Final report for GNE18-167
Project Information
Hydrogen sulfide (H2S) is a toxic and corrosive by-product in biogas produced during the anaerobic digestion process and should be removed prior to energy generation. The effect of two types of biochar addition, corn stover biochar (CSB) and maple biochar (MS), into an anaerobic digester to remove H2S in the biogas was evaluated in lab-scale systems. The study evaluated the effect of adding biochar into a digester based on: 1) biochar concentration, 2) biochar particle size, and 3) preparing the biochar with iron (Fe) impregnation. All studies were conducted using two different biochar types and evaluating the effect on both H2S and CH4 (methane) production. Additionally, a study was conducted using a separate biogas scrubbing system after digestion and adding biochar (both raw and Fe-impregnated) into the scrubber and evaluating for H2S removal. The results were compared to use of activated carbon, which is currently used as an adsorbent for H2S, mostly in European anaerobic digestion systems.
The highest biochar concentration (1.83 g biochar/g manure total solids (TS)) in the anaerobic digester had an average H2S removal efficiency of 91.2%. There was no significant effect of biochar particle size on H2S removal and CH4 production. Iron impregnation of biochar achieved an average of 98.5% H2S removal at a concentration of 0.5 g biochar/g manure TS. Methane production did not vary significantly for all three experiments. The maximum adsorption capacity of biochar when used as a digester additive was 1.74 and 1.30 mg H2S/g biochar for MB and CSB, respectively. Iron impregnation increased the adsorption capacity to 2.59 and 2.69 mg H2S/g biochar for MB-Fe and CSB-Fe, respectively. In comparison, when the biochar was used in a separate scrubbing column, MB and CSB had H2S adsorption capacities of 6.13 and 3.28 mg H2S/g biochar, respectively. In this case, Fe impregnation increased the H2S adsorption capacity to 8.16 and 23.9 mg H2S/g biochar for CSB-Fe and MB-Fe, respectively. The presence of iron oxide (Fe3O4) in the Fe-impregnated biochars highlighted the importance of reactive oxides on the biochar surface for enhancing H2S adsorption.
Activated carbon did not have a significant effect on H2S production when added to the digesters, but increased total CH4 production by 10.7% in comparison to the control and biochar amended treatments. When activated carbon was used as an adsorbent in an external scrubbing system, its H2S adsorption capacity (5.42 mg H2S/g biochar) was within the range of adsorption capacities for the two biochars tested, but lower than the Fe-impregnated biochars.
There was no significant effect on the total ammonium nitrogen (NH4-N) and dissolved phosphorus (P) concentrations using different biochar concentrations and Fe impregnation in the post-digested effluent from the reactors. While previous research has shown that biochar can act as an adsorbent for N and P, when using a complex matrix, such as manure, there is competition among the multiple ionic and organic molecules for the active binding sites and no significant adsorption of N and P occurred on the biochar surface.
Overall, the study showed that biochar addition in anaerobic digesters could significantly reduce H2S production without affecting CH4 production. However, adding biochar directly into the digester was not as effective at removing H2S compared to using biochar as an adsorbent in a separate scrubbing system. Dairy farmers with anaerobic digestion systems with high H2S concentrations in the biogas may be able to use biochar as an alternative means of H2S control.
The first objective of the study was to test the applicability of biochar as an additive in dairy manure digestion for in-situ desulfurization of biogas under mesophilic conditions. The study investigated the effect of 1) four biochar concentrations, 2) three particle sizes, and 3) surface modification through Fe impregnation on CH4 and H2S production, ammonium N (NH4-N) and dissolved phosphorus (P) removal.
The second objective of this research was to study the effect of Fe impregnation on the performance of corn stover biochar (CSB) and maple wood biochar (MB) for H2S adsorption from biogas and compare the H2S removal efficiency between biochar addition into a digester and biochar used in a separate gas adsorption column for H2S scrubbing.
Biogas produced from anaerobic digestion (AD) consists of 55-70% methane (CH4), which can be used as a source of renewable energy, but also contains carbon dioxide (CO2) and traces of hydrogen sulfide (H2S). H2S can corrode piping, mixing motors, and electric generator sets that convert CH4 into electricity. Market available solutions to high H2S concentrations in biogas can have high capital costs, operating costs and/or unpredictable efficiencies (Shelford et al., 2019). A possible alternative could be the addition of a carbon-based material (biochar or activated carbon) to the anaerobic digester for in-situ capture of H2S and simultaneous enhancement of CH4 production.
Biochar is a carbon-based material that is produced via thermal degradation of organic material under limited oxygen (pyrolysis) at temperatures between 100 °C and 700 °C (Hale et al., 2011). The lower preparation temperatures of biochar in comparison to activated carbon and no requirement of any activation steps has shown that biochar ($0.35 - $1.2/kg) is comparatively cheaper than powdered activated carbon ($1.1 - $1.7/kg) (Thompson et al., 2016). Recent studies have investigated the direct addition of biochar into an anaerobic digester (Jang et al., 2018; Meyer-Kohlstock et al., 2016; Mumme et al., 2014; Shen et al., 2016, 2015). These studies focused on increasing CH4 content or digestion stability upon addition of the biochar but did not monitor or focus on the effect of biochar addition on H2S production.
In a study by Shen et al. (2015), adding biochar made from corn stover directly to an anaerobic digester treating municipal wastewater resulted in an 86% reduction in CO2 in the biogas, resulting in a biogas with more than 90% CH4 and less than 5 parts per billion H2S (Shen et al., 2015). The experiments were conducted using thermophilic conditions, with a concentration of initial H2S (90 ppm) that was lower than the concentrations associated with biogas from dairy manure digesters (1000 – 8000 ppm). In this prior study, up to 3.64 g biochar/g substrate total solids (TS) were added using a sewage sludge as the substrate, which has 1.3% TS. In dairy manure digesters, the TS concentration can vary from 1% to 10%, which would result in large quantities of biochar being to the digesters, that could reduce the effective volume for substrate treatment. A lower biochar concentration that adequately desulfurizes biogas, while also providing a measure of CO2 sequestration may be better for on-farm dairy manure digesters. Studies have also shown that biochar can be used to uptake metal, organic, and inorganic contaminants from soil and water, and surface modified biochar can lead to enhanced uptake of these contaminants (Ahmad et al., 2014; Ahmed et al., 2016). Iron (Fe) salts are commonly used for in-situ precipitation of H2S in anaerobic digestion (Lupitskyy et al., 2018). It is likely that surface modification of the biochar through Fe impregnation could significantly enhance its H2S adsorption capacity. To the best of our knowledge, a study focusing on the use of biochar and Fe-impregnated biochar as additives, specifically for CH4 enhancement and H2S reduction from biogas produced by dairy manure digestion at mesophilic temperatures, does not exist.
There have been limited investigations into H2S removal from biogas using biochar, but these prior studies showed that the surface properties of the biochar were an important parameter in the effectiveness of biochar in removing H2S (Kanjanarong et al., 2017; Shang et al., 2013; Xu et al., 2014). Shang et al. (2013) prepared biochar from camphor, bamboo, and rice hull and compared H2S removal efficiencies to activated carbon and found higher adsorption of H2S on the biochar surface compared to activated carbon, with the H2S removal efficiency increasing as the pH of the biochar increased. However, this prior study also used a low H2S concentration (50 ppm) compared to full scale digesters. Xu et al. (2014) and Kanjanarong et al. (2017) used manure-derived biochar and mixed-wood derived biochar, respectively, for H2S adsorption and achieved >97% removal efficiencies for H2S concentrations varying from 100 – 10,000 ppm (Kanjanarong et al., 2017; Xu et al., 2014). Both studies highlighted that the alkaline nature of the biochar was an important factor for the high removal efficiencies, but they did not specifically investigate the reaction behavior for biochar samples with an acidic pH.
