Final report for GNE16-114
Project Information
The goal of this research was to approach basil downy mildew disease control by elucidating aspects of the pathogen biology for effective and precise cultural control, as well as testing a biological control treatment. We sought to determine the length of time that infective P. belbahrii sporangia remain viable in order to develop a recommendation for safe crop re-establishment in a greenhouse following a disease episode. We also investigated the biological control efficacy of Pseudozyma aphidis, a filamentous yeast closely related to biological control agent Pseudozyma flocculosa.
In order to determine the survivability of P. belbahrii sporangia, we developed a protocol to isolate and store the infective pathogen structures from plant tissue, exposing them to controlled temperature and humidity settings. This approach mimicked greenhouse conditions that sporangia could be exposed to following dispersal, and was complemented with a treatment of low temperature and high humidity that we determined to be optimal for pathogen survival in order to define maximum viability. Sporangia were stored for 24 hour intervals at 2 temperatures and 2 relative humidity conditions, and then germinated in vitro and used to inoculate plants in order to confirm viability and infectivity. Our investigation of P. aphidis as a biological control organism was performed by applying live cells of the yeast as a protective treatment 3 days prior to P. belbahrii inoculation as has been described in prior P. aphidis literature, and using disease scoring methods to evaluate the efficacy of the treatment.
We presented preliminary results of the P. belbahrii survival trial to agricultural industry, and academic professionals and included a brief discussion during the UMass Extension Vegetable Twilight Meeting, where local growers were present. The results of this objective are being finalized in follow-up experiments, and will be prepared for publication in an academic journal as well as through UMass Extension communications.
P. belbahrii sporangia remained viable beyond 144 hours when kept isolated at 20ºC in 96.5% relative humidity, while viability was reduced to 96-120 hours in 84% relative humidity at the same temperature. Temperature of 25 ºC did not significantly reduce viability of sporangia, though other literature has shown that temperatures above 25 ºC will impact P. belbahrii survival. While this project is still being finalized, we are able to conclude that high relative humidity of 84% can sustain P. belbahrii sporangia for up to 120 hours after dispersal. We recommend that greenhouse growers who have faced an outbreak of basil downy mildew clear infected plant material and asymptomatic plant material in close proximity from a space and leave it vacant for at least 5 days prior to re-establishing basil crops in the area. Additionally, raising temperatures and taking measures to reduce humidity will decrease the survival of P. belbahrii inoculum, and may be used to shorten this safe re-entry interval.
Our investigation of biological control efficacy of P. aphidis against the basil downy mildew pathogen did not yield positive results as a protective treatment. We cannot rule out the possibility that a different isolate or strain of the yeast would provide protection against basil downy mildew, but further investigation and screening would be necessary.
The results of the P. belbahrii sporangia survival objective will be directly useful to greenhouse growers, particularly during the winter and spring seasons when inoculum is not present environmentally in the Northeast. Additionally, the establishment of these research protocols will provide us with the ability to offer more targeted recommendations based on greenhouse conditions if needed. This data has also brought into question the previously understood mode of pathogen dispersal through contaminated seed, as point introductions appear unlikely by sporangia with reduced survivability over extended time in low humidity conditions. This discovery suggests that the pathogen may remain viable internally in the seed, or else a different method that our research approaches failed to elucidate. This will inform our research questions and development of methods for viability detection in the future. While the biological control investigation did not yield promising results, our protocols may be useful for future investigation of treatment efficacy in a controlled environment, which would provide a more rapid preliminary screening result as compared to field trials reliant on natural infections.
Introduction:
Basil downy mildew is caused by the pathogen Peronospora belbahrii, which results in devastating crop losses for basil growers. Basil growers have consistently reported 100% crop loss of susceptible varieties, with estimated economic losses in the tens of millions of dollars (Roberts et al., 2009; Wyenandt et al., 2015). Peronospora belbahrii is an oomycete pathogen which infects basil leaf mesophyll tissue by entering through stomatal openings on the lower surface of the leaves. Symptoms and signs of infection include interveinal chlorosis and gray, mildew-like sporulation on the underside of leaves (sometimes emerging on the adaxial surface as well) (Belbahri et al., 2005; Garibaldi et al., 2007; Koroch et al., 2013). The spore-bearing structures, sporangiophores, produce sporangia abundantly, which are wind dispersed in both field and greenhouse production settings. Multiple fungicide efficacy trials have suggested that basil downy mildew is best controlled using a preventative spray program with ample coverage of the top and bottom of foliage, but there are currently no organic fungicide options that provide effective control (Mcgrath, 2016; Mcgrath et al., 2014; Wyenandt et al., 2015). Conventional fungicides options are limited and can risk increased pathogen resistance, and the demand for organically-grown basil is a priority for consumers. Breeding efforts toward producing downy mildew-resistant lines of sweet basil are in development, but there are no sweet basil cultivars currently in production that have shown resistance to downy mildew.
