Final report for GNE17-141
Project Information
Shellfish aquaculture is an important and rapidly expanding sector in US food production (FAO, 2016). Shellfish farmers in New Jersey and along the US east coast are eager to diversify their farm plan by cultivating new species. The Atlantic surfclam (Spisula solidissima) represents an ideal target species for diversification because it is native, grows rapidly, and its growing season complements the region’s established farming framework. However, the species is known to be vulnerable to high temperature conditions (Goldberg and Walker, 1990; Munroe et al., 2013; Weinberg 2005) – an issue that will be exacerbated by rising temperatures (Munroe et al., 2016), and one that will be problematic on shallow coastal farms. Data from our previous and ongoing studies examining optimal nursery and grow-out conditions for surfclams suggest that thermal tolerance may be a heritable trait (Acquafredda et al., 2019). The purpose of this project was to evaluate whether selective breeding for a heat-tolerant surfclam stock is a viable strategy for enhancing the survival of cultivated surfclams exposed to high temperatures on shellfish farms.
To accomplish this goal, hatchery-reared surfclams were challenged with sustained heat stress and the most tolerant survivors were selected. The resulting heat-selected broodstock were bred to produce heat-selected progeny. The growth and survival of these progeny was then compared to non-selected surfclam progeny during the larval and nursery rearing phases. Then, the heat tolerance of both groups was assessed using a controlled, fully crossed laboratory experiment where the heat-selected and control progeny clams were exposed to elevated or ambient water temperature treatments. In lieu of the originally planned on-the-farm evaluation of the performance of the selectively bred surfclam stocks, we instead examined the phenotypic and transcriptomic response of surfclams to repeated heat stress.
We found that a 5-day heat shock at lethal temperatures was adequate to create a heat-selected group of broodstock with a ≈75% selection differential. The surfclam progeny from heat-selected and control broodstock groups performed similarly under hatchery and nursery conditions. During early development, growth was not compromised in the heat-selected group, yet survival may be influenced by the parental condition. Most notably, through selective breeding, heat tolerance was improved in first-generation surfclam progeny. In addition, we found that when juvenile surfclams were exposed to prolonged lethal temperatures, the adult survivors withstood subsequent thermal stress for significantly longer than clams that had no prior history of heat shock. Finally, we found that the control or naïve surfclams had a more intense response to the heat shock, differentially expressing about 3x more genes, than clams that had survived a previous heat exposure.
This study represents the first step towards developing heat-tolerant Atlantic surfclams, which will facilitate sustainable aquaculture production of this species in a warming climate.
Objective 1: Select heat-tolerant broodstock. Selecting the appropriate brood stock is paramount to any successful breeding program. We will select individuals from a large cohort of clams derived from a mix of wild stock parents. This cohort will be split into two groups: one that will be exposed to thermal stress, from which the heat-tolerant survivors will be selected, and a second that does not experience thermal stress and will act as control brood stock.
Objective 2: Produce heat-tolerant and control progeny. We will spawn and back-cross the heat-tolerant and control brood stocks to produce a thermally-selected cohort (TS-cohort) and a non-selected control cohort for comparison (NS-cohort). Through experimentation (Objectives 3 and 4), these progeny will allow us to determine if the heat-tolerance observed in the parent stock was transmitted to their offspring.
Objective 3: Evaluate survival, growth and performance of selected progeny on the farm. Clam farms experience fluctuating temperatures over tidal, diel, and seasonal temporal scales. By growing these surfclam cohorts on an actual farm for the entirety of the grow-out phase, we will be able to assess whether the thermally-selected progeny perform better than the non-selected progeny during both optimal (cooler water of late fall through early spring) and suboptimal conditions (summer), and determine differences in yield during the final harvest of market size animals.
Objective 4: Evaluate the heat tolerance of selected progeny in the laboratory. When the thermally-selected and non-selected cohorts are ready to be deployed to the farm, a portion of each cohort will be retained in the laboratory in order to assess the thermal tolerance of each group in a highly-controlled setting. By exposing the animals to more severe conditions than they would experience on the farm, we will be able to precisely determine the upper thermal tolerance of each cohort.
Objective 5: Evaluate the transcriptomic response of surfclams to heat stress. Four months after creating the selected and non-selected (control) broodstock groups, we subjected the clams to a six-hour exposure to 16 or 29˚C, and compared the transcriptome expression profiles of each temperature and group combination. We conducted a differential gene expression analysis and a KEGG pathway analysis to identify genes and pathways associated with the species’ heat stress response.
Like many other states in the Northeast region, the New Jersey shellfish farming sector is poised for growth. Shellfish aquaculture production has grown rapidly, expanding in volume by 5.1% and value by 11.1% nationally from 1998-2008 (FAO, 2016). Currently, only two species of shellfish are cultured commercially in New Jersey: the Eastern oyster (Crassostrea virginica) and the hard clam (Mercenaria mercenaria). The emerging oyster aquaculture industry in New Jersey consists of 17 oyster farms, 10 of which reported sales of $1.1 million in 2015 (Calvo 2016). Meanwhile, the more established hard clam industry consists of 39 farms and reported sales of over $2.3 million in 2013 (USDA, 2015). Although monocultures may increase efficiency, they pose a threat to food security because they are vulnerable to a variety of risks including heightened disease and predation pressure (Altieri et al., 2015). Consequently, many New Jersey farmers have expressed significant interest in expanding farm capacity and resilience by diversifying their crops (D. Munroe, personal communication).
Diversification can sustain the economic viability of farm operations. By cultivating diverse species, a farmer becomes insulated from any individual crop failure, whether it occurs from disease (Felton et al., 2016), predation (Russell et al., 1989), or fluctuating environmental conditions (Gaudin et al., 2015). Growing multiple species may also increase profitability, by allowing farmers to more easily navigate market forces as the price of each individual crop fluctuates (Chopin et al., 2012; Isaacs et al., 2016). Finally, growing multiple species in one area may enhance the environmental quality and the natural resources of the local ecosystem, upon which local aquaculture endeavors depend (Chopin et al., 2012). For example, enhancing filter feeders abundance can lead to the removal of excess suspended particles from the water column and can even provide habitat for other economically and ecologically important species (Newell, 2004; Luckenbach et al., 2016).
For the Northeast aquaculture sector, the Atlantic surfclam (Spisula solidissima) represents an ideal target for species diversification because it is native, grows rapidly, and fits into the region’s established farming framework. Surfclam aquaculture can add value to existing hard clams or oyster farm plans, especially since the rapid early growth rates of this species gives it the potential to reach marketable sizes within a year (Krzynowek et al., 1980). Moreover, this species experiences its fastest growth rates during the winter and early spring months (Goldberg and Walker, 1990), which would allow aquaculturists to stay engaged with farming operations and improve productivity during what would otherwise be a largely inactive winter season.
