Final report for GNE15-100
Project Information
Honey bees are a critical part of most agroecosystems as they provide a vast majority of the managed pollination services, increasing the yield and quality of our nutritionally and economically valuable crops. Sustainability of this pollinator species is thus, a key component of overall sustainability in agriculture. Especially in temperate areas, winter is the time of the year where most of the honey bee colony loss occurs. Beekeepers try to make up for the winter loss via helping their surviving colonies flourish in the spring and make splits, bringing their numbers back to where it was the previous summer. However, in the last decade, an average of 30% of the colonies are lost every winter, which is beyond what the beekeepers report as sustainable. Most beekeepers in the north, try to replace their loss via ordering package bees and queens from southern breeders which is not only a financial burden for the beekeeping operations but also increases genetic admixture between the southern and northern bred honey bees, decreasing the likelihood of local adaptations being selected for the northern, temperate climatic conditions.
Despite the importance of winter preparedness in honey bee colonies, little is known about how honey bees are primed to transition from the summer colony where individuals live for a relatively short time and carry out a variety of tasks inside and outside the hives to the winter colony where physiologically distinct winter bees live up to 4 times longer and mainly focus on thermoregulation via forming a cluster. The lack of a mechanistic explanation for the cues and signals that may be involved in this seasonal transition leaves the beekeepers with no control over the
timing and quantity of winter bees produced. We have developed a model describing how environmental and
social cues (pheromones) may interact to drive winter bee production (see Figure 1 below).
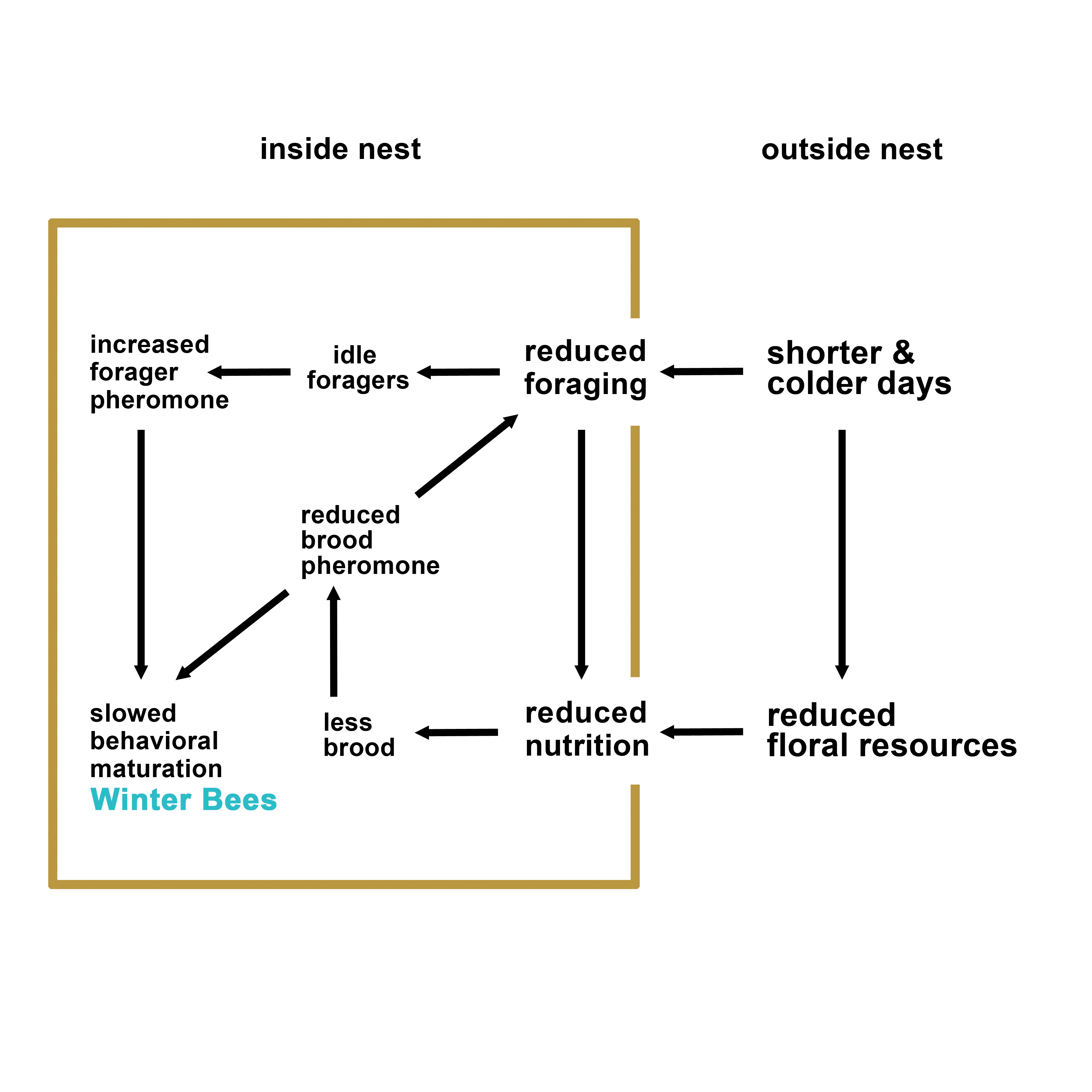
Figure 1. A theoretical model on the factors that may play a role in the initiation of winter phenotype in honey bee colonies.