The catalytic activity and selectivity towards sulfur have been reported to be improved by impregnation of transition metal salts, such as iron, copper and zinc, in activated carbon, which enhanced H2S adsorption capacities with only a small additional cost (Dalai et al., 2008; Nguyen-Thanh and Bandosz, 2005). Carbon-based adsorbents impregnated with metal salts from aqueous solutions have been shown to increase selectivity towards acidic gases. Impregnation of activated carbon with iron chloride (FeCl3) improved the adsorption capacity for H2S by 14% (Sakanishi et al., 2005). However, the authors conduced the experiments at elevated temperatures (400 °C) using H2S concentrations of 100 ppm. Biochar impregnated with Fe salts has been used to remove toxic heavy metals, such as arsenic and chromium, from aqueous solutions (Agrafioti et al., 2014; Frišták et al., 2017). However, to the best of our knowledge, there has not been a study on the use of Fe-impregnated biochar for gaseous H2S adsorption at room temperatures.
The project results from our previous NE SARE grant (LNE15-341) showed that managing and operating external scrubbing systems require technical expertise and manpower that may be unavailable on smaller-scale farms. Additionally, the lack of technical expertise from scrubber vendors (in part due to shutdown of one of the major scrubber businesses) has led to scrubber management issues for larger farms. Biochar could be a low-cost additive for AD systems that can be easily introduced with the digester feed to help reduce H2S production, without adversely affecting biogas production and farm nutrient management plans. It could also be an alternative to activated carbon as an adsorbent for H2S in the biogas.
AD systems with efficient H2S scrubbing could result in potential increases in on-farm energy production due to reduced generator down time and increased profit margins, which could make low-cost scrubbing systems a valuable investment for farmers. There could be other potential benefits, including decreased odors.
Research
Biochar characterization and properties:
Two biochar substrates, corn stover (CSB) and maple wood chips (MB), were tested in this study. The biochars were prepared through pyrolysis under an O2-free atmosphere at 500 °C with a holding time of 10 mins at the final temperature (ArtiCHAR, Prairie City, Iowa, USA). Each biochar was tested for mineral composition and pH, and then characterized using five methods (described in Section 2.6): 1) N2 adsorption isotherms for BET surface area, 2) Fourier Transform Infrared Spectroscopy (FTIR) for qualitative detection of functional groups, 3) Scanning Electron Microscopy (SEM) for imaging of the biochar surface, 4) zeta potential for biochar surface charge, and 5) electrical conductivity.
Effect of biochar concentration (Objective 1: Experiment 1):
The effect of biochar concentration on H2S production was conducted using unseparated liquid manure as the manure substrate and anaerobic digester effluent as the inoculum source collected from a covered lagoon digester at Kilby dairy farm in Rising Sun, MD. The farm co-digested 98% (by vol) flushed dairy manure and 2% (by vol) organic substrates containing cranberry waste, chicken fat, meatball fat and ice-cream waste, which was characterized by Lisboa et al. (2013) (Lisboa and Lansing, 2013). The flushed liquid manure and inoculum had TS values of 7.03 g/L and 8.63 g/L, respectively, and VS values of 4.47 g/L and 5.80 g/L, respectively.
The CSB and MB biochar were each tested at four concentrations: 1) 0.1 g biochar/g manure TS (CSB-0.1 and MB-0.1), 2) 0.5 g biochar/g manure TS (CSB-0.5 and MB-0.5), 3) 1 g biochar/g manure TS (CSB-1 and MB-1), and 4) 1.82 g biochar/g manure TS (CSB-1.82 and MB-1.82). For comparison, the highest concentration (1.82 g biochar/g manure TS) in this study was the lowest concentration in the study conducted by Shen et al. (2015).
Effect of biochar particle size (Objective 1: Experiment 2):
The study on the effect of biochar particle size on H2S production was conducted using inoculum and unseparated DM obtained from a manure digestion system at the USDA Beltsville Agricultural Research Center (BARC) in Beltsville, MD. The unseparated DM and inoculum had TS values of 30.5 g/L and 21.4 g/L, respectively, and VS values of 22.1 g/L and 13.2 g/L, respectively. Prior to digestion, both biochar types (CSB and MB) were segregated into three different particle sizes: larger biochar particles between 841 µm – 707 µm (CSB-L and MB-L), medium biochar particles between 177 µm – 149 µm (CSB-M and MB-M), small biochar particles less than 74 µm (CSB-S and MB-S). Activated carbon (Darco G-60, Fisher Scientific, Ontario, Canada) with a particle size of < 74 µm was also used as a treatment to compare its effects on CH4 and H2S production to biochar addition (AC-S). All segregated particle sizes of biochar and the activated carbon were added to the digestion reactors at a concentration of 0.5 g biochar/g manure TS.
Effect of biochar surface modification (Objective 1: Experiment 3):
The effect of biochar surface modification on H2S production was conducted using inoculum and unseparated DM obtained from a mono-digestion system at Mason Dixon farm, Gettysburg, PA. The unseparated DM and inoculum had TS values of 57.8 g/L and 40.8 g/L, respectively, and VS values of 46.8 g/L and 29.9 g/L, respectively. The two biochar surfaces were modified using a pretreatment step for metal impregnation. There were two treatments: 1) unmodified biochar (CSB and MB) and Fe impregnated biochar (CSB-Fe and MB-Fe). All biochar substrates were added to the reactors at a concentration of 0.5 g biochar/g manure TS.
For impregnation, 10 g of biochar was mixed with a solution containing 0.97 grams of hydrated iron chloride (FeCl3.6H2O) in 200 mL deionized water. The slurry was stirred using a magnetic stirrer for 48 hours and then dried in an oven for 24 hours at 105 °C. The dried composites were rinsed three times with deionized water to remove contaminants that can easily leach out, and then dried overnight at 105 °C (Frišták et al., 2017).
Experimental Design for Objective 1:
All three experiments were conducted using 300 ml serum digestion reactors and conducted in batch mode. In the experiments, the substrate (dairy manure), digester inoculum, and biochar were added into triplicate reactors, purged with N2 gas, capped, and incubated at 35 °C. An inoculum to substrate (ISR) ratio of 2:1 was utilized based on the VS concentration. Biogas, CH4 and H2S concentrations was monitored at regular intervals until biogas production had largely ceased to daily production at less than 1% of the total biogas produced. The quantity of biogas produced was measured using a graduated, gas-tight, wet-tipped 50 mL glass syringe inserted through the septa of the BMP bottles and equilibrated to atmospheric pressure. Biogas samples were collected in 0.5 mL syringes and tested on an Agilent 7890 gas chromatograph (Agilent, Shanghai, China) using a thermal conductivity detector (TCD) at a detector temperature of 250 °C and the oven temperature at 60 °C with helium as the carrier gas. The average CH4 production in the triplicates from the inoculum control was subtracted from the other treatments to present the total CH4 production from the waste substrates only and subtract the biogas attributed to the inoculum. All cumulative CH4 and H2S data presented was normalized by VS addition.