Although some of the techniques for cultivating surfclams were developed decades ago (Walker and Heffernan, 1990a, 1990b, and 1990c), recently efforts in Massachusetts (Murphy et al., 2017) and New Jersey (present authors) have focused on expanding upon established methods and innovating new approaches in order to address the challenges presented by the Northeast’s unique coastal environment. Through recent support from NJ Sea Grant and NOAA Sea Grant Aquaculture Extension, we have begun studies to optimize nursery and grow-out phase techniques. As this work continues, farm grow-out strategies and seasonal planting regimes are being evaluated, along with optimal nursery gear types. During experimental evaluation of the optimal nursery rearing temperature of early post-metamorphic juvenile surfclams (shell length = 0.600 – 3.00 mm), we demonstrated that surfclam seed under temperature stress survived less than half as well as those reared under cooler conditions (Acquafredda et al., 2019). Interestingly, surfclams produced from different parent stock responded significantly differently to temperature during these trials, suggesting that thermal tolerance is a heritable trait (Acquafredda et al., 2019). Selective breeding programs have been the foundation of viable Eastern oyster production in the Eastern US, (Haskin & Ford, 1979), and we believe this will likewise be a critical step in developing surfclams as a cultivation candidate in the region.
The purpose of this project is to evaluate whether selective breeding for heat-tolerant surfclams is a viable strategy for enhancing survival of cultivated surfclams exposed to high temperature conditions on shallow coastal shellfish farms. It is clear that this new crop (“shellstock”) species has strong potential to benefit farmers eager to build diversity and resiliency into their farm plans. Farm-raised surfclams also may provide a new domestic product for chefs eager to concoct new recipes and consumers eager to try a new, locally-sourced, high-protein food (Krzynowek et al., 1980). The development of a selectively bred heat-tolerant surfclam stock will lead to improved and consistent annual yields, providing stability to farmers and seafood consumers, alike.
Cooperators
Research
Methods to achieve Objective 1: Select heat-tolerant broodstock.
Selected and non-selected broodstock generation: 2017-2018
The broodstock surfclams used in this project were spawned at the New Jersey Aquaculture Innovation Center (AIC) in May of 2016 from a mix of wild and farm-raised parents; specifically, five wild females, one wild male, two first-generation farm-raised females, and four first-generation farm-raised males were crossed to generate these clams. The clams were out-planted onto Farm DP and Farm BA in October and November of 2016, respectively. Farm DP is located in the Lower Barnegat Bay and Farm BA is located in Absecon Bay.
A total of 505 clams were harvested from Farm DP on October 11th 2017, and 499 clams were harvested from Farm BA on November 20th 2017. During the time between the harvest and the start of the experiment, the clams were kept at the AIC in continuous flow-through upwellers supplied with raw water from the Cape May Canal. The clams from both farms were split into non-selected (NS) control groups and thermally-selected (TS) groups. The initial number of clams per group was as follows: NSBA = 235; TSBA = 239; NSDP = 240; TSDP = 240.
The experiment took place at the Haskin Shellfish Research Laboratory in Bivalve, NJ. The TSBA and TSDP clams were challenged with a warm water thermal shock. The water temperature that these clams experienced was increased from 16˚C to 30°C over a five-day period. Then, the clams were exposed to temperatures that exceeded their known tolerance limit (between 28˚C and 30˚C) for five days (Munroe et al., 2013; 2016). After the thermal shock, the TSBA and TSDP clams were cooled to 10˚C over a period of 16 days. The control groups, NSBA and NSDP, were maintained at 10°C.
Mortality was monitored daily, and dead animals were immediately removed from the treatments when detected. Each day, animals were fed algae paste (Shellfish Diet 1800, Reed Mariculture) according to the manufacturer’s recommendations of 3% of dry tissue weight. Temperature was recorded every 10 minutes with Seabird Scientific SBE 56 temperature loggers. Daily temperature and salinity point measurements were also collected. Ammonia, nitrite, and nitrate concentration levels were monitored daily; concentrations of nitrogen waste products never reached dangerous levels. Water changes during the thermal shock were conducted daily, but otherwise water changes occurred twice weekly.
Before the experiment began, the following morphometric data were collected on 25 individuals from each farm origin: shell dimensions (shell length, height, and width), whole wet weight, wet shell weight, and wet tissue weight. The wet shells and tissues of the sacrificed clams were placed in a 68˚C drying oven for at least 48 hours until all moisture was removed. Afterward, dry shell weight and dry tissue weight were measured and the following condition indices were calculated: dry body weight/shell length and dry body weight/shell volume.
Immediately following the temperature challenge and at the end of the experiment, the following data were also collected from individuals of each group: shell dimensions (shell length, height, and width) and whole wet weight. Since only a limited number of clams remained in each group after the experiment, no clams were sacrificed. Consequently, wet and dry shell weight data as well as wet and dry tissue weight data were not collected.
Following a recovery period after the thermal shock, approximately half of the clams from each group were out-planted, so that they would naturally develop gonad (ripen) over the winter. As a precaution, the remaining clams were held at the Bivalve facility. These clams were maintained in conditions that facilitated gonad development, but eventually these animals were also out-planted.
Selected and non-selected broodstock generation: 2018-2019
At the end of 2018, more broodstock groups were created using similar methods as stated above. The new broodstock surfclams were spawned at the AIC in March of 2017 from first-generation farm-raised surfclams. The clams were originally out-planted onto Farm DP in the fall of 2017 and harvested in July of 2018. The clams were kept at the AIC in raceways supplied with continuous flow-through seawater from the Cape May Canal. In September 2018, the clams were placed re-deployed at sites in the Barnegat Bay, and in December 2018, the clams were once again harvested for the thermal shock experiment.
These clams were split into one non-selected group and two thermally-selected groups that will eventually be pooled. To reduce ambiguity among the 2017 and 2018-produced broodstock groups, the new groups are coded as random control (RC) and heat-selected (HS). The initial number of clams per group was as follows: RC = 500; HS1 =566; HS2 = 566;
The experiment took place at the AIC. The HS clams were challenged with a warm water thermal shock. The water temperature that these clams experienced was increased from 12˚C to 30°C over a seven-day period. Then, the clams were exposed to temperatures that exceeded their known tolerance limit (between 28˚C and 30˚C) for five days (Munroe et al, 2013; 2016). After the thermal shock, the HS clams were cooled to 10˚C over a two-week period. The RC clams were maintained at 10-12°C for the duration of the experiment.
Mortality was monitored daily, and dead animals were immediately removed from the treatments when detected. Each day, animals were fed algae paste (Shellfish Diet 1800, Reed Mariculture) according to the manufacturer’s recommendations of 3% of dry tissue weight. Temperature was recorded every 10 minutes with Seabird Scientific SBE 56 temperature loggers. Daily temperature and salinity point measurements were also collected. Ammonia, nitrite, and nitrate concentration levels were monitored daily; concentrations of nitrogen waste products never reached dangerous levels. Water changes during the thermal shock were conducted daily, but otherwise water changes occurred twice weekly.