Two of the likely players in this system are the brood pheromone and forager pheromone which are naturally occurring compounds in the honey bee hives and are known to regulate worker physiology and behavior. In this project I aimed to put this model to test and explore the use of these pheromones by the beekeepers to modulate the production of winter bees and improve colony overwintering survival. Specifically I aimed to 1) determine the effect of fall applications of two honey bee pheromones (forager and brood pheromone) on the timing of winter bee production and the size of the winter bee population, 2) determine the effect of pheromone treatment on colony health and overwintering survival, 3) using the results from objectives 1 and 2, develop a pheromone treatment program to improve overwintering success, and partner with beekeepers to test this treatment program in real life conditions, and 4) disseminate the information from objectives 1, 2, and 3 to beekeepers and commercial bee breeders.
Applying the pheromones to separate groups of colonies of similar strength and genetic make-up, we did not see an effect on their overall overwintering behavior or physiology. Neither was the survival effected by the different pheromones applied in comparison to the control colonies which did not receive either of the treatments. In the light of these findings we did not carry on with the 3rd goal of this study which was to translate our findings into a pheromone treatment regime for the beekeepers to test in realistic conditions and hopefully increase their colonies’ survival rates through the winter. We did, however, present our findings in several scientific and beekeepers meetings in line with aim 4 above.
I personally regret not having the results to create a tool to aid the beekeepers in improving the sustainability of their operations. However, I hope the methods used and the results obtained in this project will establish a baseline for other researchers to develop their own experiments to approach the same problem from a different tangent. Thus, I am glad to have the opportunity to share them through the reports here in the SARE portal.
Introduction:
The purpose of this project was to increase overwintering success of honey bee colonies by 1) examining effects of pheromones on the timing of winter bee production and size of winter bee population, 2) comparing health, performance and overwintering success of manipulated colonies, 3) using the results from objectives 1 and 2 for developing a pheromone treatment program, and partner with beekeepers to test this treatment program in real life conditions, and 4) disseminate the information from objectives 1, 2, and 3 to beekeepers and commercial bee breeders.
Honey bee colonies consist of a single reproductive female (the queen) and up to 50,000 sterile worker bees. In the fall, honey bee queens decrease their egg laying rate gradually and cease egg-laying in mid fall. When the temperature drops below 15 degrees Celsius (~60 degrees Fahrenheit) worker bees pack together and form a thermoregulatory cluster in which the temperature is kept relatively constant. The queen will only resume egg-laying in the early spring. Thus, during the fall, the production of long-lived winter bees that will form the thermoregulatory cluster and carry the colony to the consecutive spring is crucial.
The results of surveys consecutively conducted from 2006 to 2014 show that US beekeepers lose on average 30% of their colonies through the winter. In the multi-year survey conducted by Bee Informed Partnership, beekeepers have consistently reported annual colony losses are higher than what they see acceptable. Such high losses can cause an imbalance of the supply-demand ratio in pollination services. In the short term there is a risk of increased cost for pollination, which may affect the production cost of various crops; and in the long term beekeepers may fail to provide the number of colonies needed for pollination in US.
Reviewing the published literature on honey bee overwintering, maturation, and longevity, we developed a model which hypothesizes that interacting environmental and social (pheromonal) factors play a role in the timing of winter bee production and therefore overwintering success (see Figure 1 in the Abstract). Beekeepers have little to no control over the environmental factors such as day length or ambient temperature and can only regulate nutrition to a certain extent by selecting apiary sites that have abundant and diverse flowering plants. However, social factors are excellent targets that may be exploited to manipulate the colonies in fall in order to improve their winter readiness and overwintering success.