Current understanding of the epidemiology of P. belbahrii spread and infection is incomplete, and therefore research approaches are limited. Basil downy mildew was first introduced to the United States and the Northeast in 2007, and is re-introduced each growing season via inoculum traveling north on southerly winds, or through infected transplants and seed. This pathogen is the primary cause of basil yield loss due to biotic factors in the Northeast, and there is currently no policy in place for controlling shipment of infected material. This is of special concern during cold winter and spring months, during which time the pathogen does not survive in the Northeastern environment, and the only source of infection would be through infected material. There is no known resting spore stage produced by the pathogen and sporangia cannot overwinter in Northern climates, thus destruction of the affected crop following a disease outbreak should be an effective means of preventing further disease spread (Wyenandt et al., 2015). Sporangia that do not come into contact with plant tissue may be released onto greenhouse benches, potting materials, and other surfaces. A previous study based on plant inoculation bioassays suggested that detached sporangia kept at minimum temperatures of 25°C were unable to infect plants after 96 hours (Cohen et al., 2017). In order to improve control strategies for this pathogen, we examined survival of infective pathogen structures isolated from plant material and stored under conducive environmental conditions to improve cultural control of the disease.
Pseudozyma aphidis, a filamentous yeast species, appears to utilize Peronospora belbahrii as a mode of travel into basil mesophyll tissue. Pseudozyma is a genus within the phylum Basidiomycota, and shares common ancestry with plant pathogenic smut fungi (such as the corn smut fungus Ustilago maydis) (Boekhout, 2011). Pseudozyma aphidis was reported to activate a local and whole-plant defense response in plants challenged by fungal and bacterial pathogens (Buxdorf et al., 2013; Raacke et al., 2006). P. aphidis has also been reported to reduce the incidence of cucurbit powdery mildew (caused by Podosphaera xanthii) on squash plants by modes of ectoparasitism and antibiosis of the powdery mildew fungus (Gafni et al., 2015). Pathogen-infected plants may recognize and respond to biotic pathogens by triggering a defense response via stress hormone signaling, production of antimicrobial compounds, and deposition of callose in the cell walls to fortify the plant against pathogen attack (Dangl and Jones, 2001). In the future, activating a defense response in basil plants prior to infection could confer a beneficial effect for resisting pathogen infection (Raacke et al., 2006). After screening several P. aphidis isolates for colonization of P. belbahrii infective structures, we selected one for investigation as a potential biological control organism.
Literature Cited
Belbahri, L., Calmin, G., Pawlowski, J., and Lefort, F. (2005). Phylogenetic analysis and Real Time PCR detection of a presumbably undescribed Peronospora species on sweet basil and sage. Mycol. Res. 109, 1276–1287. doi:10.1017/S0953756205003928.
Boekhout, T. (2011). Pseudozyma Bandoni emend . Boekhout ( 1985 ) and a comparison with the yeast state of Ustilago maydis ( De Candolle ). Elsevier B.V. doi:10.1016/B978-0-444-52149-1.00153-1.
Buxdorf, K., Rahat, I., Gafni, a., and Levy, M. (2013). The Epiphytic Fungus Pseudozyma aphidis Induces Jasmonic Acid- and Salicylic Acid/Nonexpressor of PR1-Independent Local and Systemic Resistance. Plant Physiol. 161, 2014–2022. doi:10.1104/pp.112.212969.
Cohen, Y., et al. (2017). Epidemiology of Basil Downy Mildew. Phytopathology 107, 1149-
- doi: 10.1094/PHYTO-01-17-0017-FI.
Dangl, J. L., and Jones, J. D. G. (2001). Plant Pathogens and Integrated Defence Responses to Infection. Nature 411, 826–833.
