Final Report for GNE14-089
Project Information
Environmental and health risks associated with the products from Mixed Crop-Livestock Farming (MCLF) systems due to cross contamination with zoonotic bacterial pathogens are major safety concerns. Current intervention strategies such as, administration of antibiotics for livestock production are of limited use due to the emergence of resistant pathogens and/or presence of antibiotic residues in foods. Further, organic MCLFs are not allowed to use antibiotics or chemicals. As a result, alternative approaches are critical to address the current issue. Natural bioactive compounds from plant origin can play crucial role in such a situation. Berry fruits are well documented as sources of natural antimicrobials which primarily consist of polyphenols and their bioactive derivatives. These antimicrobial components are present in berry byproduct called “pomace”, which are primarily seeds and skins of fruits after juice is squeezed out. These components show promising antimicrobial activity against various zoonotic pathogens especially Campylobacter and Salmonella. In this project, we used bioactive extracts from berry pomaces as an alternative intervention to reduce Campylobacter and Salmonella colonization in poultry gut. These bioactive extracts showed promise to replace antibiotic growth promoters by increasing weight gain in chickens without altering blood parameters or modulating the expression of inflammation mediating genes. Microbiological quality of the compost was unaltered. Findings from this study suggest potential applicability of bioactive extracts from berry byproducts to reduce pathogen contamination and livestock productivity.
Introduction:
The proximity of livestock to crops in MCLF is regarded as one of the major threats to the microbiological safety of its products. The gut microbiota of farm animals, especially poultry, contain numerous pathogenic bacteria that include Campylobacter and Salmonella as natural reservoirs which remain viable in the compost and ultimately contaminates farm environment and crops. Together these two pathogens account for a significant portion of foodborne bacterial infections in the U.S. In addition to food poisoning, these bacterial pathogens have been associated with the development of myocarditis, endocarditis, pericarditis, acute atrial fibrillation and Guillain-Barré syndrome as post infection sequelae to certain percentage of patients. Antibiotic is the major tool available for the farmers to make their products safer by reducing pre-harvest colonization of these bacterial pathogens in animal gut. Antibiotics are also being used for growth promotion of farm animals. But high prevalence of antibiotic resistant bacteria in the environment and antibiotic residues in the food products have been reported and excessive use of antibiotics in farm animal production is one of the prominent reasons behind this phenomenon. In response to increased public health concern about antibiotic resistance, the U.S. Food and Drug Administration (FDA) have announced that they will gradually withdraw non-therapeutic use of antibiotics from farm animal production (Kuehn, 2014). This might mitigate the emergence of antibiotic resistance in pathogenic bacteria but bacterial colonization and/or cross contamination of products with zoonotic bacterial pathogens will be out of control. Moreover in the U.S., specifically in the Mid-Atlantic and Corn Belt regions, a large number of farming practices are organic MCLF which are not allowed to use antibiotics or chemicals as growth promotants. These factors warrant the need for alternative antimicrobials and growth promoters to make the products of MCLF safer as well as profitable for the farmers.
Bioactive components from berry fruit by-products as natural antimicrobial may offer unique advantages to the farmers. Berries, such as, blackberry or blueberry are significant sources of polyphenols, phytosterols, tocopherols (mostly α-tocopherol), fiber and biotin (Puupponen-Pimiä et al., 2005). Many of these compounds exhibit a wide range of biological effects, such as antimicrobial, antioxidant, anti-inflammatory and vasodilatory activities. As berry fruit juices are expensive, bioactive extracts possessing these compounds can be extracted from berry fruit byproducts. The fruit processing industry in the U.S. generates significant amount of byproducts called “pomace” which account for 20-30% of the weight of processed fruit. Fruit that is not sold fresh or processed immediately is generally frozen to provide a constant, year-round supply for juice production which makes pomace to also be available year-round. Uses of pomaces are limited, and disposal is problematic due to acidic pH and limited use as animal feed due to low protein content. Drying, storage, and transportation of pomaces are major economic concerns to the industries. Therefore, fruit processing industries are becoming increasingly interested in exploring economically advantageous ways to utilize and recycle their by-products.