The two pheromones that we hypothesize function in regulating the production of winter bees are brood and forager pheromone. Honey bee larvae produce brood pheromone which accelerates maturation and aging in worker bees. Bees reared without brood or brood pheromone physiologically resemble winter bees, mature later, and live longer. Thus, the application of brood pheromone in late fall should delay the production of winter bees and be detrimental to overwintering success of the colonies.
Forager pheromone has the opposite effect, compared to brood pheromone, on behavioral maturation of young bees: it slows down maturation, and maintains bees in the nursing state longer before they transition to foraging. When foragers are confined in the colony in the fall (due to inclement weather conditions), this should increase the amount of forager pheromone in the colony and slow the maturation of young bees. Thus, the application of forager pheromone in the fall should facilitate the production of winter bees and increase overwintering success of the colony.
Our previous studies demonstrated that the size of the worker population was strongly correlated with overwintering success in honey bee colonies in Pennsylvania. Thus, if fall applications of forager pheromone can induce earlier production of winter bees and result in a higher population of winter bees, it may greatly improve overwintering success. In contrast we predict that fall applications of brood pheromone will cause delayed production of winter bees, a smaller population of winter bees, and reduced overwintering success. Interestingly, previous studies found that year-long brood pheromone treatment has a positive effect on overwintering survival of first year package bees. This may be due to the timing of brood pheromone application: if flowering resources are available (e.g., spring, summer, and early fall) brood pheromone enhances foraging activity which leads to increased brood production, and thus may have increased worker population size overall. As an outcome of this this project we wanted to provide beekeepers a novel method to support the colony's natural efforts to prepare for winter, and thereby improve overwintering success. Significantly reducing overwintering losses will increase the sustainability of beekeeping operations while reducing the economic burden resulting from replacing a large portion of colonies after each winter.
Objective 1. Test the effects of two honey bee pheromones, forager pheromone and brood pheromone, on timing of winter bee production in fall and size of overwintering population in honey bee colonies.
Objective 2. Determine the effect of different pheromonal treatments in fall on colony health (colony weight, colony size, storage levels, virus and parasite loads) and overwintering survival.
Objective 3. Using the results from Objectives 1 and 2, develop a pheromone treatment program to improve overwintering success in honey bees, and partner with beekeepers to test this treatment program in real life conditions.
Objective 4. Disseminate the results from Objectives 1 and 2, and the treatment program developed in Objective 3 to beekeepers and commercial bee breeders.
Cooperators
Research
Honey bee colonies and their maintenance. In April 2016, package honey bees (~3lbs of worker bees and a mated queen) purchased from Gardener’s Apiaries, Baxley GA were installed into 30 Langstroth hives at apiaries near Penn State campus in State College, PA (40.848172, -77.839832 & 40.874540, -77.851841). All study colonies were maintained using standard beekeeping practices besides the experimental pheromone applications. By the end of summer, when the pheromone treatments were to begin, we had 26 surviving colonies due to queen loss. Colony weight and strength was normalized at the beginning of the pheromone applications by exchanging frames of honey and brood between hives. All hives were made up of two standard ten-frame deep boxes (with base and cover/lid) and weighed 29 ± 1kg (without equipment). For varroa management, colonies were treated with oxalic acid in 1:1 sucrose solution following provider’s recommendations (Brushy Mountain Bee Farm) at the time of package installation and a second time with formic acid (Mite-Away Quick Strips™) in August after finding above-threshold varroa loads (>5 varroa/100 worker bees) in a sugar roll test.
Pheromone treatments. In September 2016, 26 surviving colonies were randomly assigned to one of the treatment groups: 9 brood pheromone, 9 forager pheromone, and 8 control. Colonies from each treatment group were distributed evenly between two Penn State apiaries (5 vs 4 in the case of groups with 9 colonies). Treatments were applied continuously from beginning of September to the end of November, 2016. Brood pheromone was delivered as a commercial product (SuperBoost®; Phero Tech International Inc.) in the form of a pouch which releases 70 – 80 mg (1,446 and 1,250 larval equivalents) of synthetic brood pheromone mix (ethyl linoleate 1%, ethyl linolenate 13%, ethyl oleate 8%, ethyl palmitate 3%, ethyl stearate 7%, methyl linoleate 2%, methyl linolenate 21%, methyl oleate 25%, methyl palmitate 3% and methyl stearate 17%; Pankiw, Lafontaine, & Avelino, 2010) per day (Lait et al., 2012) placed between the frames at the center of the hive. Forager pheromone was delivered daily, mixed in fondant made of honey and powdered sugar as in Leoncini et al., 2004, because the natural route of transmission for this pheromone is trophallaxis between the workers in the colony.