Gafni, A., Calderon, C. E., Harris, R., Buxdorf, K., Dafa-Berger, A., Zeilinger-Reichert, E., et al. (2015). Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front. Plant Sci. 6, 1–11. doi:10.3389/fpls.2015.00132.
Garibaldi, A., Bertetti, D., and Gullino, M. L. (2007). Effect of leaf wetness duration and temperature on infection of downy mildew (Peronospora sp .) of basil. J. Plant Dis. Prot. 114, 6–8.
Koroch, A. R., Villani, T. S., Pyne, R. M., and Simon, J. E. (2013). Rapid Staining Method to Detect and Identify Downy Mildew (Peronospora belbharii) in Basil. Appl. Plant Sci. 1, 1–4. doi:10.3732/apps.1300032.
Mcgrath, M. T. (2016). Tips for Managing Basil Downy Mildew in Greenhouse and Field.
Mcgrath, M. T., LaMarsh, K. A., Simon, J. E., Pyne, R. M., and Wyenandt, C. A. (2014). Assessment of downy mildew resistance in basil breeding lines and experimental hybrids, 2014.
Raacke, I. C., von Rad, U., Mueller, M. J., and Berger, S. (2006). Yeast increases resistance in Arabidopsis against Pseudomonas syringae and Botrytis cinerea by salicylic acid-dependent as well as -independent mechanisms. Mol. Plant. Microbe. Interact. 19, 1138–1146. doi:10.1094/MPMI-19-1138.
Roberts, P. D., Raid, R. N., Harmon, P. F., Jordan, S. A., and Palmateer, A. J. (2009). First Report of Downy Mildew Caused by a Peronospora sp. on Basil in Florida and the United States. Plant Dis. 93, 199. doi:10.1094/PDIS-93-2-0199B.
Wyenandt, C. A., Simon, J. E., Pyne, R. M., Homa, K., Mcgrath, M. T., Zhang, S., et al. (2015). Basil Downy Mildew (Peronospora belbahrii): Discoveries and Challenges Relative to Its Control. Phytopathology 105, 1–45.
Our central objective was to discover and communicate information to basil growers regarding the basil downy mildew pathogen in an effort to improve control efforts. Specifically:
- Determine the length of time that Peronospora belbahrii sporangia remain viable in order to establish a safe time interval at which time greenhouse basil production can be re-established after a disease episode has occurred
- Determine the efficacy of Pseudozyma aphidis applied as a biological control to reduce basil downy mildew infection
- Communicate our findings with basil growers and researchers through extension educational programing, fact sheets and the UMass extension web site, as well as professional organization meetings.
Cooperators
- (Researcher)
Research
Objective 1
Inoculum preparation and inoculation
P. belbahrii sporangia was collected by forcing sporulation of infected plants overnight 12 hours prior to inoculation in a humidity chamber kept at 100% relative humidity (RH). Sporulating leaves were collected in sterile water, vortexed, and then filtered through a double layer of cheesecloth to remove leaf tissue. The sporangial suspension was then re-filtered through quadruple-layered cheesecloth to remove sporangiophores, soil particles, and other debris. The double-filtered suspension was vacuum- filtered onto 8 µm porosity filter paper to remove bacterial contaminants, re-suspended in sterile water, and quantified using a haemocytometer. The sporangial suspension was adjusted to a concentration of 1 x 104 sporangia/mL. This suspension was then used for inoculation of 6-8 week old plants by spraying until run-off and placing into a humidity chamber for 24 hours.
P. belbahrii inoculum storage
P. belbahrii sporangia suspensions were prepared as described previously, then separated into 50 mL aliquots. These aliquots were vacuum filtered onto 55 mm sterile filter discs with 8 µm porosity to remove them from suspension. Two temperatures were chosen to test sporangia survivability: 20 and 25°C, representing optimal temperatures for P. belbahrii infection and average daily temperatures in a greenhouse climate during the Northeastern winter months. Two humidity treatments were included in this study, with saturated salt solutions of potassium chloride and potassium sulfate mixed to retain 84% and 96.5% relative humidity in vitro, respectively (Winston & Bates, 1960). Platforms were constructed using 23-guage galvanized hardware cloth and 16-guage wire to fit 100 X 80 mm Pyrex crystallizing dishes. The filter discs were placed on the wire mesh platforms and positioned in Pyrex crystallizing dishes containing 25mL of saturated salt solution and sealed using 2 layers of parafilm. The salt solution treatments were randomly assigned time and temperature, and placed accordingly into incubation chambers.