In this study we show a novel approach to use bioactive extracts from berry pomaces as an alternative to antibiotics as well as chemical growth promoters. We believe berry pomace extracts will reduce the colonization of pathogenic bacteria, such as Campylobacter jejuni and Salmonella Typhimurium in animal gut and as a result the compost made from their manure will have reduced contamination with these pathogens. Consequently, cross contamination of crops with pathogenic bacteria will be reduced especially in the organic MCLF where compost from the manure serve as sole source of fertilizer. Moreover, bioactive berry extracts will work as organic growth promoters just like antibiotic growth promoters used in farm animals. We believe the use of organic and natural antimicrobials, instead of chemicals and antibiotics, will improve the biosecurity of products in the MCLF as well as reduce the environmental and health risks in agriculture. It will reduce the costs related to the administration of antibiotics and chemical growth promoters. In addition, natural or “green” antimicrobials will improve consumer satisfaction.
In this study, we proposed to:
- Assess the effects of berry pomace extracts (BPE) on the colonization of Campylobacter and Salmonella in poultry gut;
- Evaluate the effects of bioactive components of BPE on the poultry productivity, feed conversion efficiency and overall health;
- Compare the microbiological quality of the composts produced from the manure of birds grown in presence or absence of BPE.
Cooperators
Research
Preparation of pomace extracts:
Extraction solution was prepared in water (pH 7.7). One hundred gram blackberry or blueberry pomace was suspended in 2 L previously prepared extraction solution and incubated for 18-24 h at various temperatures. After the appointed time point, the solid portion was separated using centrifugation at 2000 g for 20 min and the supernatant was sterilized with 0.45 µm filters (AcroVac Filter Unit, Pall Life Sciences, NY). The extracted solution was dried at 40˚C in oven with continuous airflow and dried powder was collected. Dried pomace extract was stored at 4˚C and resuspended in deionized water whenever necessary. Total phenolic content in each extract was determined using spectrophotometric method (Singleton et al., 1999). Total phenolic content was expressed as Gallic Acid Equivalent (GAE). The pH of the crude extracts were 4.5-5 and pH varied depending on the treatment concentration.
Pomace extract as water supplement in chicks:
Natural colonization of Campylobacter and Salmonella in chick model provided with various concentrations of BPE was determined. One hundred 1-day-old Cobb-500 broiler chicks were obtained from Longenecker’s Hatchery Inc, PA. They were assigned into four groups of 25 chicks each in floor pans, using a Completely Randomized Design. Chicks were provided with commercially available crumbles (Purina Animal Nutrition, MO) with no antibiotic supplementation and this way the chicks were raised for seven weeks. After three weeks, five chicks from each group were euthanized to check the natural colonization level of Campylobacter and Salmonella in chick cecum. After seven weeks, all the birds were euthanized and ceca were separated. To check the Campylobacter and Salmonella colonization, approximately 200 g of cecum content was homogenized in one mL PBS, serially diluted and plated on Karmali Campylobacter agar and Xylose Lysine Deoxycholate (XLD) agar for enumeration. Three representative presumptive isolates from each group were tested with Campylobacter and Salmonella specific PCR. Quantitative Real Time PCR (qPCR) was also used to enumerate the number of Campylobacter and Salmonella in the cecum samples. In brief, about 2 gm fecal sample was suspended in 5 mL PBS, vortexed vigorously to dislodge attached bacteria to the solid particles. It was centrifuged at low speed (500 × g) for 5 min to precipitate solid particles. The supernatant (1 mL) was used to isolate DNA with QIAmp DNA Stool Mini Kit (Qiagen, CA) according to manufacturer’s instructions. This DNA was used to detect and quantify Campylobacter and Salmonella with the specific primers using PerfeCTa SYBR Green FastMix (Quanta, MD). All the amplifications were performed in an EcoTM Real Time PCR system (Illumina, CA). The amplification profile consisted of 50 cycles: 30 s at 95ºC; 30s at 62ºC; and finally 30 s at 72ºC. Serial dilutions of an overnight culture of C. jejuni RM1221 (ATCC BAA-1062) and S. Typhimurium (ATCC 19585), containing approximately log 8 CFU/mL was prepared in PBS and 1 mL of each dilution will be subjected to DNA extraction. Calibration curves were constructed plotting the threshold cycle (Ct) by the DNA against the log CFU/mL. Correlations coefficients (R2) and amplification efficiency (AE) will be calculated.These two quantification methods were compared to each other for consistency. Cecum from each bird was considered an experimental unit for statistical analysis. The number of birds colonized by Campylobacter and Salmonella was compared using Fisher’s exact test. Differences in the level of colonization (CFUs/g cecum content) were compared by first ranking the data and performing one-way analysis of variance (ANOVA) on the ranked data. Comparison of mean ranks was performed using Tukey’s test.