Assessing the timing of production of winter bees (Objective 1). Every two weeks after the treatments begin, we collected frames of maturing brood from the experimental colonies, allow them eclose in an incubator set at 34oC (~93oF) and 60% relative humidity. Once eclosed, callow workers were paint-marked and 100 marked workers per colony were introduced back to their hives (see Figure 1 for paint-marked callow workers). Every two weeks marked bees were censused to determine the remaining percentage of each cohort in each colony (see Figure 2 with 1 marked individual on a deep frame mostly covered by unmarked worker bees). We continued the censuses until late November. Censusing is a standard method to detect the timing of winter bee formation because cohorts containing long-lived winter bees exhibit higher survivorship throughout the fall and survive into the winter (H. R. Mattila et al., 2001).


RNA isolation and cDNA synthesis (Objective 1). For molecular experiments, we used workers bees of second cohort collected in the beginning of October. We chose this cohort because they are the earliest that have been exposed to the treatments from larval stage to 2-week into their adulthood, increasing the likelihood of observing both summer and winter bees depending on colony or treatment. Fat body tissues (eviscerated abdomens) of 5 bees/group were dissected and pooled for homogenization using an automated homogenizer (Thermo Savant FastPrepTM FP120 Cell Disrupter System) for 3 intervals of 45 seconds at highest speed setting using 4-6, 2mm zirconia beads (BioSpec Products, Inc.). Homogenates were immediately used for RNA extraction or were temporarily stored at -80oC. RNA was extracted from these samples using RNeasy Plus Mini Kit (QIAGEN) and cDNA was synthesized from RNA isolates using SuperScript® III First Strand Synthesis Kit (Thermo Fisher Scientific).
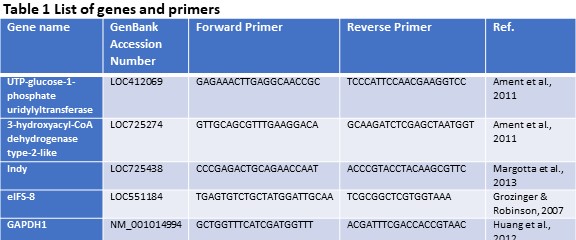
Quantification of gene expression (Objective 1). Using the findings of our previous study (Döke et al., 2017 – Submitted), we selected three candidate genes (INDY, UTP--glucose-1-phosphate uridylyltransferase, and 3-hydroxyacyl-CoA dehydrogenase type-2-like) that are differentially expressed in the fat body tissues of summer and winter bees. Gene expression levels were assessed via quantitative PCR using gene-specific primers and SYBR® Green Real-Time PCR Master Mixes (Thermo Fisher Scientific) on an 7900HT Fast Real-Time PCR System (Applied Biosystems Inc.). Each sample was run in triplicate and two housekeeping genes (eIFS-8 and GAPDH1) were included to normalize expression levels. A dissociation curve and negative control (cDNA reaction without reverse transcriptase, RT, enzyme) were used to ensure primer specificity and lack of genomic DNA contamination. Relative expression levels were calculated using the ΔΔCt method as described in Winer, Jung, Shackel, & Williams, 1999. See Table 1 for a detailed list of genes and primers used in this study.
Measuring colony level parameters (Objective 2). Colonies were monitored for colony health via biweekly measurements during the pheromone treatments (see Figure 3 for the set-up used during assessments in adverse weather). Net colony weight was determined by weighing the colonies and then subtracting the weight of equipment (boxes, frames, lids, and bottom boards). Number of frames occupied by workers (partially occupied frames represented by fractions) were used as an estimate of colony size (number of worker bees). Nectar, honey, and pollen storage levels were determined in a similar fashion via assessing the frames. In spring 2017, colonies were inspected for overwintering survival.