Experimental Design for Objective 2:
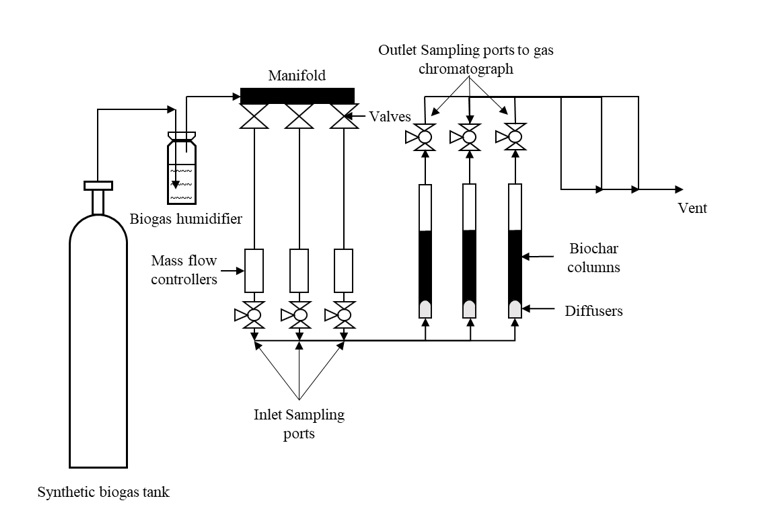
The experimental set-up is shown in Figure 1. In order to promote chemical adsorption along with physical adsorption, the moisture content of the biochar and activated carbon samples were increased to 25% by adding deionized water (Kanjanarong et al., 2017; Xu et al., 2014). The samples were packed into a vinyl tube (25.4 mm internal diameter, 200 mm height) strapped to steel rods using zip ties to keep it vertically oriented and plugged using rubber stoppers at the top. An air diffuser was connected to the bottom of the tube to ensure uniform distribution of biogas. The experiment was conducted at room temperature (25 °C) in triplicates where 3 g of each adsorbent was packed up to a height of 75 mm in each column. Each triplicate run tested one of five adsorbents used in the study (CSB, MB, CSB-Fe, MB-Fe and AC). Prior to entering the column, the biogas passed through a biogas humidification system to ensure a low, constant moisture in the biogas tested. Sampling points near the inlet and outlet of the column were added to measure the H2S concentrations before and after treatment. The biogas flow rate was kept constant at 100 mL/min using mass flow controllers (MCS-1SLPM-D/5M, Alicat, USA). The outlet H2S concentrations were tested hourly, and the experiment was stopped when the average outlet concentration of the triplicates was equal to the inlet H2S concentration, which was assumed to be the saturation point. In order to keep the influent H2S concentrations constant, synthetic biogas containing 1000 ppm H2S, 40% CO2, and 59.9% CH4 (Airgas, Air Liquide, France) was used for the experiment. Biogas samples at the inlet and outlet sampling ports were collected in 500 µL syringes and tested on an Agilent 7890 gas chromatograph (Agilent, Santa Clara, USA) using a thermal conductivity detector (TCD) at a detector temperature of 250 °C and the oven temperature at 60 °C with helium as the carrier gas. The adsorption capacity was calculated using the following formula (Shang et al., 2016):
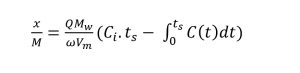 --------------------------------------> 1
--------------------------------------> 1
In equation 1, x/M is the adsorption capacity (mg/g of sorbent), Q is the inlet flow rate (m3/s), Mw is the molecular weight of H2S (g/mol), ω is the weight of biochar in the column (g), Vm is the ideal gas molar volume (L/mol), Ci is the inlet concentration (ppm), ts is the saturation time (s), and C(t) is the outlet H2S concentration at time = t. The integral was calculated using experimental data and numerical methods and input into the equation to find the adsorption capacity.
Figure 1 Laboratory set up for evaluating the efficiency of H2S adsorption using biochar
Biochar testing:
Nitrogen adsorption isotherms were measured at 77 K (-196.15 °C) using a BET Analyzer (ASAP2020 Micromeritics, Norcross, GA) for the biochar samples. The samples were heated to 150 °C and degassed under a vacuum of <5 µm Hg for six hours. The adsorption isotherms were used to calculate the specific surface area, SBET (BET method) in the range of 0.1 < p/p0 < 0.55, and micropore volume Smicro (t-plot Method).
The topographic analysis and the elemental composition of the biochar surface before and after modification, and after completed digestion was carried out by scanning electron microscopy (SEM-EDS) with a magnification range between 2000x and 10000x using a XEIA3 FIB-SEM (Tescan, Czech Republic). Biochar samples were mounted on a stub and gold coated prior to viewing. The powder XRD patterns of the raw, Fe-impregnated and H2S saturated biochar were recorded using Bruker D8 Advance Powder X-ray Diffractometer (Billerica, MA, USA), over a scanning interval (2θ) from 5° to 90° with CuKα radiation (λ = 1.54 Å).
The electrical conductivity and zeta potential were measured on the suspensions using a Zetasizer Nano ZS90 (Malvern Instruments, Westborough, MA). The zeta potential can be used to determine the pH at the point of zero charge (pHpzc), which is an important indicator of the biochar surface charge in a solution. For this test, 10 mg of biochar samples were added to 100 mL of deionized water. The solution was agitated on a shaker for 24 h at 25 °C. The point of zero charge (pHpzc) was obtained by measuring zeta potential values at different equilibrium pH values. The pH was adjusted using 0.05 M NaOH and 0.05 M HCl. All measurements were conducted in triplicates.
The carbon (C), hydrogen (H), nitrogen content (N), and metal analysis (including heavy metals) of the raw and Fe-impregnated biochar were conducted at Soil Control Labs Inc, California, using dry combustion for C, H, N, and EPA methods (EPA3050B/EPA 6010, 6020) for metals.
Manure sampling:
All manure and inoculum samples were brought to the laboratory on ice and tested for TS and VS within 24 hours in triplicate according to Standard Methods (APHA-AWWA-WEF, 2005). For TS analysis, triplicate 10.0 ml samples were pipetted into pre-weighed porcelain crucibles. The samples were then dried at 105 °C until a constant weight was obtained for the TS concentration. The crucibles were then placed in a furnace at 550 °C until a constant weight was obtained to determine VS concentration.
For ammonia-N, samples before and after digestion were acidified to pH < 2, and centrifuged at 15000 rpm for 30 min. The supernatant was filtered through a cellulose acetate membrane with pore size of 0.45 µm to obtain a filtrate that was analyzed for ammonium-N using a Lachat Quikchem 8500 (Method 10-107-06-2-O; Lachat Instruments, Loveland, CO).
Dissolved phosphorus was analyzed by modifying the tests for total phosphorus. In the tests, post digested samples were filtered first using 0.45 µm membrane filters to prevent possible dissolution of adsorbed and precipitated P species and then acidified to pH < 2. The samples were then digested with concentrated sulfuric acid and tested using Method 13-115-01-1-B rev 2006 with the Lachat Quikchem 8500 to obtain the dissolved P fraction.
Statistical Analysis:
Statistical analysis was conducted to determine significant differences in CH4, H2S, TS, VS, NH4-N, and dissolved P, using t-tests, analysis of variance (ANOVA) and Tukey–Kramer multiple comparisons. All p-values <0.05 were considered significant. All triplicate values are reported as averages with standard errors (SE).
The physical and chemical characteristics of CSB and MB are shown in Table 1. MB had lower iron (Fe), magnesium (Mg), potassium (K), nitrogen (N) and phosphorus (P) concentrations than CSB, but a higher C and H concentrations. CSB and MB had a BET surface area of 23.5 and 161 m2/g respectively, which were within the ranges seen for biochar prepared at 500 °C (2 – 400 m2/g), but lower than the surface area of activated carbon (>1000 m2/g) (Sitthikhankaew et al., 2014; Weber and Quicker, 2018). The micropore volume followed a similar trend, with MB (0.095 cm³/g; 3.5 nm) having a higher pore volume and lower pore width than CSB (0.011 cm³/g; 6.0 nm). Both the biochars were alkaline in nature due to the high preparation temperature (500 °C), with the pH of CSB higher than MB (10.2 and 9.1, respectively) due to the higher metal concentrations and ash content (Mireles et al., 2019).
Table 1. Physical and chemical properties of the two biochar types and AC.