Before the experiment began, the following morphometric data were collected on 20 individuals from the RC group and 10 from each HS group: shell dimensions (shell length, height, and width), whole wet weight, wet shell weight, and wet tissue weight. The wet tissues of the sacrificed clams were placed in a 68˚C drying oven for at least 48 hours until all moisture was removed. Afterward, dry tissue weights were measured and the following condition indices were calculated: dry body weight/shell length and dry body weight/shell volume. After the selection process took place, the HS and RC broodstock groups were deployed into the Barnegat Bay to become naturally conditioned to spawn (ripen).
Methods to achieve Objective 2: Produce heat-tolerant and control progeny.
2018 progeny production
All work towards Objective 2 was conducted at the AIC. Since the AIC water pumping system was damaged by ice during the severe winter of 2017-2018, the facility was not able to function for much of the early spring. Therefore, the breeding of the broodstock groups was postponed until late April. Surfclams that had developed ripe gonads were thermally induced to spawn (Jones et al., 1993; Loosanoff and Davis, 1963). The broodstock groups TSBA and TSDP were backcrossed in order to produce cohorts of heat-selected progeny, and the broodstock groups NSBA and NSDP were backcrossed in order to produce cohorts of non-selected, control progeny.
Surfclam larvae were reared using methods adapted from Acquafredda et al., 2019. Specifically, larvae were reared in static tanks containing aerated seawater, which had been UV sterilized and filtered to 1 µm. The salinity and temperature of each larval culture was recorded daily using a YSI 85 handheld probe and maintained at 31.0+/-0.5 ppt and 20.5 +/-1.1˚C, respectively. Water changes were conducted 2-3 times weekly. The initial stocking density of each culture ranged from 8.3 to 35.5 fertilized eggs/ml. By day 2, the stocking density was lowered to <8 larvae/ml. Reductions in stocking density continued to occur during each water change. The target stocking density of <1 larva/ml was achieved prior to metamorphosis. Larvae were fed a diet of live algae (Tisochrysis lutea and/or Pavlova pinguis), and the initial feeding ration was 1*104 cells/ml on Day 0. The feeding ration was increased over the culture period, ultimately reaching 7*104 cells/ml by metamorphosis. Shell length and height growth data were collected from each larval cohort 2-3 times weekly by measuring a random sample of 25 individuals (Acquafredda et al., 2019). Repeated volumetric abundance estimates were conducted to determine the approximate survival of each cohort (Acquafredda et al., 2019).
To facilitate metamorphosis, pediveliger were placed in 125 and 150 µm downwellers. After metamorphosis, the early juvenile clams were reared in 200 µm downwellers. During the peri-metamorphosis period, the clams were fed a diet of live algae (Pavlova pinguis) at a feeding ration between 6*104 cells/ml and 8*104 cells/ml. By day 49, all surviving progeny had a shell height larger than 500 µm and were moved to continuous flow-through upwellers supplied with unfiltered seawater. The stocking density remained low for the duration of the nursery period, initially less than 7 clams/cm2 on day 49 and successively reduced to <1 clam/cm2. Temperature was recorded every 10 minutes with Seabird Scientific SBE 56 temperature loggers. Daily temperature and salinity point measurements were also collected. Approximately once per week, shell growth and survival data were collected as previously described. Between September and December 2018, the clams were moved to the Bivalve facility and reared in static tanks, fed algae paste, and maintained at a temperature and salinity of 12-18˚C and 30-33 ppt, respectively.
2019 progeny production
In May 2019, the HS and RC broodstock groups were backcrossed at the NJ Aquaculture Innovation Center at Rutgers University in North Cape May, NJ. Ripe surfclams were induced to spawn using thermal manipulation (Loosanoff and Davis, 1963; Jones et al., 1993). Two males and two females from each broodstock groups contributed to their respective progeny groups. RCF1-19 refers to the F1 progeny of the non-selected broodstock group (RC). HSF1-19 refers to the F1 progeny of the heat-selected broodstock group (HS). The larvae and juvenile clams were reared using culture methods nearly identical to what was conducted in 2018.
Methods to achieve Objective 3: Evaluate survival, growth and performance of selected progeny on the farm.
2018 farm evaluation
The only surfclam seed produced this year were offspring from the thermally-selected broodstock group, TSBA; this progeny cohort group is coded as TSBA-1. Since no progeny from a non-selected broodstock group were successfully cultured, a “common garden” experiment comparing the survival, growth and performance of selected and non-selected surfclam seed on a coastal New Jersey clam farm could not be conducted.
2019 farm evaluation
In September 2019, surfclams from both the heat-selected and random control progeny cohorts were deployed to three partner farms situated across the Barnegat Bay. Clams were deployed in standard 6 mm ADPI oyster bags and placed into bottom cages. Each cage held four bags. Two cages were deployed on each farm, and each cage contained two bags of HSF1-19 and two bags of RCF1-19. Each bag was stocked with 1000 clams initially. We anticipate collecting growth, survival, and condition data on these clams during spring and summer 2020.
Methods to achieve Objective 4: Evaluate heat tolerance of selected progeny in the laboratory.
2018 laboratory-based evaluation of heat tolerance
The only surfclam seed produced this year were offspring from the thermally-selected broodstock group, TSBA; this progeny cohort group is coded as TSBA-1. Since no progeny from a non-selected broodstock group were successfully cultured, a comparison of the heat tolerance of the selected and non-selected surfclam seed could not be conducted.
However, the heat tolerance of the sole surviving cohort was compared to two other groups of surfclams that were present from other projects. The first, HS_2016, is a cohort that was spawned in 2016 and underwent a selection event two-weeks post-metamorphosis that was caused by a month-long exposure to 26˚C water. The second, the NS_2016, was of the same cohort as HS_2016, but did not experience the early heat stress. In addition to being two years older than TSBA-1, the HS_2016 and NS_2016 groups were also significantly larger (>40 mm compared to TSBA-1 <20 mm). The experiment was conducted in 15 L buckets, stocked so that the biomass stayed equivalent across the different cohorts; each bucket contained six individuals from the 2016 cohorts and 105 individuals from the TSBA-1 cohort.
In the heated treatment, clams were exposed to water maintained between 28 and 30˚C (mean= 29.4˚C), after an acclimation period where the water temperature was slowly increased from 16˚C. Controls were maintained at 9-11˚C. There were at least four replicates buckets for each cohort for both the heated treatment and control. Buckets were aerated, salinity was 30 ppt, and clams were fed algae paste. Water changes occurred daily. Continuous temperature data were logged with SBE 56 (Seabird Scientific) devices using a 10-minute sampling scheme.
2019 laboratory-based evaluation of heat tolerance
In September 2019, we compared the heat tolerance of the heat-selected (HSF1-19) and random-control (RCF1-19) surfclam seed in a controlled laboratory setting. The methods used here closely matched those outline above. One notable difference was that only 100 seed clams were placed in each 15 L bucket.
Methods to achieve Objective 5: Evaluate heat tolerance of selected progeny in the laboratory.
