wt3MxN5k
Oral Presentations
Bickerman-Martens, K. F. Drummond, and B. Swartz. The Status of Native Bumblebees in Maine. Maine Bumble Bee Atlas Volunteer Training Workshop. Orono, ME, June 11, 2016.
Bickerman-Martens, K. F. Drummond, and B. Swartz. The Status of Native Bumblebees in Maine. Maine Bumble Bee Atlas Volunteer Training Workshop. Gorham, ME, May 7, 2016.
Bickerman-Martens, K., F. Drummond, and B. Swartz. The Status of Native Bumblebees in Maine. Maine Bumble Bee Atlas Volunteer Training Workshop. Gorham, ME, July 18, 2015.
*The Maine Bumble Bee Atlas workshops are capped at 40 people and consist of an approximately 7 hour day during which we go over bumble bee biology, sample and data collection methods, and answer any questions. These sessions include a catered lunch for the workshop volunteers and everyone is given collection equipment (net, cooler, vials, data forms) with the possibility that volunteers can contribute to the project multiple years and not only the year of their attended workshop. Two workshops are held each year. 2015 was the first year and volunteers submitted over 4000 combined image and specimen vouchers.
Bickerman-Martens, K.. Birds, sea turtles, and bumblebees – the life of a biologist. Summer Camp at the Challenger Learning Center of Maine. Bangor, ME, July 16, 2015.
*This presentation was at the request of the camp coordinator of the Summer Camp at the Challenger Learning Center of Maine, Kirsten Hibbard. The Challenger Learning Center of Maine is non-profit organization that offers simulated space science missions for classrooms, and programs throughout the year that focus on the promotion of STEM learning. My presentation to a small group of 4 children and a chaperone was included in their animal science unit.
Bickerman, K.E. and F. Drummond. The Status of Native Bumblebees in Maine. Maine Bumble Bee Atlas Volunteer Training Workshop. Orono, ME, May 16, 2015.
Bickerman, K.E. and F. Drummond. The Status of Native Bumblebees in Maine’s Wild Blueberry Fields. Androscoggin Beekeepers Club Meeting. Lewiston, ME, May 13, 2015.
*The Androscoggin Beekeepers Club meets once a month and is for beekeepers in Maine’s Androscoggin County. There were around 30 or so members in the room during my hour long talk. Meeting announcement can be found here: http://androscogginbeekeepers.org/may-2015-abc-meeting-announcement/
Bickerman, K.E. and F. Drummond. Bumblebee Health and Conservation in Maine’s Lowbush Blueberry Fields and Differential Insecticide Susceptibility of Maine’s Native Bumblebee Species. Thesis Proposal Defense. Orono, ME, May 1, 2015.
*The University of Maine Graduate School requires that all graduate students host an hour-long proposal defense (including questions) to set the course of their dissertation. In attendance were all five of my committee members (two of whom watched remotely through the internet), labmates, and faculty in the Ecology and Environmental Sciences, Wildlife Ecology, and the School of Biology and Ecology departments. Approximately 20 people were in attendance.
Bickerman, K.E.. The Health of Maine’s Native Bees. Maine Arborist Association Spring Workshop. Portland, ME, April 8, 2015.
*The Maine Arborist Association holds a yearly meeting during which attendees can gain Pesticide Applicator credits and International Society of Arboriculture credits. Approximately 40 certified Maine arborists were in attendance.
Bickerman, K.E. and F. Drummond. Individual and colony effects of dietary imidacloprid on managed Bombus impatiens in Maine’s lowbush blueberry fields. The Entomological Society of America Annual Meeting. Portland, OR, November 17, 2014.
*This talk was presented in a ten-minute paper competition at the Entomological Society of America’s annual meeting in the Physiology, Biochemistry, and Toxicology section.