|
Parameter |
Corn Stover Biochar (CSB) |
Maple Biochar (MB) |
Activated Carbon (AC) |
|
C (%) |
62.9 |
81.5 |
N/A |
|
H (%) |
3.1 |
3.4 |
N/A |
|
N (%) |
0.95 |
0.55 |
0.91 |
|
P (%) |
0.21 |
0.05 |
0.14 |
|
K (%) |
2.34 |
0.49 |
0.15 |
|
S (%) |
0.04 |
0.02 |
0.12 |
|
Ca (%) |
1.45 |
0.96 |
0.49 |
|
Mg (%) |
0.31 |
0.09 |
0.11 |
|
Zn (ppm) |
56 |
43 |
14 |
|
Fe (ppm) |
5500 |
1100 |
423 |
|
Cu (ppm) |
12 |
9.2 |
8 |
|
pH |
10.2 |
9.10 |
8.3 |
|
Ash (%) |
29.6 |
5.8 |
3.5 |
|
Moisture (%) |
1.3 |
0.8 |
6.4 |
|
Surface Area (m2/g) |
23.5 |
161 |
>1000 |
|
Micropore Volume (cm3/g) |
0.011 |
0.095 |
N/A |
Due to the acidic nature of FeCl3, the pH decreased with the increasing Fe concentrations to 2.8 for CSB-Fe and 6.97 for MB-Fe. The iron concentrations increased from 5,500 (CSB) to 17,700 ppm in CSB-Fe, and from 11,000 (MB) to 29,700 ppm in MB-Fe. The impregnation process also changed the micropore volumes of the biochars. MB-Fe had a 58% lower micropore volume (0.04 cm3/g) compared to MB, while CSB-Fe had a 64% lower micropore volume (0.004 cm3/g) compared to CSB. Micropore volume decreases in biochar due to impregnation can be attributed to the blockage of micropores by the impregnating agent and a change in the pore size distribution (Ahmed et al., 2016). The surface area of MB-Fe was reduced by 63%, but the surface area of CSB-Fe increased by 48%. The contrasting effect could be due to the difference in distribution of the resulting Fe-composites on the biochar surface and pores of CSB-Fe and MB-Fe. The XRD spectra showed the formation of Fe3O4 crystals on both CSB-Fe and MB-Fe, but MB-Fe had additional crystals of FeO(OH) that may have lowered the surface area.The carbon content of both biochars were >50%, with MB (81.5%) having a higher C content than CSB (62.9%). The higher ash content in CSB (29.6%) was composed mostly of silica, as previously reported by Shen et al. (2015) and validated by the intense peaks associated with silica in the SEM-EDS and XRD data (Shen et al., 2015). Zhao et al. (2018) prepared biochar from corn straw at 500 °C with similar physicochemical parameters (61.8% C, 20.7% ash, and a surface area of 7.7 m2/g) (Zhao et al., 2018). Similarly, maple biochar prepared by Wang et al. (2015) at 500 °C with a residence time of 30 mins had a carbon content of 78.9%, 1.4% ash content, and a surface area of 257 m2/g (Wang et al., 2015). The EDS data verified that the biochar was primarily composed of carbon with smaller amounts of calcium (Ca), magnesium (Mg) and oxygen (O) in MB compared to CSB. The high Ca content in both biochars was mostly in the form of CaCO3, with intense peaks at 2θ = 28.3° and 29.4°. Heavy metal concentrations in both biochars were low or below the detection limit.
Effect of biochar concentration (Objective 1: Experiment 1):
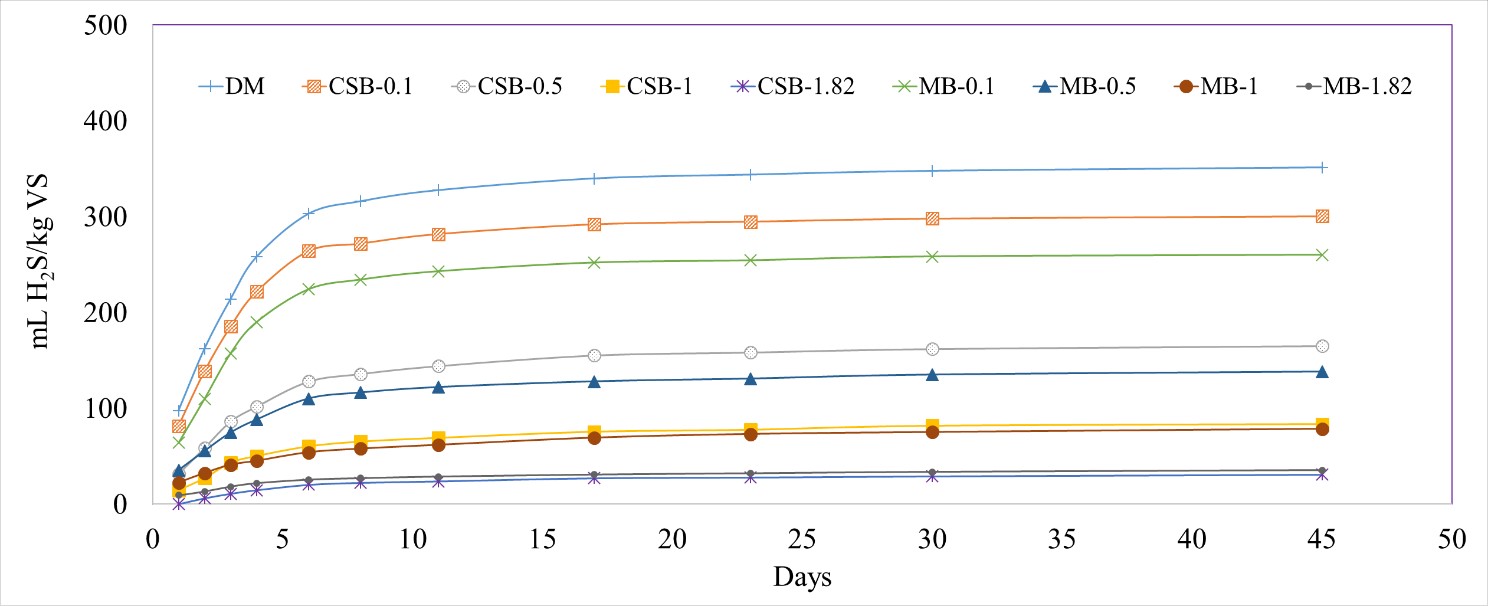
Addition of biochar to the reactors significantly decreased the H2S production compared to the DM control (p-value < 0.0001). The normalized H2S concentration in the biogas decreased as the concentration of biochar increased in the treatments. When no biochar was added into a digester, 351 ± 9.4 mL H2S/kg VS was produced. At the highest concentration of biochar added (1.82 g biochar/g manure TS), only 35.4 ± 5.8 mL H2S/kg VS was produced for MB and 30.9 ± 2.4 mL H2S/kg VS was produced for CSB, a reduction of 90 and 91%, respectively (Figure 2).
Figure 2. Cumulative hydrogen sulfide (H2S) production normalized by kilograms of volatile solids (VS) with different biochar concentrations, with dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested concentrations (0.1, 0.5, 1, 1.82 g/g manure TS) are used to differentiate each treatment.
The total volume of H2S captured increased with the concentration of added biochar. However, a decreasing trend was observed when the volume of H2S was normalized by the weight of biochar added (Table 2). This effect was likely due to limited H2S production and excess amount of biochar added into the reactors. Lower concentrations of added biochar (0.1, 0.5, and 1 g/g manure TS) were not able to completely reduce the H2S concentrations even though the biochar was not fully saturated with H2S, probably due to the lack of vigorous mixing resulting in a lower contact between the biochar and H2S. Increasing the biochar concentration led to an increase in the available biochar surface for interaction with the H2S, thereby, leading to an increase in the percent reduction. Over the duration of the incubation period, the biochar pores were also coated with microbial biomass, thereby reducing its effectiveness (Figure 3).
Table 2 Normalized mass and volume of hydrogen sulfide (H2S) removed per gram of biochar added into the reactor, and percent reductions in comparison to dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested concentrations (0.1, 0.5, 1, 1.82 g/g manure TS) are used to differentiate each treatment.
|
Treatment |
H2S volume reduction (uL) |
Normalized H2S reduction (mg H2S/g biochar) |
Percent Reduction (%) |
|
CSB-0.1 |
17.87 |
0.45 |
14 |
|
CSB-0.5 |
65.43 |
0.33 |
53 |
|
CSB-1 |
94.08 |
0.24 |
76 |
|
CSB-1.82 |
112.6 |
0.16 |
91 |
|
MB-0.1 |
32.00 |
0.81 |
26 |
|
MB-0.5 |
74.91 |
0.38 |
61 |
|
MB-1 |
95.89 |
0.24 |
78 |
|
MB-1.82 |
111.0 |
0.16 |
90 |
Figure 3. SEM image of biochar after digestion showing microbial biomass layers on the biochar surface and pores
The importance of solution pH is an important factor determining the adsorption of different ions on the biochar surface. The pH of the biochar amended treatment solutions stayed constant with little variation after (7.18 – 7.28) after the incubation period. The zeta potential results showed that pHpzc < 2 for CSB and 2.35 > pHpzc > 2.03 for MB. Below the pHpzc, the functional groups present on the biochar surface are protonated and provides an overall positive charge to the biochar surface (Tan et al., 2015). pH values higher than pHpzc favor the adsorption of positively charged contaminants due to electrostatic attraction by the negative charge on the biochar surface. The presence of metal component traces in the biochar provided microsites with a positive charge that may have aided the precipitation of sulfur on the biochar surface.