Four months after creating the selected and non-selected (control) broodstock groups, a controlled experiment was conducted to determine whether surfclams that survived a lethal heat stress had different gene expression patterns upon re-exposure to similarly stressful thermal conditions compared to clams that never experienced a severe heat stress. Eighteen individuals were randomly selected from each of the two aforementioned groups, HS and RC. Nine from each group were placed into one of the two treatments, a six-hour heat shock (29.0±0.1˚C) and designated either RC29 or HS29, or six-hours in favorable control conditions (16.0±0.5˚C) and designated RC16 or HS16. Within each treatment, the nine clams from each group were divided into three replicate buckets, each containing three clams and 15 l of treated (1 µm filtered, UV-sterilized) seawater. After the six-hour experiment, we collected 12 pooled samples (2 clam groups x 2 temperature treatments x 3 replicate buckets), each comprised of gill tissue from the three clams from each replicate bucket. Samples were then sent to Novogene Bioinformatics (Beijing, China) for RNA extraction, library construction, and sequencing. Upon receiving the sequencing data, DESeq (Anders et al., 2010) was used for the differential gene expression analysis, and genes found to have an adjusted p-value < 0.05 were designated as differentially expressed genes (DEGs). KEGG pathway analysis was alsoc conducted to identify significantly enriched metabolic or signal transduction pathways, by comparing the frequency of differentially expressed genes to the background frequency of genes associated with a given pathway.
Objective 1 Results:
Selected and non-selected broodstock generation: 2017-2018
At the onset of the experiment, the average shell length of DP and BA clams was 32.37+/-2.03 mm and 32.21+/-4.13 mm, respectively. The average whole wet weight of DP and BA clams was 9.661+/-1.421 g and 9.245+/-2.706 g, respectively. The average wet tissue weight of DP and BA clams was 3.094+/-0.535 g and 2.820+/-0.795 g, respectively. None of these morphometric variables differed significantly between the clams of the two farm origins (Student t-test, p>0.16).
Conversely, the initial health or condition of the clams, as measured by dry tissue weight/shell length, was significantly greater for clams from Farm DP as compared to those from Farm BA (Student t-test, p<0.001). The average condition of DP and BA clams was 1.25e-2+/-3.43e-3 g/mm and 7.43e-3+/-1.97e-3 g/mm, respectively.
During the experiment, no mortality was observed for either control group, NSBA or NSDP. Immediately after the warm water thermal shock, the survival of the TSBA group was reduced to 23.8% while survival of the TSDP group was reduced to 60%.
Some mortality continued to occur after the thermal shock, even as the water temperature became more hospitable. By the end of the experiment, the survival of the TSBA group was reduced to 11.7% while survival of the TSDP group was reduced to 45.4%.
After one day into the thermal shock, TSBA and TSDP clams displayed behaviors that suggest the animals were succumbing to heat stress. The clams were frequently observed gaping. Clams were also seen extending their feet into the water column for prolonged periods of time, but they did not attempt to walk or explore their immediate surroundings by feeling the bottom of the tank. Similarly, the clams in the thermal shock responded more slowly to touch stimuli than clams in the control conditions; when probed, gaping clams in the thermal shock did not immediately close their valves or retract their extended feet or siphons.
After five days in the thermal shock, the shell margins of TSBA and TSDP clams had become very soft, almost pliable. The shell margins easily cracked or chipped under moderate finger pressure. During the thermal shock, the soft tissues of TSBA and TSDP clams took on a moribund appearance, becoming thin, watery, and translucent. Sixteen days into the cool down period, the soft tissues of most TSBA and TSDP clams had regained their color and turgor, their shell margins hardened, and their response times to touch stimuli were closer to that of the non-selected control clams.
Shell length, height, width, and whole wet weight did not differ significantly among the four groups immediately following the thermal shock or at the end of the experiment (ANOVA, p>0.05).
Selected and non-selected broodstock generation: 2018-2019
Initially, the average shell length of the clam population used to generate the 2018 broodstock groups was 36.55+/-2.46 mm. The average whole wet weight of was 9.16+/-1.81 g and the average wet tissue weight was 2.48+/-0.66 g. The initial condition of the clams, as measured by dry tissue weight/shell length, was 8.1+/-1.9*e-3 g/mm.
During the experiment, no mortality was observed for the control group, RC During the heat challenge used to select the HS broodstock group, mortality reached 53.8%. However, latent mortality continued to occur for approximately one month after the clams were returned to favorable conditions. Consequently, the final selection differential for the HS group was 74.8%. In terms of behavior, physical response, mortality rate, and post-shock recovery, the clams in the HS groups responded similarly to the TSBA and TSDP groups.
Objective 2 Results:
2018 progeny production
Ten attempts were made to spawn the different broodstock groups. Spawns of the TSDP and NSBA broodstock groups were each attempted three times. Spawns of the TSBA and NSDP broodstock groups were each attempted two times.
The only surfclam larvae that survived to metamorphosis and ultimately reach the size necessary to conduct Objectives 3 and 4 were progeny from a single spawn (=cohort) derived from two male and two female individuals from the TSBA broodstock; this progeny cohort group is coded as TSBA-1. All other larval cohorts survived between 2 and 24 days (Figure 1A-D). Numerous attempts at culturing surfclam larvae were unsuccessful due to an unidentified seawater contaminant at the AIC. This contaminant affected all bivalve larvae reared at the facility, impacting this project as well as the commercial oyster production that normally occurs at the AIC.
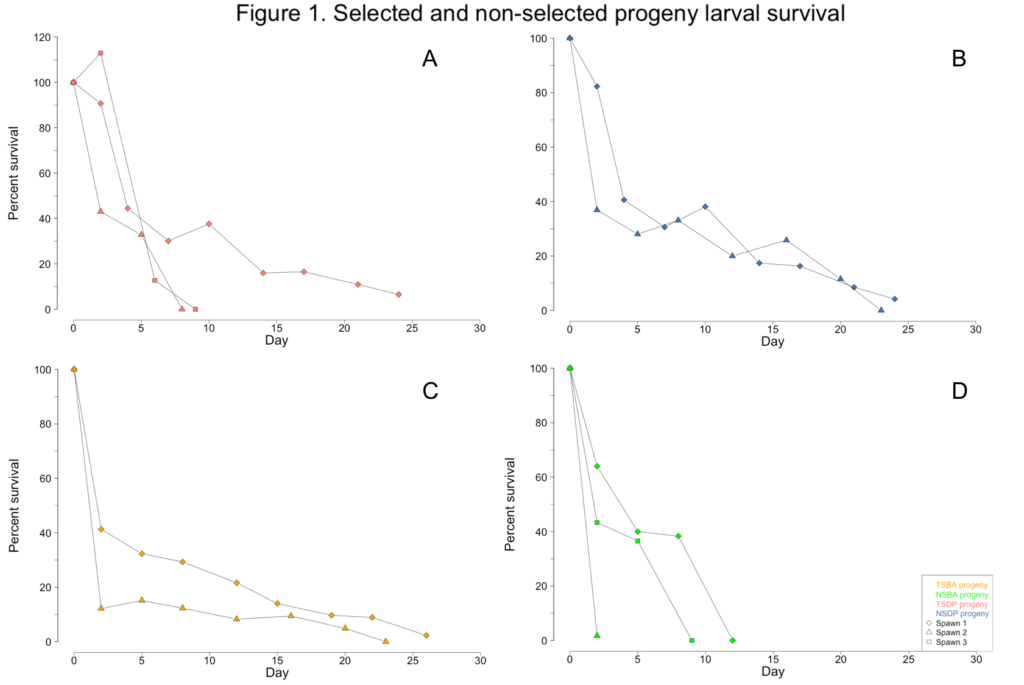
The unknown contaminant initially caused the larvae to display aberrant swimming behavior. Unlike the directed spiral movement typically associated with veliger motility, the affected larvae were often observed rapidly twitching or rotating in place. The larvae were also observed struggling to exude their velums. Growth stalled as the larvae diminished their swimming and feeding activities (Figure 2A-D). Eventually, all the larvae in a culture would cease their swimming and feeding activities, sink to the bottom of the tanks, and succumb to mortality. By June, the broodstock clams no longer had sufficient gamete material present in their gonads and were unable to spawn for the remainder of the year.