Statistical analyses (Objectives 1 and 2). A Mixed Model Repeated Measures ANOVA was used to compare the treatments and apiary locations for the colony level measurements. One-way ANOVA was used to compare the expression levels of selected genes between treatment groups, followed by Student’s t test for comparing each group when there was a significant difference (p≤0.05). K-means clustering (a partitional clustering method) was used to group our samples using expression patterns of all three genes. Clustering analysis was repeated with the number of clusters set to 1, 2, and 3 to compare Cubic Clustering Criterion (CCC) numbers as an indicator of highest between cluster and lowest within cluster variation to obtain best fit. All statistical analyses were performed using JMP Pro 10 software (SAS Institute Inc., Cary, NC).
Developing a pheromone treatment regime (Objective 3). In the original proposal, we aimed to use the results from this experiment to develop a pheromone treatment regime for the beekeepers in the northeast to enable them with a tool to improve the overwintering success of their colonies. However, our experimental treatments did not yield in any measurable difference in colony strength or winter survival between treatments. Thus, we were not able to develop a treatment regime as was initially planned (further explained in the Results and Discussion section).
Dissemination of results to beekeeper and other stakeholders (Objective 4). In order to disseminate the results from Objectives 1 and 2, I have presented our findings to beekeepers and researchers in Pennsylvania State Beekeepers Association Annual Meeting in State College, PA (November 11-12, 2016) and American Bee Research Conference in Galveston, TX (January 12-13, 2017). In these two events I was able to reach a large number of stakeholders. Many beekeepers and researchers engaged with the presentation and I had the chance to not only explain the details of the project to them but also get excellent feedback and ideas on how to move on from here.
Cohort assessments (Objective 1). Based on previous studies of exposure to brood pheromone and forager pheromone, we anticipated exposure to brood pheromone would delay the production of winter bees, while forager pheromone would accelerate it (Leoncini et al., 2004; Mattila & Otis, 2007; Smedal et al., 2009). This impact on winter bee production should have been apparent from our census of the survival of cohorts of introduced bees during the fall or in expression of our biomarkers of the winter bee phenotype in these bees. However, low recovery rate of marked worker bees prevented any meaningful statistical comparison over the survival rate of cohorts. In the future, I would consider using number tags that can be glued on the thoraxes of workers rather than paint-marking and marking a greater number of workers per release to improve the recapture rate.
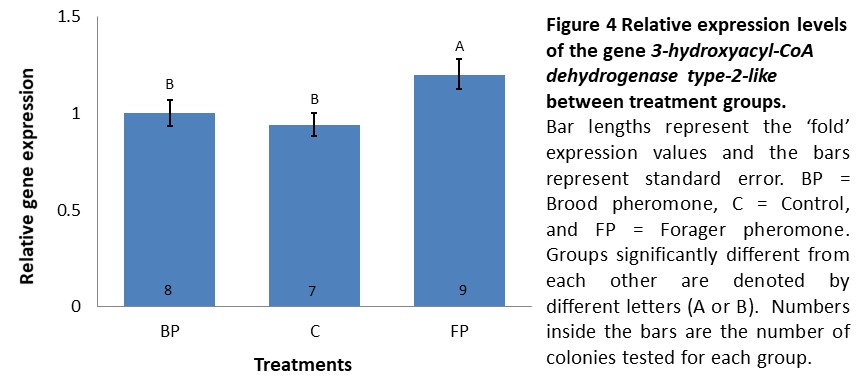
Gene expression (Objective 1). We anticipated to observe the impact of different treatments in expression of our biomarkers of the winter bee phenotype in these bees. In the second cohort (introduced in mid-September and sampled two weeks later), expression levels of INDY and UTP--glucose-1-phosphate uridylyltransferase did not significantly differ between treatments (data not shown) while 3-hydroxyacyl-CoA dehydrogenase type-2-like was expressed 1.2 fold higher in colonies receiving forager pheromone in comparison to controls and brood pheromone colonies, which exhibited similar expression levels (F=3.87, p=0.037, see Figure 4). However, K-means clustering analysis using the expression levels of all three genes did not demonstrate a clear separation between the treatments where a single cluster with all the individuals tested having the optimal CCC value (data not shown). Thus, if there was an effect of pheromone treatment, it was very weak.
Colony assessments (Objective 2). A Mixed Model Repeated Measures ANOVA showed that stored pollen differed by apiary location (F=49.36, p<0.001), treatment (F=4.94, p=0.014), and their interaction (F=5.41, p<0.01, see Figure 5) and stored honey/nectar differed by apiary location (F=8.95, p=0.006, see Figure 6). Colony weight and size, and number of brood were not significantly different between treatments, apiaries or their interaction.