FCR calculation:
Total feed intake: (original weight of feed) - [(feed left at specified time points) - (weight of empty container)]
Average feed intake per chick: (total feed consumed)/ (number of chicks)
Average chick weight gain (g): (average weight at specified time points) – (average weight at birth)
Average feed conversion ratio for a group: (average feed intake per chick)/ (average weight gain)
At the end of experiments, 5 birds from each group was randomly selected and sacrificed to examine the internal organs, such as liver, spleen, breast meat, abdominal fat and gizzard.
Composting:
Composting procedure was be carried out at the ANSC departmental farmland. Chick manure and wooden chips which was used as bedding, was be collected from each group of birds and compiled into 2 ft3 container separately. Since nitrogen content in poultry manure is high, a ratio of 2:1 for bedding material and manure was maintained. When the correct ratio of bedding to manure was compiled, moisture was added on the top with sprayer and the compost piles were maintained at 130-150°F. Temperature was checked with a thermometer. After maintaining this temperature of the core for 3 days, center portion was pulled apart and moved to the edges, whereas the edge material was put into the center to heat up. This procedure was repeated 3 times. After 40 days, composts were ready to check the microbiological quality. The procedure mentioned at section 3.3. was followed to check the number of pathogenic bacteria present in the composts made from the manure of the different groups using culture and qPCR.
Events and activities of your project over that past year in sequence:
- March 12 to April 30, 2015: Trial 1 with 80 chicks.
- June 1 to July 13, 2015: Trial 2 with 80 chicks.
- July 27, 2015: Presented data from Trial 1 to IAFP 2015.
- October 6 to November 17, 2015: Trial 3 with 80 chicks.
- November 18, 2015 to December 31, 2015: Sample processing and data analysis.
- January 1, 2016 to July 31, 2016: Manuscript preparation and submission to International Journal of Food Microbiology; answering to the review comments; manuscript accepted for publication.
In Trial 1, we used 80 chicks, which were divided into 4 groups. Group 1, provided with only tap water; Group 2, provided with 0.1 mg Gallic Acid Equivalent (GAE)/mL BPE in water; Group 3, provided with 0.5 mg GAE/mL BPE in water; and Group 4, provided with 1.0 mg GAE/mL BPE in water. Each week, fecal samples were collected to check bacterial shedding and weights were measured to check growth rate. After 7 weeks, all the chicks were euthanized, cecum were collected to check the colonization of Campylobacter and Salmonella.
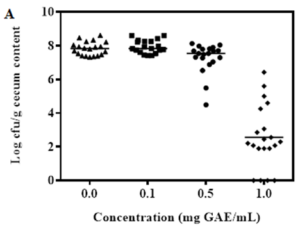
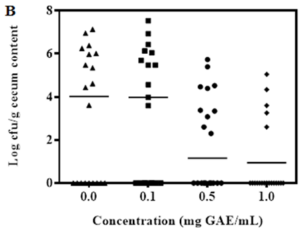
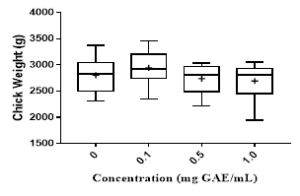
Figure 1: Chicks provided with various concentrations (mg GAE/mL) of BPE. Colonization of Campylobacter (A) and Salmonella (B) per gram of chick cecum content. Growth performance of chick (C) when provided with various concentrations of BPE as water supplement.
Results indicated that 0.5 and 1.0 mg GAE/mL of BPE significantly reduced the colonization of two major pathogens, Campylobacter and Salmonella in chick cecum (Figure 1A, 1B). However, these two concentrations (0.5 and 1.0 mg GAE/mL) of BPE reduced the median chick weight compared to the control (Figure 1C). Reduction in chick weight will result in economic loss, hence may not be practical to be used in poultry industry. Interestingly, 0.1 mg GAE/mL BPE increased the median weight of the chicks compared to the chicks provided with only water but this concentration did not show any effect on the colonization of bacterial pathogens. As our target is two-fold, “reduction of environmental risks and improving livestock productivity,” we adopted a novel approach to use the BPE in our following trials. In this approach, the control group was provided with only water while the test group was provided with water supplemented with 0.1 mg GAE/mL BPE; and we increased this treatment concentration to 1.0 mg GAE/mL BPE in water for the last 72 h before euthanization.