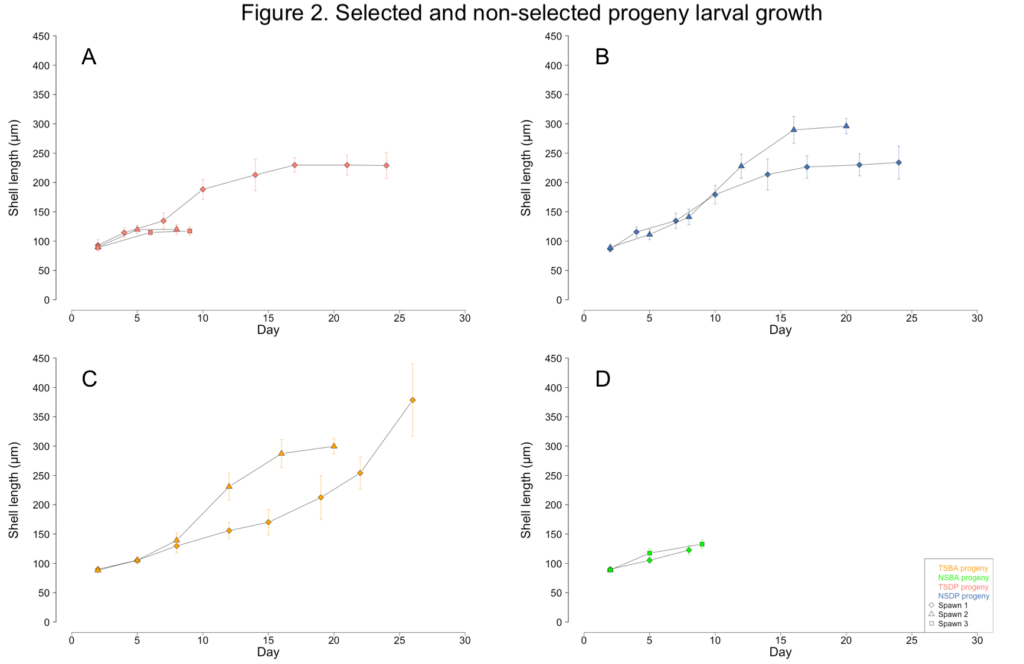
Less than one percent of TSBA-1 larvae survived to the upweller stage (Figure 1C; Figure 3A). However, after reaching the upweller stage, very little mortality was observed (Figure 3B). Despite the severe early mortality, the surviving TSBA-1 clams showed growth that was comparable to that observed in other studies of juvenile surfclams (Figure 4; Acquafredda et al., 2019).
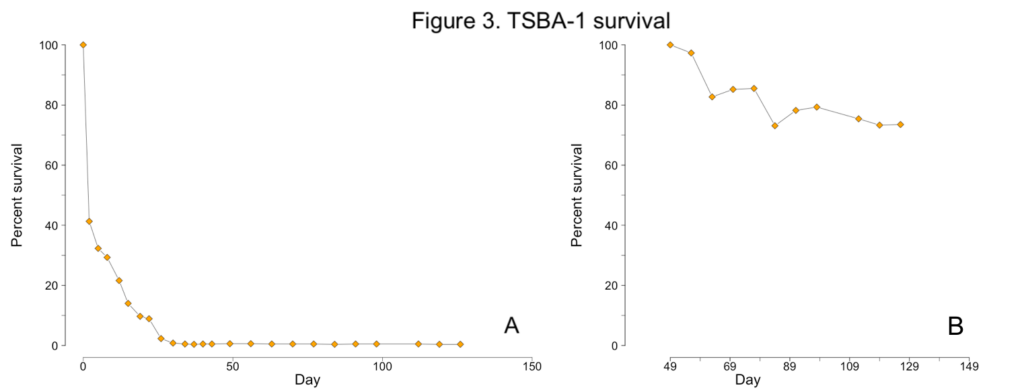
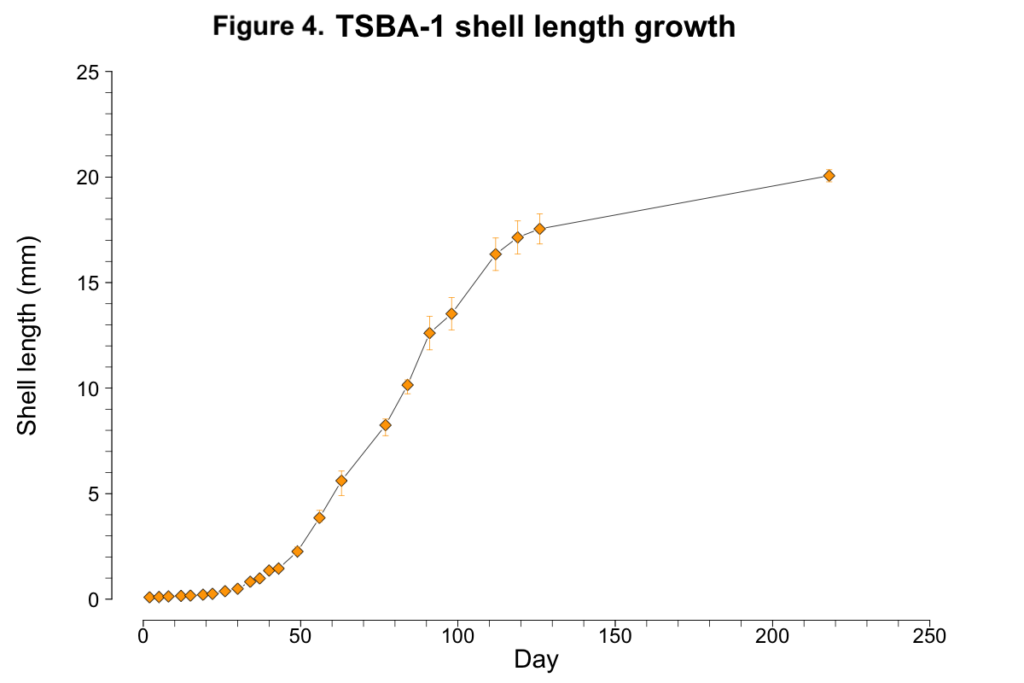
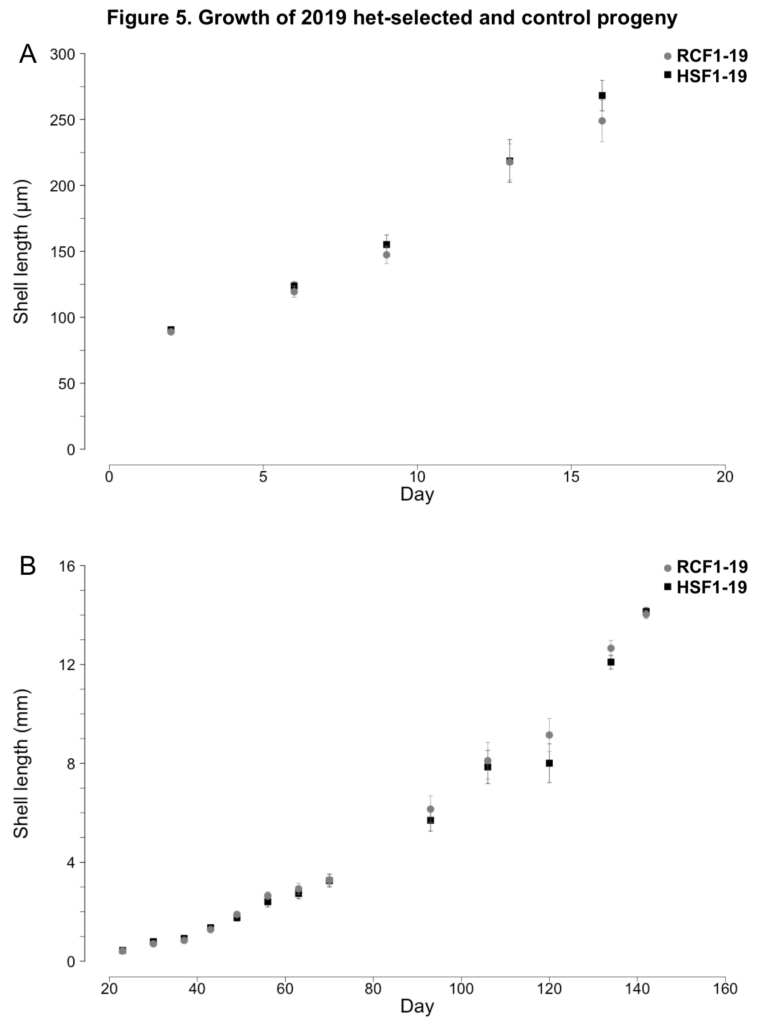
2019 progeny production
No notable differences in larval or juvenile growth were observed between the HSF1-19 and NSF1-19 surfclam progeny groups (ANCOVAs; p ≥ 0.35, Figure 5). During the larval phase, HSF1-19 and NSF1-19 clams grew at rates of 12.7 and 11.4 µm day-1, respectively (Figure 5A). During the nursery phase, HSF1-19 and NSF1-19 progeny grew at rates of 0.115 and 0.114 mm day-1, respectively (Figure 5B).
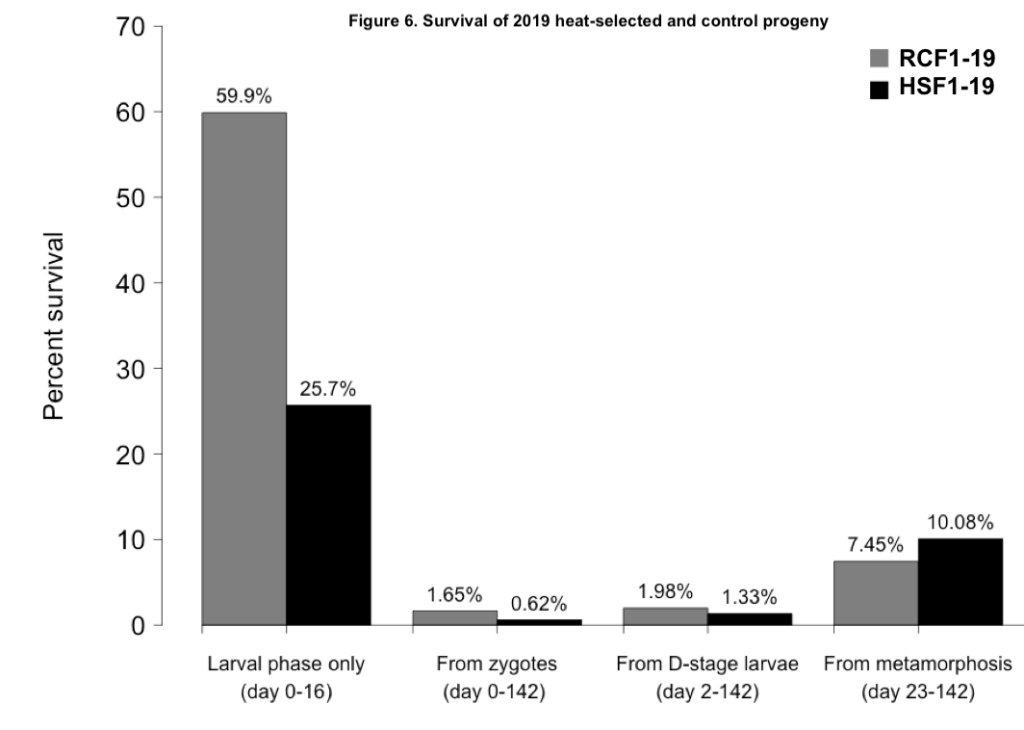
The two progeny groups did show some variation in survival over the course of their larval and nursery rearing (Figure 6). Larval survival was much greater in the RCF1-19 group than the HSF1-19 group. However, juvenile survival was greater in HSF1-19. Measured over the entire study period, from fertilization to farm deployment, the survival of RCF1-19 was 168% greater than that of HSF1-19.
Objective 3 Results:
2018 farm evaluation
Since no control progeny clams could be successfully reared, no farm evaluation was conducted this season.
2019 farm evaluation
By September 2019, progeny clams reached 12-15 mm in shell length. In total, 24,000 clams were deployed, 12,000 of each progeny group. These clams were evenly distributed across three partner farms in the Barnegat Bay. However, no resampling data could be collected before the end date of this award. Since we leveraged this award to receive other funding (Sea Grant #NA18-OAR4170357) to expand the project, we look forward to continuing our farm evaluations of these selectively bred surfclams in spring 2020 and beyond.