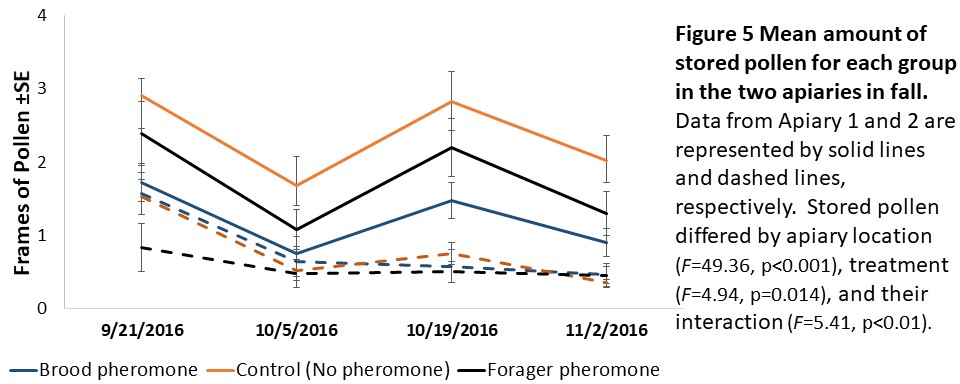
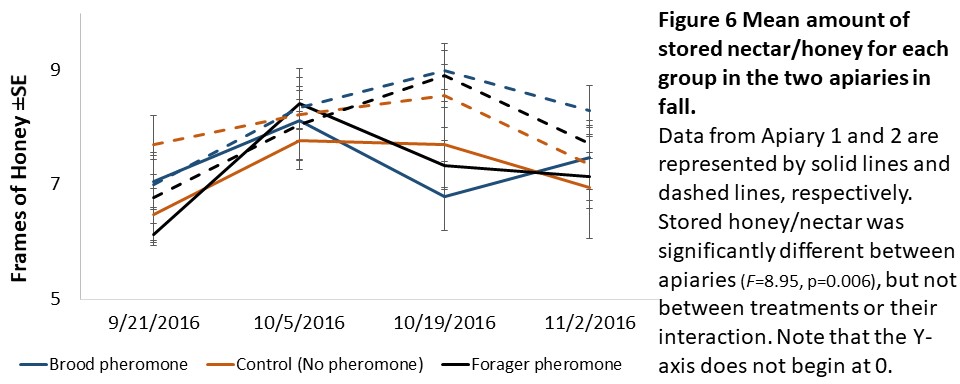
We expected to see impacts of pheromone exposure on colony resource acquisition and use, weight, and survival. Indeed, previous studies had shown that brood pheromone exposure increase pollen foraging (Pankiw et al., 1998) and enhance productivity and survival of colonies started from package bees (Lait et al., 2012). However, we did not find an effect of pheromone treatments on any colony level factor except stored pollen. In our study, pheromone treatment, apiary location, and their interactions all significantly impacted stored pollen levels. However, the colonies treated with brood pheromone had less pollen stored than the others. At first seeming contradictory to previous research, this can be a result of increased protein consumption in brood pheromone treated colonies (Pankiw et al., 2008) masking the actual pollen foraging rates.
Overwintering survival (Objective 2). Overwintering survival was highest among colonies treated with brood pheromone (3/9), followed by colonies treated with forager pheromone (2/9), and lastly the control colonies (1/8). Low survival rate (6/26 colonies) prevented any meaningful statistical comparison of survival rates between treatments. This was likely a result of size-limiting the experimental colonies (they were only allowed to grow into 2 deep Langstroth boxes) which was necessary for accomplishing the other goals in Objective 2. See Research Conclusions for a discussion of how to overcome this limitation in future research.
Overall, the results of this study do not support our hypothesis that changes in environmental conditions lead to changes in colony demography and pheromone profiles, which in turn induces the production of winter bees. However, there were a number of technical issues with this study which may have reduced our ability to observe differences in our treatment groups, and other biologically relevant factors which may have played a role. First, we limited the hive size by 2 ten-frame deep Langstroth boxes to keep census assessments manageable. Other researchers had done more extreme size limitations (1 ten-frame deep box) for the same reason (Heather Mattila – personal communication), yet they did not measure colony survival under size-limited conditions (Mattila et al., 2001). The size limited set-up also caused swarming in most colonies, which has a negative effect on the strength of first year colonies established from package bees. However, it would be virtually impossible to complete the cohort census assessments in a timely manner with full-sized colonies.