Based on our data from the 1st trial, we divided 80 chicks into 4 groups: group A, tap water for 6 weeks; group B, tap water with commercial antibiotic growth promoter; group C, tap water with 0.1 mg GAE/mL BPE; and group D, like group C except 1.0 mg GAE/mL BPE during last 72 h before euthanize. Results indicated that addition of 1.0 mg GAE/mL of BPE for the last 72 h significantly reduced the colonization of two major pathogens, Campylobacter and Salmonella in chick cecum (Figure 2A, 2B). Colonization of these two pathogens remained unaltered in the presence of 0.1 mg GAE/mL of BPE. However, addition of 0.1 mg GAE/mL BPE increased the chicken weight by >6% compared to the group provided with only tap water (Figure 2C).
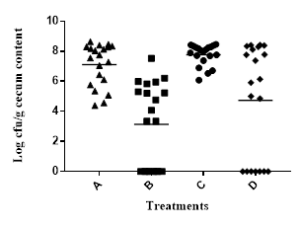
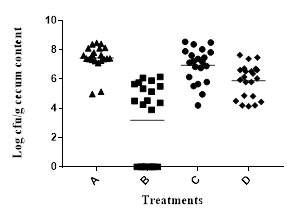
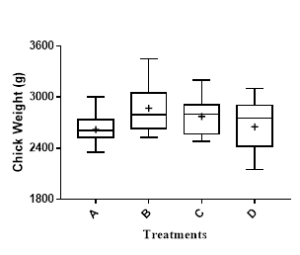
Figure 2: Trial 2&3, Colonization of Campylobacter (A) and Salmonella (B) per gram of chick cecum content. Growth performance of chick (C) when provided with various concentrations of BPE as water supplement.
We calculated the feed conversion efficiency (FCE) values. Lower FCE values were observed in the bird group with 0.1 mg GAE/mL BPE supplementation compared to the control group (Table 1).
Table 1: Effect of BPE on feed conversion efficiency of chickens
|
Age (d) |
Treatments |
|||
|
0.00 |
0.10 |
0.50 |
1.00 |
|
|
1 |
- |
- |
- |
- |
|
7 |
1.40 |
1.40 |
1.29 |
1.36 |
|
14 |
1.27 |
1.27 |
1.22 |
1.26 |
|
21 |
1.33 |
1.31 |
1.35 |
1.41 |
|
28 |
1.67 |
1.61 |
1.69 |
1.74 |
|
35 |
1.84 |
1.68 |
1.78 |
2.02 |
|
42 |
1.90 |
1.81 |
1.95 |
1.98 |
|
49 |
1.97 |
1.89 |
2.01 |
2.08 |
*Amount of feed consumed (g) per gram of weight gain in chicken
We observed, depending on the concentrations of BPE, percent spleen weight and cecum lengths in chickens increased significantly (Table 2). The other tested organs remained unaltered due to the use of BPE as water supplement in chicken.
Table 2: Effect of BPE on various chicken organs
|
Organs |
Treatments |
||||
|
0 |
0.1 |
0.5 |
1 |
P-value |
|
|
Spleen* |
0.118a |
0.129ab |
0.146b |
0.149b |
0.012 |
|
Liver* |
1.982a |
1.929a |
2.119a |
1.913a |
0.134 |
|
Heart* |
0.564a |
0.572a |
0.615a |
0.598a |
0.152 |
|
Pancrease+Duodenal Loop* |
0.709a |
0.698a |
0.764a |
0.723a |
0.139 |
|
Cecum** |
10.585a |
12.626ab |
14.405bc |
15.8c |
<0.0001 |
Values not sharing same letters within the same row are significantly different.
*Percentage of live body weight
** Length in centimeter of one cecum
We determined the relative expression of important genes involved in the modulation of immune system in chickens. Determination of gene expression was carried out with qRT-PCR technique. Data suggests, BPE provided as water supplement did not alter the expression of immune modulatory genes in chickens (Figure 3).