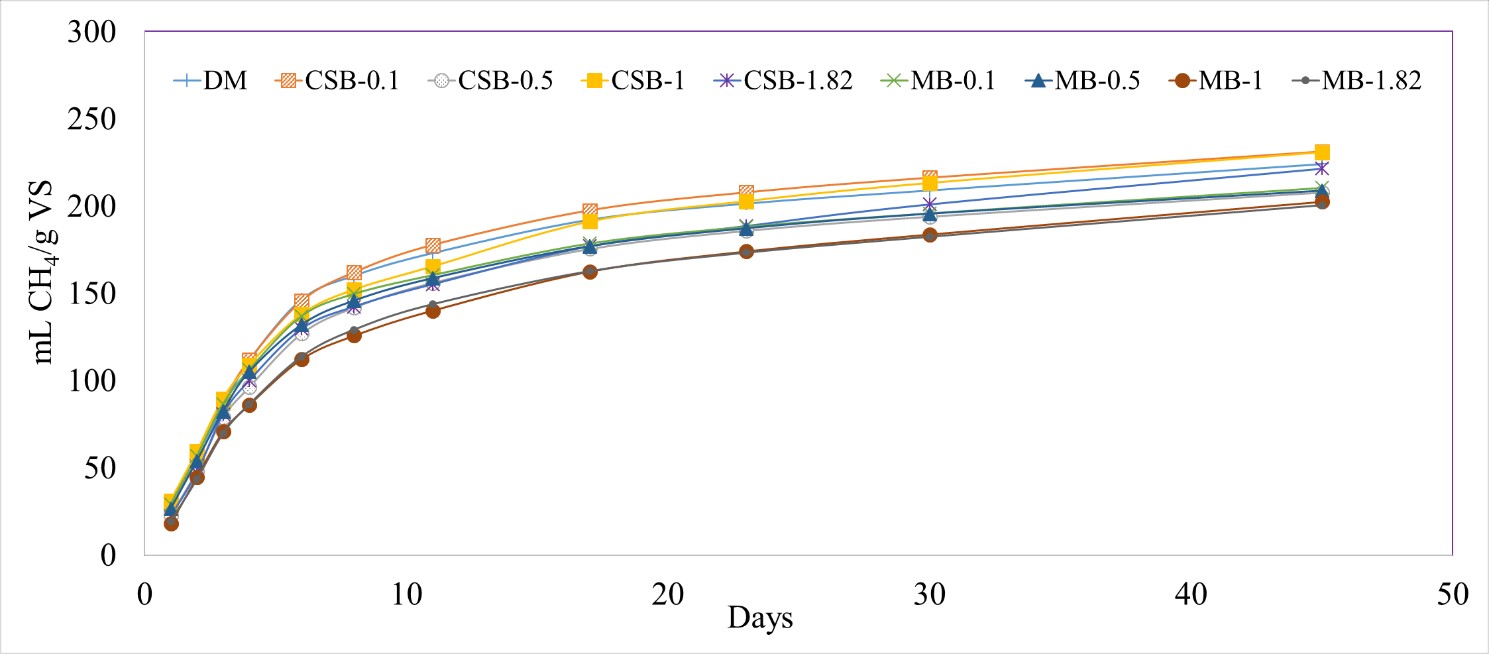
Addition of different biochar concentrations into the reactors did not lead to any significant differences between the treatments in terms of CH4 production (0.0801 < p-value < 1.000). The cumulative methane production varied between 231 ± 6 mL/g VS (CSB-0.1) and 201 ± 2 ml/g VS (MB-1.82) when normalized by the grams of VS added (Figure 4).
Figure 4 Cumulative methane (CH4) production normalized by grams of volatile solids (VS) with different biochar concentrations, with dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested concentrations (0.1, 0.5, 1, 1.82 g/g manure TS) are used to differentiate each treatment.
Nitrogen and Phosphorus results:
The addition of different biochar concentrations did not significantly affect the NH4-N (p-value = 0.3640) and dissolved P (p-value=0.1204) concentrations. The NH4-N concentrations in the biochar amended reactors varied from 143 ± 22 mg/L (CSB-0.1) to 215 ± 46 mg/L (CSB-1.82) with 143 ± 18 mg/L for the DM control. The dissolved P concentrations in the biochar amended reactors varied from 5.62 ± 0.22 mg/L (MB-0.5) to 6.99 ± 0.12 mg/L (CSB-0.1) with 6.21 ± 0.43 mg/L for the DM control (Table 3).
Table 3. Ammonium nitrogen and dissolved phosphorus (P) in the post-digested biochar treated treatments in comparison to dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested concentrations (0.1, 0.5, 1, 1.82 g/g manure TS) are used to differentiate each treatment.
|
Treatment |
Ammonium N (mg/L) |
Dissolved P (mg/L) |
|
DM |
143 ± 18 |
6.21 ± 0.43 |
|
MB-0.1 |
179 ± 4.2 |
6.50 ± 0.10 |
|
MB-0.5 |
201 ± 23 |
5.62 ± 0.22 |
|
MB-1 |
172 ± 15 |
5.78 ± 0.57 |
|
MB-1.82 |
215 ± 46 |
5.59 ± 0.33 |
|
CSB-0.1 |
143 ± 22 |
6.99 ± 0.12 |
|
CSB-0.5 |
172 ± 13 |
5.96 ± 0.41 |
|
CSB-1 |
148 ± 19 |
6.71 ± 0.49 |
|
CSB-1.82 |
145 ± 34 |
6.56 ± 0.31 |
Several authors have conducted studies on the use of biochar for NH4-N and PO43- removal from aqueous solutions. Hou et al. (2016) showed that NH4+ ions were adsorbed onto the biochar surface due to ion exchange and the best results were seen at pH values ranging from 7-9 (Hou et al., 2016). However, most of these studies were conducted on single component systems with no competing ions interacting with each other and the biochar surface. Dairy manure contains multiple cations, anions and organic matter in the system, and it is likely that the species with the highest affinity to the binding sites would be preferentially captured. The presence of organic matter in the manure also contributed to steric hindrance for the NH4+ and PO43- ions, in addition to competition with HS- ions for the available binding sites (Villar da Gama et al., 2018). SEM images (Figure 3) of the biochar samples after incubation showed layers of microbial biomass on the surface of the biochar that could also have prevented access to the binding sites for NH4+ and PO43- ions. Liu et al. (2010) showed that the presence of zinc, aluminum, bicarbonate and phosphate ions directly reduced the NH4+ adsorption capacity of the adsorbent (Liu et al., 2010). Kizito et al. (2015) conducted experiments on using biochar to remove NH4+ from swine manure digestate and found that the presence of most metal cations (K, Ca, Mg, Fe, Zn, etc.) negatively affected the sorption capacity of the biochar due to competition for active binding sites (Kizito et al., 2015). Similarly, phosphate ion adsorption has been shown to be affected by the presence of chloride ions and high concentrations of bicarbonate ions, leading to precipitation inside the biochar pores and adsorption sites (Fang et al., 2014; Yin et al., 2017). The N and P results obtained from the current study suggest that biochar addition into an anaerobic digester may not be effective at reducing NH4-N concentrations due to the presence of interfering cations and anions.
Effect of biochar particle size (Objective 1: Experiment 2):
Different biochar particle sizes significantly lowered the normalized H2S volume compared to DM control and AC-S (p-value <0.0001). The normalized H2S production for the biochar treated reactors varied from 519 ± 24 (MB-S) to 675 ± 23 (CSB-M) mL H2S/kg VS (Figure 5). Even though the differences in H2S production between the biochar treated reactors were not significant, MB had a slightly higher percent H2S reduction than the corresponding CSB particle sizes, similar to the results seen in the first batch test. The mid-range particle sized biochar (CSB-M and MB-M) had the lowest treatment efficiencies (26% for CSB-M and 29% for MB-M, compared to DM control). Overall, the added biochar led to a 26 to 43% reduction in total H2S volume when compared to DM digestion (Table 4). A trend of lowered H2S percent reductions over time was also observed in this batch study, with 57 to 96% reductions from Day 1 to 2 and decreasing to 13 to 32% reduction by Day 21.
Figure 5 Cumulative hydrogen sulfide (H2S) production normalized by kilograms of volatile solids (VS) with different biochar particle sizes, with dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested patricle sizes (L,M,S) are used to differentiate each treatment.