Objective 4 Results:
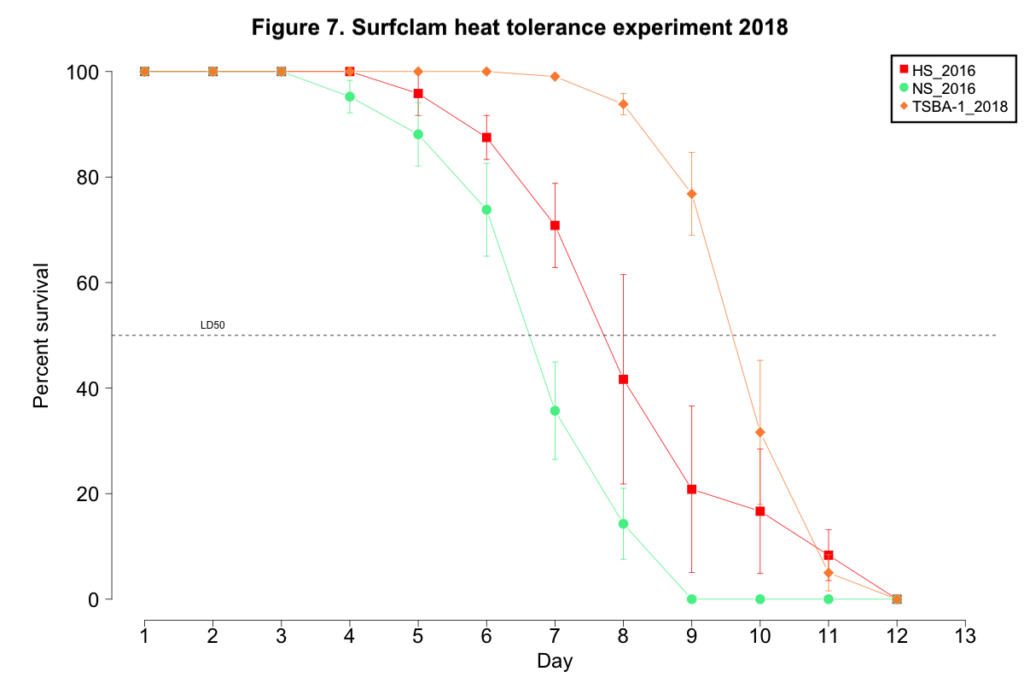
2018 laboratory-based evaluation of heat tolerance
No clam from any cohort succumbed to mortality when maintained at the control conditions of 9-11˚C. Mortality for clams exposed to the heat stress began on day 4 for NS_2016, day 5 for HS_2016, and day 7 for TSBA-1. (Figure 7). All clams in all NS_2016 replicates died by day 9, while 100% mortality was not observed for the HS_2016 or TSBA-1 clams until day 12 (Figure 7). The LD50 for the NS_2016 cohort, the HS_2018 cohort, and the TSBA-1 cohort was 6.57 days, 7.93 days, and 9.65 days, respectively.
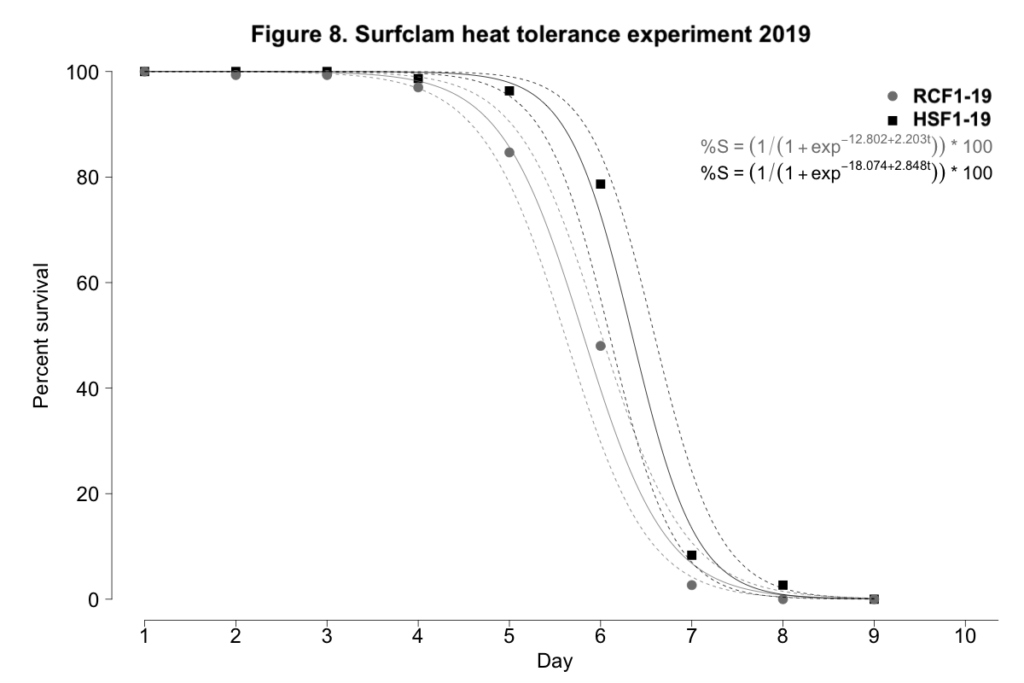
2019 laboratory-based evaluation of heat tolerance
When exposed to control conditions between 9-15˚C, surfclam mortality was negligible for both progeny groups. When exposed to heat stress, again both experimental day and progeny group were significant predictors of survival for surfclams exposed to temperatures between 28 and 30˚C (p < 0.001, Figure 8). The largest difference in survival was observed on day 6. On this day, survival was 48.0±13.7% and 78.7±12.4% for RCF1-19 and HSF1-19, respectively. However, on day 7, the survival of NSF1-19 and HSF1-19 clams was much more similar at 2.7±3.3% and 8.3±10.7%, respectively. RCF1-19 succumbed to 100% mortality by day 8; HSF1-19 endured for an additional day.
Objective 5 Results:
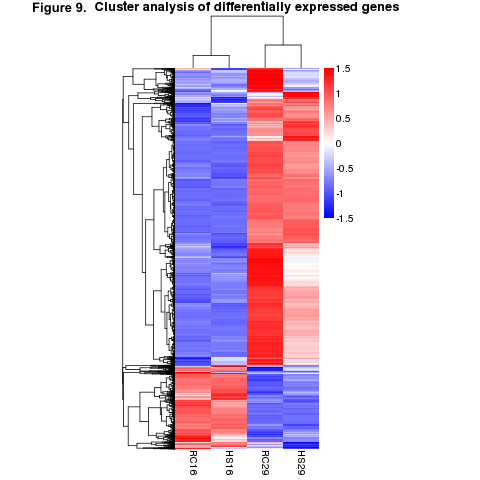
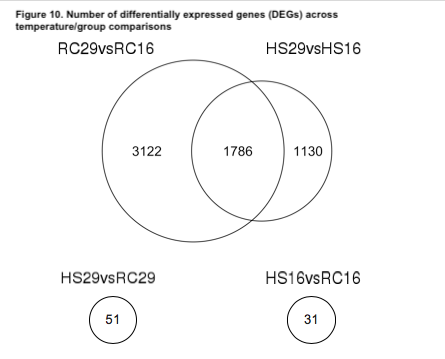
We first examined the differentially expressed genes (DEGs) of the within-group/across-temperature comparisons to determine how naïve surfclams respond to heat stress and how it may differ from the way clams that survived a prior heat stress responded to a repeated exposure. Heat shock induced a significant response in both the RC and HS groups (Figure 9). The RC clams exhibited a more robust response than did the HS clams (Figure 9). We found nearly 2000 more DEGs in the RC29vs.RC16 comparison than the HS29vs.HS16 comparison (Figure 10). In total, 4908 DEGs were detected in RC29vs.RC16 clams, in contrast to 2916 DEGs observed in HS29vs.HS16 clams (Figure 10).