It could be worth repeating the experiment in a different set-up where winter survival is assessed using colonies that are allowed to grow naturally while a separate set of size-limited colonies are used for assessing cohort survival and gene expression. It should be noted that forager pheromone (ethyl oleate) is not commercialized in a heat-stable form and the fondant containing the pheromone has to be kept frozen until feeding and the pheromone has to be delivered in daily doses. A commercial form of this pheromone with a steady release rate like SuperBoost® would make such experiments significantly less labor intensive.
Education & outreach activities and participation summary
Participation summary:
I first shared our preliminary findings to beekeepers and researchers in Pennsylvania State Beekeepers Association Annual Meeting in State College, PA (November 11-12, 2016) in a poster presentation. Several beekeepers engaged with the presentation, asked questions, and expressed opinions.
Later I had the opportunity to attend the American Bee Research Conference (ABRC) and North American Beekeeping Conference & Tradeshow (NABC) in Galveston, TX (January 12-13, 2017). In the ABRC, I shared this project and the findings with other researchers in an oral presentation. In the joined NABC event, I gave a poster presentation to beekeepers and researchers. In these events I was able to reach a large number of stakeholders. Many beekeepers and researchers engaged with the presentation and I had the chance to not only explain the details of the project to them but also get excellent feedback and ideas on how to move on from here.
Project Outcomes
Even though our research here did not yield in a readily translatable outcome for the beekeepers to implement in their operations, we believe that the idea that honey bees can be managed further through the use of pheromones to improve survival and sustainability was an engaging perspective for the beekeepers with whom we interacted.
Both my advisor and I have been conducting research on pollinator ecology and health. Our previous experience with the colony collapse disorder (CCD) and the bee crisis in the last decade encouraged us to conduct research that will support initiatives to improve the sustainability of honey bees as a managed pollinator. Applying, receiving, and implementing this project fortified our dedication to this goal.
We were enthusiastic about the possibility of generating not only knowledge but also a practical tool for the beekeepers to use in their operations to improve the overwintering success of their colonies and thus, become more sustainable. Unfortunately, the findings from our project did not yield in an applicable pheromone treatment as we initially intended. In fact, within the limitations of our experimental design, different pheromone treatments did not result in measurable and consistent differences in survival outcomes or colony productivity. Thus, we could not translate this research to the beekeepers in a practical perspective. However, many beekeepers who became aware of the project kept inquiring for results, insights, and recommendations - indicating the relevance of the idea to use pheromone-based management practices in order to improve overwintering success of honey bee colonies in US.
As I was concluding this project, I also defended my dissertation and finished my graduate degree at Penn State. Currently, I am transitioning into a post-doctoral position at University of Puerto Rico in Rio Piedras (UPRRP). The Island was recently devastated by hurricane Maria which destroyed most of the vegetation along with urban sites. This destruction left the honey bees on the Island with very limited shelter and food. Tugrul Giray, with whom I will work at UPRRP, is strongly dedicated to helping the sustainability of feral and managed honey bee population in Puerto Rico (PR). I aim to support him in this effort by conducting workshops, training, and seminars with local beekeepers who are few in number and low on resources. We hope, sustainability of the honey bees in PR will benefit from outreach, education, and stakeholder inclusion.
Our pheromone research also attracted the attention of researchers from diverse backgrounds with whom we may collaborate for future work building up on this idea we tested here.
In this project we undertook several experimental challenges which became clearer during the implementation. One of the largest obstacles was committing ourselves to several measurements and assessments which had conflicting prerequisites in the beekeeping/colony maintenance aspect of this study. We needed size-limited colonies to conduct visual census of marked worker bees, whereas we needed colonies to have room to build and grow in order to assess their capacity to survive the winter. Thus, we compromised and chose a limited colony size of 2 deep ten-frame Langstroth boxes which is twice the size of colonies used in similar census experiments but still vastly limited compared to how large a honey bee colony can get if not restricted by the beekeeper. In hindsight, we are aware that this compromise likely hindered successful assessment of the treatments on both census and colony survival (as well as other measures such as stored pollen and honey, and brood). As we discussed in the Conclusion of 2017 annual report, we believe future research on this hypothesis can avoid the pitfalls we discovered by a few simple modifications in the methodology.