Table 4 Normalized mass and volume of hydrogen sulfide (H2S) removed per gram of biochar added into the reactor, and percent reductions in comparison to dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested patricle sizes (L,M,S) are used to differentiate each treatment.
|
Treatment |
H2S volume reduction (uL) |
Normalized H2S reduction (mg H2S/g biochar) |
Percent Reduction (%) |
|
CSB-L |
246 |
0.50 |
35 |
|
CSB-M |
185 |
0.37 |
26 |
|
CSB-S |
237 |
0.48 |
34 |
|
MB-L |
321 |
0.65 |
43 |
|
MB-M |
221 |
0.44 |
29 |
|
MB-S |
292 |
0.59 |
40 |
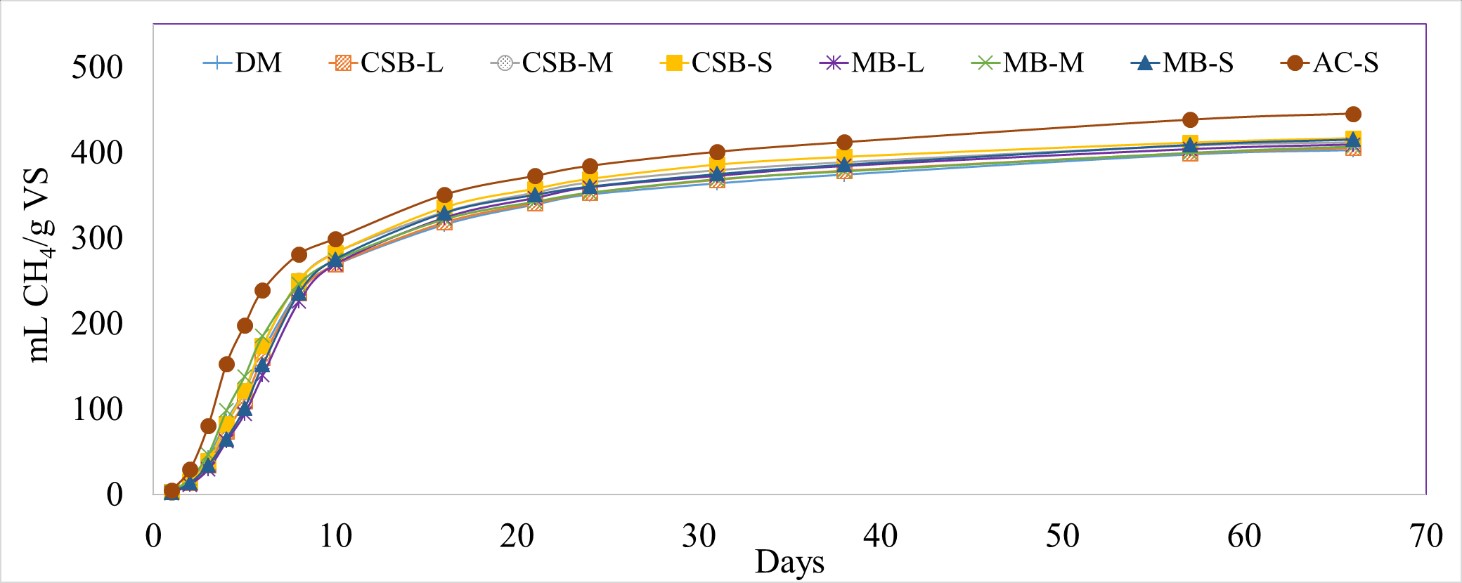
Different particle sizes of biochar did not significantly impact the CH4 production (0.8668 < p-value < 1.000) (Figure 6). However, addition of activated carbon led to a significant increase (10.7%) in the normalized CH4 production (445 ± 3.15 mL CH4/g VS) compared to DM (402 ± 3.42 mL CH4/g VS) (p-value = 0.0082). Previous studies have attributed this enhancement in CH4 production to direct interspecies electron transfer (DIET) (Park et al., 2018; Zhao et al., 2018). Additionally, the AC treatment also led to a significantly higher CH4 production rate (48.2 ± 5.01 mL CH4/day) compared to DM (30.9 ± 4.06 mL CH4/day) from Days 2 – 5 (p-value = 0.0069).
Figure 6 Cumulative methane (CH4) production normalized by grams of volatile solids (VS) for different biochar particle sizes, and dairy manure (DM). Corn stover biochar (CSB) and maple biochar (MB) coupled with the different tested patricle sizes (L, M, S).
Effect of biochar surface modification (Objective 1: Experiment 3):
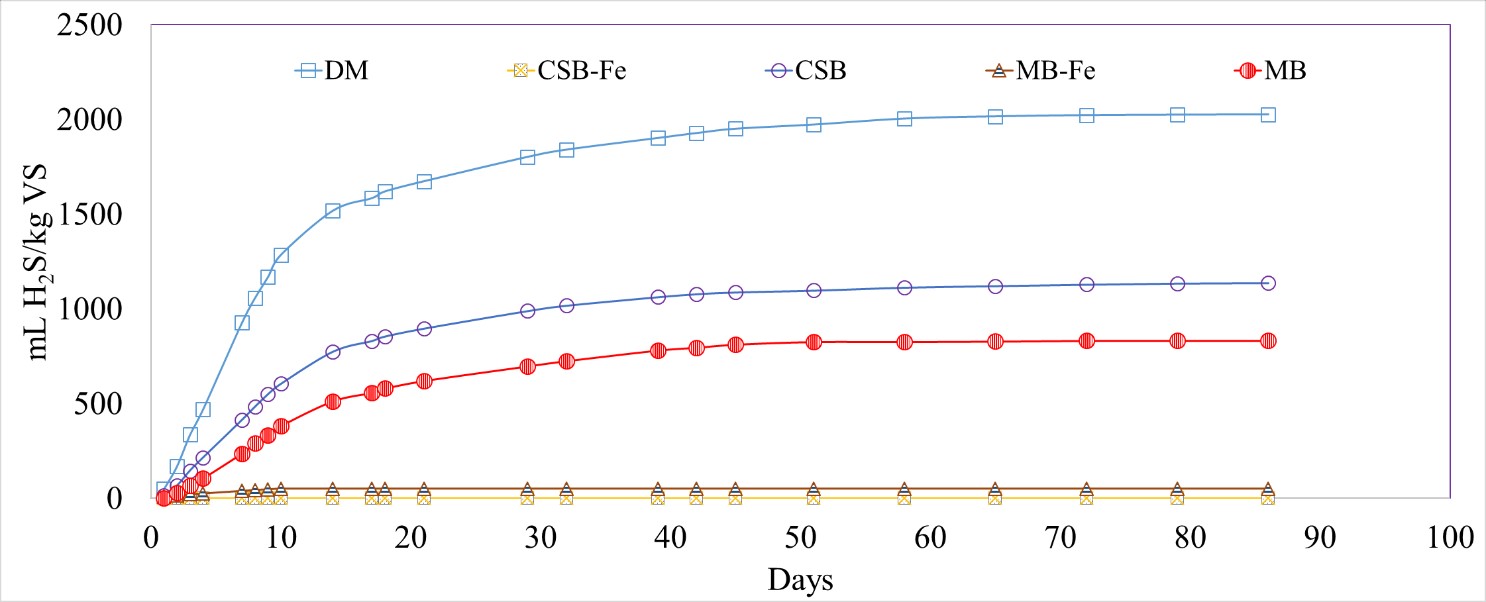
Iron-impregnated biochar (CSB-Fe and MB-Fe) led to a significant reduction in the normalized volume of H2S produced compared to unmodified biochar. The normalized H2S production for the Fe-impregnated biochar treated reactors varied from 0 to 51.3 ± 3.7 mL H2S/kg VS for CSB-Fe and MB-Fe, respectively, compared to DM-only (2025 ± 33 mL H2S/kg VS), with removal efficiencies of 100% and 97%, respectively (Figure 7; Table 5). The Fe-impregnated biochar substrates exhibited a constant effectiveness of H2S reduction over time, while the effectiveness of the unmodified biochars decreased over time.
Figure 7. Cumulative hydrogen sulfide (H2S) production normalized by volatile solids (VS) for dairy manure (DM), unmodified and Fe-impregnated corn stover biochar (CSB, CSB-Fe) and maple biochar (MB, MB-Fe).