We also examined the across-group/within-temperature comparisons to determine if the different groups had distinct expression patterns when experiencing the same conditions. When exposed to 16˚C, the gene expression patterns were largely similar between the two groups; only 31 genes were differentially expressed in the HS16vs.RC16 comparison (Figure 10). Likewise, at 29˚C, the gene expression patterns between the groups were comparable. We identified 51 DEGs in the HS29vs.RC29 comparison (Figure 10).
KEGG analysis demonstrated that at least 15 cellular pathways are significantly activated during heat stress (HS29vs.HS16 and RC29vs.RC16). This includes “Protein processing in endoplasmic reticulum”, which predominantly features members of the heat shock protein (HSP) family, and pathways that regulate cell growth, proliferation, apoptosis, and mounting an immune response. No KEGG pathways were identified in the HS16vs.RC16 comparison. Notably, KEGG pathways and DEGs that regulate fatty acid biosynthesis and lipid homeostasis were significantly upregulated in RC29 compared to HS29. By contrast, Sn1-specific diacylglycerol lipase, which is involved with lipid catabolism, was downregulated in RC29 and upregulated in HS29. Finally, the molecular chaperone Hsp70, and the immune-response gene Toll2 showed significantly upregulation in HS29 compared to RC29.
Objective 1 Conclusions:
A 5-day heat shock at lethal temperatures is adequate to create a heat-selected group of surfclam broodstock with a ≈75% selection differential.
Objective 2 Conclusions:
Surfclam progeny from heat-selected and control broodstock performed similarly under hatchery and nursery settings. Growth was not compromised in the heat-selected group. Survival, particularly larval survival, may be influenced by the parental condition.
Objective 3 Conclusions:
NA
Objective 4 Conclusions:
We found that when juvenile surfclams were exposed to prolonged lethal temperatures, the adult survivors withstood subsequent thermal stress for significantly longer than non-selected individuals (HS_16 and NS_2016). Moreover, we found that through selective breeding, heat tolerance was improved in first-generation surfclam progeny (HSF1-19 and RCF1-19).
Objective 5 Conclusions:
The control or naïve surfclams had a more intense response to the heat shock, differentially expressing about 3x more genes, than the heat-selected clams that had survived a previous heat exposure.
Education & outreach activities and participation summary
Participation summary:
JOURNAL ARTICLES
Acquafredda, MP, Guo, X., Munroe, D. (in prep). Phenotypic response of farmed Atlantic surfclams (Spisula solidissima) to repeated heat stress and the feasibility of selective breeding for greater heat tolerance
Acquafredda, MP, Guo, X., Munroe, D. (in prep). Transcriptomic response of farmed Atlantic surfclams (Spisula solidissima) to heat stress
CONFERENCE POSTERS & PRESENTATIONS
Acquafredda, MP, Guo, X, Munroe, D. (March 2020). Phenotypic and transcriptomic response of farmed Atlantic surfclams (Spisula solidissima) to repeated heat stress and the feasibility of selective breeding for greater heat tolerance. National Shellfisheries Association. Baltimore, MD. Oral Presentation.
Acquafredda, MP, Deck, N, Whiteside, M, Bushek, D, De Luca, M, Ragone Calvo, L, Munroe, D, Guo, X. (January 14, 2020). Diversification of Bivalve Aquaculture in New Jersey: New Species and Seed Lines for High-Salinity Back Bay Habitats. Milford Aquaculture Seminar. Milford, CT. Oral Presentation.
Acquafredda, MP, Munroe, D, Calvo, LM, DeLuca, M. (November 11, 2018). Temperature effects on the survival and growth of farmed Atlantic surfclams (Spisula solidissima). USDA Graduate Student Climate Adaptation Partners (GradCAP) 2018 Webinar Series. Webinar. https://www.climatehubs.usda.gov/hubs/northeast/events/gradcap-webinar-aquaculture
Acquafredda, MP, Munroe, D, Calvo, LM, DeLuca, M. (August 22, 2018). The Effect of Rearing Temperature on Survival and Growth of Early Juvenile Atlantic Surfclams (Spisula solidissima). 148th American Fisheries Society Annual Meeting. Atlantic City, NJ. Oral Presentation
Acquafredda, MP, Munroe, D, Calvo, LM, DeLuca, M. (February 7, 2018). Diversifying the Northeast aquaculture sector by developing culture techniques for the Atlantic surfclam (Spisula solidissima). Virginia Institute of Marine Science Extension Series. Wachapreague, Virginia. Oral Presentation
Acquafredda, MP, Munroe, D, Calvo, LM, DeLuca, M. (December 7, 2017). Diversifying the New Jersey aquaculture sector by developing culture techniques for the Atlantic surfclam. Rutgers Cooperative Extension - Marine Extension Program Series. Tuckerton, NJ. Oral Presentation
SOCIAL MEDIA
Instagram: @surfclamstragram
Project Outcomes
The surfclam is an ecologically and commercially valuable species. There is a lucrative wild fishery for surfclams and it is emerging as an attractive alternate aquacultured species in the region. Due to climate change and the warming ocean, understanding how the surfclam responds to heat stress is critical for ensuring environmental and economic stability in the Northeast, USA.
In this study, we evaluated the phenotypic response of farmed surfclams to heat stress, and the ability for that phenotype to be passed to subsequent generations. We found that when juvenile surfclams were exposed to prolonged lethal temperatures, the adult survivors withstood subsequent heat stress for significantly longer than non-selected individuals. We also found that selective breeding enhanced heat tolerance in first-generation surfclam progeny. Moreover, growth of the heat-selected progeny was not significantly different from that of control clams. Finally, we identified differences in gene expression between the control and selected broodstock groups. This could facilitate marker-assisted selection as well as more advanced methods of breeding via genetic techniques. In summary, this work suggests that selective breeding may be a viable strategy for enhancing survival of cultivated bivalves at risk from stressors related to climate change.
In addition to the benefits afforded to fishers and farmers, this work also benefits the academic community interested in how species can adapt to climate change. This work represents a significant portion of M. Acquafredda's dissertation, and two scientific journal articles are on track to be published in peer-reviewed journals.
My advisor and I developed a deeper understanding of how the Northeast's aquaculture sector can be made more sustainable through effective shellfish breeding and research. Specifically, we gained information on the phenotypic and transcriptomic response of surfclams to increased temperature conditions, and how future generations of surfclams may adapt or be bred to cope with climate change.
In the near future, I will be a Knauss fellow working for the NOAA Ocean Acidification Program. After finishing the fellowship and my PhD, I hope to continue researching sustainable aquaculture.