Figure 3: Expression of immune modulatory genes in chicken spleen at various concentrations of BPE.
Several blood parameters also serve as major indicators of inflammation in animals. We tested the deviations in blood parameters in chicken provided with BPE. No significant variation was observed in any of the blood parameters tested in this study (Table 3).
Table 3: Effect of BPE on blood composition of chickens
|
Blood parameters |
Treatments |
P |
|||
|
0.00 |
0.10 |
0.50 |
1.00 |
||
|
RBC (×106/uL) |
2.506±0.19 |
2.76±0.23 |
2.79±0.10 |
2.74±0.14 |
0.07 |
|
WBC (×103/uL) |
551.52±212.57 |
644.19±159.17 |
565.72±100.25 |
774.56±87.32 |
0.11 |
|
Neutrophil (×103/uL) |
532.42±206.92 |
617.77±150.52 |
544.66±93.69 |
744.37±71.31 |
0.11 |
|
Lumphocyte (×103/uL) |
13.52±2.64 |
17.50±6.74 |
15.41±6.09 |
17.78±5.37 |
0.58 |
|
Monocyte (×103/uL) |
0.37±0.36 |
0.46±0.31 |
0.65±0.43 |
0.77±1.48 |
0.85 |
|
Eosinophil (×103/uL) |
2.49±3.17 |
5.79±5.79 |
3.72±5.30 |
8.64±13.46 |
0.64 |
|
Basophil (×103/uL) |
2.71±1.66 |
2.66±1.48 |
1.27±0.49 |
2.98±0.96 |
0.16 |
|
Hemoglobin (g/100mL) |
8.24±0.50 |
8.8±0.64 |
9.1±0.43 |
8.78±0.25 |
0.07 |
|
Hematocrit (%) |
29.38±2.05 |
30.76±1.66 |
31.44±1.52 |
30.74±0.88 |
0.26 |
ProCyte DX Hematology Analyzer
Current results from this project show the effectiveness of bioactive phenolic extracts from berry byproducts to reduce pathogenic bacterial colonization in chick gut while promoting the growth in chicks. This indicates, use of this extract as water supplement will result in reduced cross contamination of crops with pathogenic bacteria especially in the organic MCLF where animal manure serve as sole source of fertilizer. Moreover, bioactive berry extracts will work as organic growth promoters just like antibiotic growth promoters used in farm animals. We believe the use of organic and natural antimicrobials, instead of chemicals and antibiotics, will improve the biosecurity of products in the MCLF as well as reduce the environmental and health risks in agriculture. It will reduce the costs related to the administration of antibiotics and chemical growth promoters. In addition, natural or “green” antimicrobials will improve consumer satisfaction.
Education & Outreach Activities and Participation Summary
Participation Summary:
Printed poster: Outcomes from this study have been presented at the International Association for Food Protection conference 2015.
S. Salaheen IAFP 2015 Cj poster
Peer reviewed manuscript: A manuscript containing outcomes of this study has been accepted for publication at International Journal of Food Microbiology on 19th August, 2016.
For both of the cases, Northeast SARE graduate student grant GNE14-089 was acknowledged.
Project Outcomes
Natural bioactive extracts from blackberry and blueberry fruit by-products can play crucial role in reducing pathogenic bacterial colonization in animal gut and as alternative to antibiotic growth promoters. The fruit processing industry in the U.S. generates significant amount of byproducts called “pomace” which account for 20-30% of the weight of processed fruit. This huge amount of pomace can be used as raw material for preparation of BPE which will provide the fruit juice industry an additional source of revenue. Growth promotion of chicken with BPE will provide organic poultry growers option to increase their profit.
Farmer Adoption
We have presented our work at the International Association for Food Protection, a well-known meeting participated by farmers, food distributers, vendors, researchers, students and academicians. Our work was highly appreciated by the participants. A manuscript has recently been accepted for publication, which can be a major source of information to the farmers regarding the findings from this study.
Areas needing additional study
The mechanism behind the growth promotion of chickens with BPE is an important area to investigate into. We hypothesize, alteration of gut microbiota in chicken is responsible for the growth promotion and reduction of pathogenic bacteria from chicken gut. Metagenomic shotgun sequencing analysis of the cecum contents will provide significant insights into the ecology and dynamics in chickens provided with BPE as water supplement.