In vitro germination assessment
Preliminary trials of the in vitro germination assay revealed that P. belbahrii sporangia require a gradual rehydration period prior to re-suspension in water (similar to Phytophthora infestans (Minogue & Fry, 1981)), and germination recovered after rehydration in a humidity chamber stored in a 10°C incubator for 2 hours. Following rehydration, stored filter discs were suspended in 50mL sterile water, vortexed, and used for in vitro germination and plant inoculations. 100 uL of the 1X104 sporangial suspensions was plated onto 1.5% water agar in triplicate and spread using a sterile bent glass rod. The plates were then stored in a 10°C incubation chamber for 24 hours. Germination was assessed by examining the agar plates under a dissecting microscope and rating the presence of germ tubes on 100 random sporangia per plate.
Plant inoculation bioassay
Re-hydrated sporangial suspensions were used to inoculate 8 week old basil plants in triplicate per temperature and humidity treatment. Plants were spray inoculated using a handheld sprayer until run-off, and placed in humidity chambers for 48 hours. A set of plants was inoculated with sterile water as a negative control for each time-point to ensure that there was no cross-contamination of inoculum. Plants were returned to humidity chambers after 6 days in order to induce sporulation. Disease incidence was calculated as a percentage of the number of sporulating leaves per plant.
Data Analysis
Analysis of variance (ANOVA) was performed using the PROC GLM procedure in SAS 9.4, and means were separated using Tukey’s HSD (⍺ = 0.05).
Objective 2
P. belbahrii and P. aphidis Inoculum preparation
P. belbahrii sporangia was collected as described previously, and P. aphidis isolates were cultured onto Potato Dextrose Agar and stored in a 22°C incubation chamber. P. aphidis inoculum was prepared by flooding cultures to suspend conidia, then creating a spore lawn by pipetting 100 uL of the suspension onto a fresh PDA plate and spreading it using a sterile bent glass rod. Spore lawns were flooded after 7 days, and the suspension was adjusted to a concentration of 1 x 106 conidia/mL. Four unique isolates were screened for their ability to colonize P. belbahrii sporangiophores and infected leaf tissue, and the most prolific isolate was chosen for biological control trials. Conidia suspensions were used to inoculate 6-8 week old plants 3 days prior to P. belbahrii inoculation by spraying until run-off and placing into a humidity chamber until P. belbahrii inoculation. One set of plants was pre-inoculated with sterile water prior to P. belbahrii inoculation, and another set of plants was inoculated with sterile water at both inoculation points as a negative control to ensure that there was no cross-contamination. P. belbahrii inoculation was performed as described previously.
Biological Control Assessment
Plants were kept in humidity chambers for 7 days after P. belbahrii or the second sterile water inoculation, and then removed for disease incidence rating. Disease incidence was calculated as a percentage of the number of sporulating leaves per plant.
Data Analysis
Analysis of variance (ANOVA) was performed using the PROC GLM procedure in SAS 9.4, and means were separated using Tukey’s HSD (⍺ = 0.05).
Literature Cited
Minogue, K.P., Fry, W.E. (1981). Effect of Temperature, Relative Humidity, and Rehydration Rate on Germination of Dried Sporangia of Phytophthora infestans. Phytopathology 71, 1181–1184.
Winston, P.W., Bates, D.H. (1960). Saturated Salt Solutions for the Control of Humidity in Biological Research. Ecology 41(1), 232-237.
Objective 1
The length of storage time was a highly significant interaction across all treatments (P<0.0001), with significant reductions in germination in vitro after 24 hours (Fig. 1), and significant reduction of disease incidence after 48 hours (Fig. Y) (P<0.0001). Humidity was a significant factor in germination and disease incidence reduction (P<0.05), with relative humidity of 96.5% yielding higher average germination than 84%. While the higher temperature of 25°C reduced germination and disease incidence compared to 20°C, it was not found to be a significant interaction.