Table 5. Normalized mass and volume of hydrogen sulfide (H2S) removed per gram of unmodified and Fe-impregnated corn stover biochar (CSB, CSB-Fe) and maple biochar (MB, MB-Fe) added into the reactor and percent reductions in comparison to dairy manure (DM).
|
Treatment |
H2S volume reduction (uL) |
Normalized H2S reduction (mg H2S/g biochar) |
Percent Reduction (%) |
|
CSB-Fe |
2002 |
2.69 |
100 |
|
CSB |
960 |
1.30 |
44 |
|
MB-Fe |
1930 |
2.59 |
97 |
|
MB |
1292 |
1.74 |
59 |
The sorption of anions on the surface of modified biochar has been attributed to chemical adsorption or electrostatic attraction to the positively charged metal oxide particles embedded on the surface (Sizmur et al., 2017). Surface area and selectivity for cations and anions can be changed by the activation (chemical or physical) or surface modification of biochar, which can affect sorption of different pollutants. The high surface area of biochar was ideal for embedding Fe-particles that provided a positive charge and chemical properties to increase H2S capture. Metal-impregnated biochar-based composites have been shown to remove negatively charged anions from aqueous solutions (Sizmur et al., 2017; Yao et al., 2013).
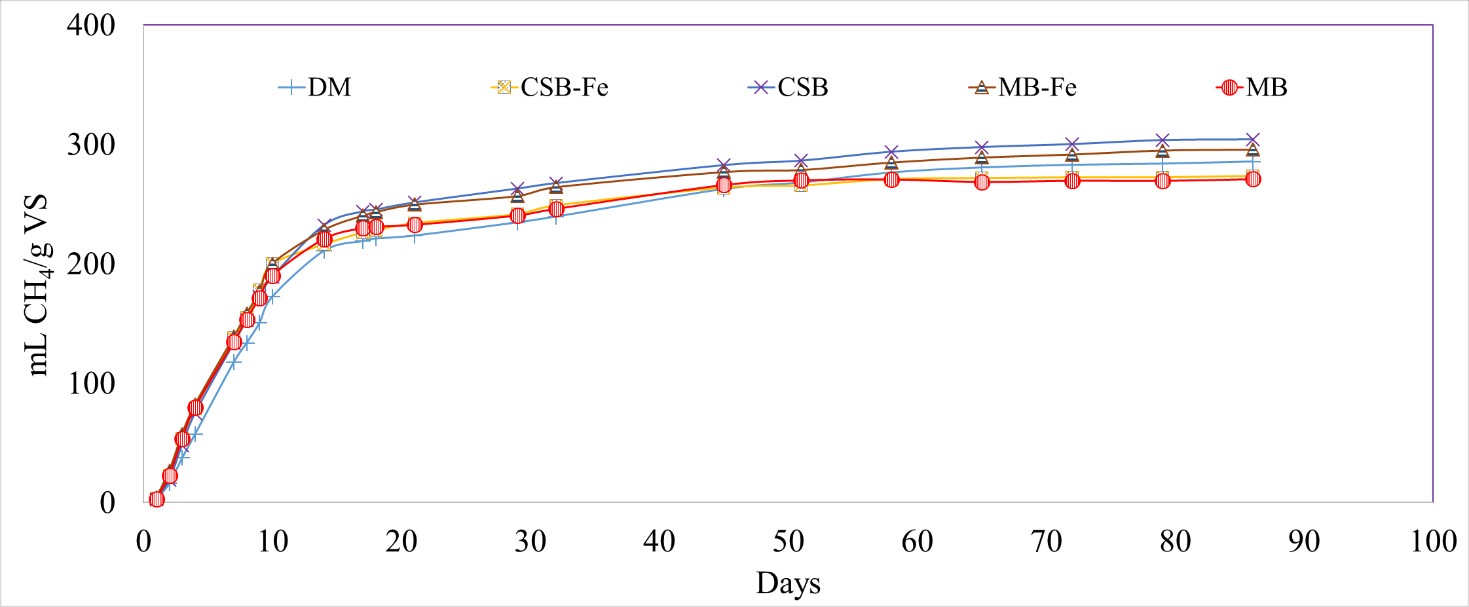
Surface modification affected the conductivities of the biochar, however, it was four orders of magnitude lower than biochars prepared at >700 °C seen in other studies (18.3 µS/cm for modified CSB and 44.7 µS/cm for MB). The change in the conductivities did not significantly impact the CH4 production for the Fe-impregnated biochar treatments (273 ± 6 and 296 ± 11 mL CH4/g VS for CSB-Fe and MB-Fe, respectively) compared to the DM-only treatment (285 ± 17 mL CH4/g VS; p-value = 0.7860, 0.8594) (Figure 8). All three experiments showed no significant differences on the rate and total CH4 production due to biochar addition.
Figure 8 Cumulative methane (CH4) production normalized by volatile solids (VS) for dairy manure (DM), unmodified and Fe-impregnated corn stover biochar (CSB, CSB-Fe) and maple biochar (MB, MB-Fe).
H2S adsorption capacity in a scrubbing system (Objective 2):
The breakthrough curves for the four biochar types and activated carbon are shown in Figure 9. The total saturation time and adsorption capacities of each biochar and activated carbon are shown in Table 6. The breakthrough time was highest for MB-Fe (210 mins), while it was less than 10 mins for CSB. The adsorption capacity also followed a similar pattern, with MB-Fe having the highest sorption capacity for sulfur (23.9 mg S/g biochar), and CSB having the lowest (3.28 mg S/g biochar). Iron impregnation increased the sorption capacity by a factor of 2.5 for CSB-Fe (8.16 mg S/g biochar) compared to CSB and by a factor of 3.9 for MB-Fe compared to MB (6.13 mg S/g biochar).
Figure 9 Breakthrough curves for H2S adsorption on raw and iron-impregnated corn stover biochar (CSB and CSB-Fe), maple biochar (MB and MB-Fe) and activated carbon (AC), where C/C0 is the ratio of the outlet to the inlet H2S concentration.
Table 6. Saturation time and adsorption capacity of the different adsorbents tested.
|
Adsorbent |
Saturation Time (min) |
Adsorption capacity (mg S/g) |
|
Corn stover biochar (CSB) |
240 |
3.28 |
|
Maple biochar (MB) |
300 |
6.13 |
|
Iron-impregnated corn stover biochar (CSB-Fe) |
540 |
8.16 |
|
Iron-impregnated maple biochar (MB-Fe) |
840 |
23.9 |
|
Activated carbon (AC) |
300 |
5.42 |
Iron impregnation led to a 1.5 to 4.4 factor increase in the H2S adsorption capacity of the biochars compared to AC. While the AC had a much larger surface area (> 1000 m2/g) compared to both unmodified (23.5 m2/g for CSB and 161 m2/g for MB) and Fe-impregnated biochar (34.2 m2/g for CSB-Fe and 59.8 m2/g for MB-Fe), it did not have a higher adsorption capacity. This result validates that even though surface area is an important parameter, it is not the most important factor for H2S adsorption in carbon-based adsorbents and highlights the importance of reactive oxygen or oxides on the biochar surface for the catalytic oxidation of H2S. Sun et al. (2016) reported that the removal of H2S by biochar is not controlled by the pore filling physisorption process that is commonly used to determine surface area by N2 adsorption on the adsorbent (Sun et al., 2016).
In our study, the confirmation of the presence of Fe3O4 in CSB-Fe and MB-Fe highlighted the importance of reactive oxides on the biochar surface, as a multifold increase in H2S adsorption capacity was observed, likely due the chemical oxidation via redox reactions of the reactive oxides with H2S (Wallace et al., 2017). The iron oxides from the impregnation process were primarily deposited on the biochar surface and pores and significantly affected the H2S adsorption capacity. The quantity of iron oxide composites on the surface of CSB-Fe (12,200 ppm) was 57% lower than the amount deposited on MB-Fe (28,600 ppm) and led to a 66% decrease in the adsorption capacity of CSB-Fe compared to MB-Fe. Huang et al. (2006) conducted similar studies on Cu-impregnated activated carbon and found that increasing the Cu content from 16,000 ppm to 40,000 ppm resulted in an increase in H2S adsorption capacity from 20.3 mg S/g AC to 46.4 mg S/g AC (Huang et al., 2006).