Poster Presentations
Bickerman, K.E. and F. Drummond. Bumblebee Health in Maine’s Lowbush Blueberry Fields. Wild Blueberry Spring Grower Conference. Bangor, ME, March 20, 2015. Poster presentation. PDF here: 3.17Bickerman_2015BlueberrySchool_Poster
*The Spring Growers’ Conference draws wild blueberry growers from Maine and maritime Canada and includes presentations by graduate students, researchers, and industry representatives.
Publications
Bickerman, K.E. and F. Drummond. 2014. The health of bumble bees in blueberry fields in Downeast Maine. In: Insect Pest Management Experiments on Wild Blueberry in Maine. J. Collins and F. Drummond, eds. School of Biology and Ecology, Cooperative Extension, University of Maine. Maine Blueberry Commission Report.
Project Outcomes
I do not believe that this section is applicable to my work.
Farmer Adoption
This project only worked directly with blueberry growers to receive permission to use their blueberry fields for the experiment. The colonies within the electric fencing were either set up on the outskirts of the fields or on a nonbearing portion of the field to be out of the way during day-to-day duties. The results of this experiment should not impact the Maine Organic Farmers’ and Gardeners’ Association (MOFGA) certified growers as they do not use field treatments with neonicotinoids. I do not imagine the results drastically changing the spraying routines of conventional growers imidacloprid is primarily used in two forms: Admire Pro® and Montana. These formulations are used to combat blueberry maggot fly, spotted wing drosophila, tip midge and thrips. The University of Maine’s Cooperative Extension, in its 2016 Maine Wild Blueberry Pesticide Chart (https://extension.umaine.edu/blueberries/media/pdf/2/0/1/2016-Maine-Wild-Blueberry-Pesticide-Chart-Insecticides.pdf) classifies neonicotinoids as Group 4A and recommends that they are not to be used prior to and during bloom.
All of the growers I worked with for this project had a previous relationship working with UMaine researchers and were therefore very open and responsive when asked about any spraying (including insecticides, herbicides, and fungicides) taking place in the field that would limit our access. I was even alerted on occasion by a grower if a deer had run through my electric fencing and repair was necessary or if they thought a bear or raccoon was in the vicinity and may possibly be a threat.
Because most neonicotinoid toxicity research has been done with honey bees to date, studies such as this one using bumble bees will be a valuable tool when reassessing application recommendations. In the wild blueberry system, many growers rely not only on rented honey bee hives for pollination for also supplementation by either commercial Bombus colonies and wild queens.
Areas needing additional study
Effects on native queens and nest establishment
Although wild blueberry growers do use commercial colonies of Bombus impatiens for pollination from mid-May through mid-June, it is important to consider that all of the native Bombus that would be pollinating lowbush blueberry would be queens. Bombus queens in Maine emerge in mid- to late April and can be observed to be foraging well into late June in some areas of the state. Workers are generally not seen in substantial numbers until mid-June. Therefore, future work into the effects of neonicotinoid exposure on bumble bees foraging in wild blueberry should focus on native species of queens and ideally would not only look at direct effects, but also successful colony establishment rates as well as the progress of those colonies through the season. From this experiment I was able to observe that queens within the higher dosed colonies seemed to be more physically affected (decreasing movements, death) than workers, even though they were much larger. Whether this is due to increased feeding because of the energy demands of reproduction or physiological differences in their detoxification systems are potential areas to study.
Native species compared to Koppert Bombus impatiens
Although Bombus impatiens is a native Maine species, Koppert Biological Systems creates the commercial colonies it sells using a wild caught population of B. impatiens that is not from the Northeast. Also, these colonies are artificial colonies, meaning that a laying queen is paired with a group of workers that are not her biological daughters. Therefore, in order to fully investigate the effects of imidacloprid on Maine’s native bees, a future research direction would be to expose native colonies to dietary imidacloprid such as was done with B. impatiens in this study. In order to do this in the laboratory setting, an effective rearing protocol must first be established. Although this area is not beyond the scope of this SARE project and rearing attempts were unsuccessful, it can be considered an important next step to investigate further.