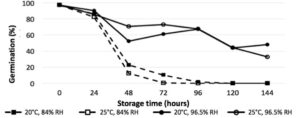
Figure 1. In vitro germination of P. belbahrii sporangia stored at 2 relative humidity (RH) treatments and temperatures over time
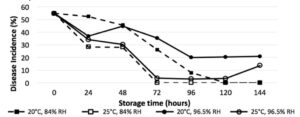
Figure 2. Plant inoculation bioassay results show disease incidence of basil inoculated with stored P. belbahrii sporangia at 2 RH treatments and temperatures over time
Objective 2
After two rounds of trials, there was no significant reduction in basil downy mildew disease in plants that had been pre-treated with P. aphidis as compared to plants that were pre-treated with sterile water.
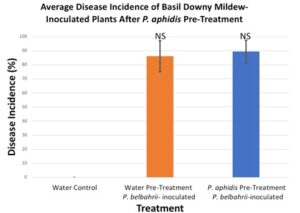 Figure 3. Pre-treatment with P. aphidis did not provide protection against basil downy mildew.
Figure 3. Pre-treatment with P. aphidis did not provide protection against basil downy mildew.
Objective 1
Humidity is critical to long-term survival of P. belbahrii inoculum, demonstrated in vitro and in planta. Greenhouse growers are still advised to remove and destroy infected plant material and plants in vicinity. Combining destruction of affected crops with reduced humidity and increased temperatures when possible will provide a less favorable environment for basil downy mildew infection. Under typical greenhouse conditions with relative humidity below 84%, greenhouse growers who have removed and destroyed infected plant material should be able to establish new crops as early as 72 hours after sporulation. Further research is being performed to identify maximum survivability, and may help to elucidate the currently questioned epidemiology of basil downy mildew spread.
Objective 2
Our results found that P. aphidis provided no biological control against basil downy mildew infection. These results are dependent on many factors, including identification of a suitable P. aphidis isolate, handling of the organism, and growing conditions. It may be possible that a different P. aphidis isolate could provide biological control against P. belbahrii through direct antagonism or by stimulating the plant immune response. The protocols developed for this experiment may be useful in future investigations of potential biological control organisms.
Education & outreach activities and participation summary
Participation summary:
The results of the Objective 1 trials were reported at the American Phytopathological Society 2017 Annual Meeting in San Antonio, TX, and at the Northeastern Division American Phytopathological Society Meeting in November of 2017. We also shared a report of our preliminary data at the UMass Extension Vegetable Twilight Meeting in September of 2017. Several agricultural extension professionals were interested to see the data, and provided insight into future directions for our research. Additionally, we were able to share the data with a local grower, and have established a working relationship with his greenhouse operation. We are currently in the process of replicating this experiment for publication and extending the timeframe in order to determine maximum viability in optimal conditions.
Presentations
Allen, K.S., Higgins, G., Ma, L-J, Wick, R.L. (November 2017). Survival of Peronospora belbahrii sporangia affected by temperature and relative humidity. Oral session presented at: American Phytopathological Society Northeastern Division Annual Meeting; Quebec City, Canada.
Allen, K.S., Ma, L-J, Wick, R.L. (September 2017). Suppression of Fusarium oxysporum f. sp. basilicum using common chives in a crop rotation system. Extension talk presented with Rob Wick during the UMass Extension Vegetable Program’s Twilight Meeting.
Allen, K.S., Higgins, G., Ma, L-J, Wick, R.L. (August 2017). Survival of Peronospora belbahrii sporangia isolated from plant tissue. Poster session presented at: American Phytopathological Society Annual Meeting, San Antonio, TX; UMass Amherst Plant Biology Symposium (October 2017), Amherst, MA.
Project Outcomes
The assessment of P. belbahrii sporangia viability is particularly important for sustainable agriculture practices in controlling basil downy mildew in greenhouse operations. This data can be used by growers to inform their intervals for re-establishing basil crops in greenhouses with previous downy mildew outbreaks. We have recently started working with a local greenhouse basil grower, and we have continued to target our research efforts to provide useful information for large-scale greenhouse operations through this collaboration. Though our assessment of P. aphidis as a biological control organism did not yield positive results, we feel that our methodology may provide basil downy mildew researchers with a more rapid approach for screening biological and chemical control products.
Not only did this project provide me with the opportunity to hone my research skills and methodology, I have also learned a great deal from my direct and indirect interactions with extension professionals, growers, and through interacting with SARE. I am more focused and attuned to asking research questions that will have direct application to sustainable agriculture. I will be using the data that I generated during this project as preliminary data for my PhD project once I have completed my Master’s work.