The oxides in MB and CSB were mostly in the form of quartz SiO2, a very stable, non-porous and unreactive oxide and most likely did not take part in the oxidation process of H2S to elemental sulfur or sulfates. It is also important to note that the inherent iron content of the biochar was not a factor in H2S adsorption in CSB and MB, as CSB had five times more Fe content (5,500 ppm) and yet, had a lower H2S adsorption capacity.
Bamdad et al. (2017) stated that since biochar has a heterogeneous surface with many different functional groups, it is complicated to predict a suitable mechanism for the adsorption of acidic gases on the biochar surface (Bamdad et al., 2018). They stated that the original mechanism proposed by Adib et al. (1999) for activated carbon is likely the same for adsorption of acidic gases on biochar, with differences created by the presence of alkali metals and basic functional groups in biochar (Adib et al., 1999). Sun et al. (2016) compared the H2S adsorption performance of biochar to AC and found that adsorption capacity of biochar (70 mg S/g biochar) was 3.7 times higher than AC (19 mg S/g AC) (Sun et al., 2016). Ciahotný et al. (2019) also reported that H2S adsorption on AC is a physical process that takes place mostly because of van der Waals force interactions, and hence, leads to a lower adsorption capacity compared to biochar (Ciahotný and Kyselová, 2019). Both biochars used in our study had higher metal concentrations compared to AC that should have aided in the process of chemical oxidation in addition to the physical adsorption process. In addition, CSB had higher alkaline metal (Ca and K) concentrations compared to both MB and AC. However, the H2S adsorption capacity of CSB in the current study was 39.5% and 46.5% lower than AC and MB, respectively. It is possible that most of the metals in CSB were embedded in the carbon matrix of the biochar that prevented access to the sulfide molecules on the biochar surface. Another reason could be the chemical form of K and Ca on the biochar surface. The XRD results showed that K was primarily present as KCl and Ca as CaCO3. KCl, being a neutral salt, would not participate in an acid-base reaction with H2S. On the other hand, CaCO3 can participate in an acid-base reaction with H2S, but it cannot catalytically oxidize H2S to elemental sulfur/sulfate like its oxide form (CaO), and once it is exhausted further reaction with H2S is not possible (Wallace et al., 2017).
The study showed that biochar can be used in-situ to reduce H2S concentrations in an anaerobic digestion system. The study showed an increasing trend in the percent reduction of H2S as the biochar concentrations increased, with the highest tested concentration showing > 90% H2S removal. Differences in biochar particle size had no significant impact in the H2S removal efficiency. Iron impregnation resulted in 97 – 100% H2S removal at 73% lower concentrations for both biochars in the study. Iron impregnation significantly increased the H2S adsorption capacity of biochar by a factor of 1.5 to 4.4 compared to activated carbon when used in an external scrubbing system. Adding biochar directly into the digester was not as effective at removing H2S when compared to using it in a scrubbing column due to the presence of competing species inside the digester. While previous studies have shown that biochar can remove NH4+ and PO43-, the biochar was not effective with the dairy manure substrate due to competition among all the other compounds present in the digester. Additionally, the formation of a microbial biofilm on the biochar surface over time decreased its effectiveness for H2S removal inside the digester. The adsorption capacity for the Fe-impregnated biochar added inside the digester likely had additional H2S absorption capability, as it was not completely saturated before H2S production declined to negligible concentrations in the digestion system, which operated in batch mode. A continuous anaerobic digestion system with stable H2S production could have helped determine the maximum H2S adsorption capacity of the Fe-impregnated biochar added inside a digester environment. Future studies should further investigate the H2S removal capacity of biochar and its effect on lag times in AD systems with substrates not acclimatized to the inoculum source.
Education & Outreach Activities and Participation Summary
Participation Summary:
Preliminary results on the effect of different biochar concentrations on biogas desulfurization were presented at the US Biochar Initiative (USBI) conference held at Wilmington, DE (Aug 20 - 23, 2018), USBI conference 2018. USBI-conference-2018-Abhinav-Choudhury-23
The results on the effect of particle size distribution was presented at the annual American Ecological Engineering Society conference, held at Houston, TX (June 12 - 14, 2018). Poster-Abhinav-AEES-2018-Conference-color-21
The results from the entire study on using biochar as a digester additive for H2S control was presented at the Department of Environmental Science and Technology Graduate Seminar at College Park, MD on May 5th, 2019. ENST-Exit-Seminar-2019-Abhinav-Choudhury (2)The audience present at the conferences and seminars included academics, students, producers, anaerobic digestion practitioners and consultants working with farmer owners with anaerobic digester systems on their farms. The results were disseminated with the people present at the conference during networking events. The studies formed a major part of the graduate student’s dissertation and two manuscripts are currently in preparation for publication in peer reviewed journals, which will be submitted by December 2019.
Multiple follow-up correspondence and meetings were also held with government officials and consultants in Vermont and Virginia, who were interested in this research and its applicability in the future. Government officials were interested in the future potential for biochar, including modification of the surface, for lowering H2S. Phone conversations were held where topics such as funding sources, connections to researchers and sharing research on biochar, and trials on Vermont digesters for N and P capture, improved biogas production and creating a market for low-value wood were discussed. There was interest in this research from Vermont consultants trying to create a market for biochar prepared from anaerobically digested dairy manure solids. Discussions on current advances in biochar applications and providing assistance in determining a possible market value to make biochar production profitable was held over phone calls. Waste management officials in the county of Vienna, VA were interested in H2S control due to the problems associated with odors in sewage systems and conversations about possible solutions, including the use of biochar, and these items were discussed. In addition, we collaborated and helped train a Fulbright scholar from Nigeria who incorporated biochar into their study on anaerobic digestion of cassava waste. The scholar is currently working on preparing a manuscript for publication in a peer reviewed journal.
Project Outcomes
It is expected that biochar will play an important role in anaerobic digestion by promoting a stable AD process, improving soil characteristics when used as a soil amendment, and acting as a means of H2S control. We hope that the project results lead to more investigations into the use of biochar as a low-cost alternative and more sustainable solution to H2S scrubbing when compared to bioscrubbers and iron sponge adsorbents. This particular project did not work directly with farmers, but we had meetings with consultants currently working on pilot scale research on biochar production from digested manure solids in Vermont in collaboration with farmers.
Our work on this project resulted in working collaborations and consultations with practitioners interested in biochar and its uses and opened up dialog between researchers on the need to understand the interaction of biochar with different chemical species. Presentation of our results at national conferences and conversations with other biochar researchers has highlighted the importance of biochar characterization. Characterization is extremely important for understanding how biochar functions and future applications of biochar due to its heterogeneous nature and inherent variability.
Our understanding of biochar interactions has led to more conversation and/or research in its use for phosphorus capture, preparation of biochar from digested manure solids, and incorporation of nanoparticles into the biochar surface to enhance its adsorption capacities. We were also able to gain a preliminary understanding of biochar and its effect on co-digestion of cassava waste with manure from the collaborative work with the Fulbright scholar.
The PhD student on this project is currently interested in pursuing waste management and waste to energy technologies in the industry, and would like to further explore the potential for biochar to play an important role in a multitude of study areas including environmental remediation, adsorption, energy production, biotechnology, among others.
Future studies should further investigate the H2S removal capacity of biochar and its effect on lag times in AD systems with substrates not acclimatized to the inoculum source. In addition, co-digestion of organics with manure has the capability to significantly enhance methane production but high loading rates can lead to digester instability. There has been limited research on biochar stabilizing the anaerobic digestion process and biochar amended digesters that co-digest manure and organics with a focus on H2S production. Preliminary understanding of co-digestion of cassava waste with manure can help aid future studies on this topic for areas where cassava waste has a high environmental impact on surrounding waterways. Further studies should be also conducted on the impregnation of other transition metals (nickel, zinc, etc.) and its effect on H2S adsorption and the regeneration capabilities of biochar (for reuse in scrubbing columns) to further enhance its adsorption efficiency and lower its cost as an adsorbent.
Information Products
- Effect of biochar addition on H2S production in an anaerobic digester (Conference/Presentation Material)
- Renewable biochar for hydrogen sulfide and nutrient reduction in anaerobic digesters (Conference/Presentation Material)
- Evaluation of biochar for biogas desulfurization in dairy manure digesters (Conference/Presentation Material)