Final report for GNE18-171
Project Information
Dairy cattle health and physiology is challenged when animals are exposed to high ambient temperature (i.e., heat stress). In attempts to increase evaporative cooling, animals divert the blood from visceral organs towards the body periphery. This in turn causes disturbances in the small intestine epithelium and directly affects tight junction proteins, which allows for bacterial translocation into the circulation and cause inflammation. This condition is commonly called ‘leaky gut’, which is characterized by heightened levels of bacteria-derived endotoxin in blood. The rise in circulating endotoxin and increase in intestinal permeability may explain why heat-stressed cows are at risk of experiencing hepatic steatosis and inflammation. Unfortunately, these detriments may compromise cow health, fertility, and milk production; however, our understanding of this interplay in dairy cows experiencing leaky gut is profoundly inadequate. Moreover, the dairy industry demands dietary therapies which increase nutrient absorption and intestinal barrier to combat leaky gut. Thus, we hypothesized that heat stress triggered gut dysbiosis and heightened intestinal permeability. Moreover, we considered the possibility that rumen-protected organic acid and plant botanicals (OA/PB) supplementation may restore the intestinal barrier, lower circulating endotoxin, and improve productive performance in cows challenged by heat stress. To test our hypothesis, we enrolled forty-five mid-lactating multiparous pregnant Holstein cows in a complete randomized design study. Cows were housed in temperature and humidity controlled environmental chambers with tie-stalls at the Cornell Large Animal Research and Teaching Unit (LARTU). Following acclimation (7-d), cows were assigned to one of four treatments (14-d): (i) thermoneutral or (ii) heat stressed conditions, (iii) pair-fed in thermoneutral conditions to match the intake of heat stressed cows, or (iv) heat stressed with supplemental rumen-protected organic acids and plant botanicals (OA/PB; AviPlus R® at 75 mg/kg of body weight [BW] per d; contains 25% citric acid, 16.7% sorbic acid, 1.7% thymol, 1% vanillin and 55% lipid encapsulate [for rumen protection]; Vetagro) as a top-dress. Unsupplemented cows not receiving OA/PB were supplemented with an equivalent level of lipid encapsulate. For thermoneutral conditions, daily ambient temperature was maintained at 22 to 23 °C. For heat stress conditions, ambient temperature increased at 0600 h from 28 to 37°C and decreased at 1800 h from 37 to 28°C. Cows were milked twice daily at 0600 and 1600 h. Daily feed and water intakes, milk, blood, and fecal swabs were collected throughout the trial. Although we are still processing the fecal swabs and intestinal permeability assay in the lab, we were able to complete our objective of evaluating OA/PB supplementation as a nutritional therapy for heat stress. We found that OA/PB tended to sustain a higher feed intake and total volume of milk produced, improved milk fat and protein yields, and improved energy-corrected and 4% fat-corrected milk. And although OA/PB did not modify blood markers when compared to unsupplemented heat stressed cows, the lactation performance improvements may already justify the use of such nutritional therapy. Such applications have the potential to reduce economic losses to favor sustainability, especially when we consider the projected negative impact of climate change on the northeast dairy industry.
Objective 1: Determine whether rumen-protected OA/PB supplementation prevents endotoxemia and liver injury in lactating dairy cows experiencing leaky gut. Rationale: Leaky gut increases circulating endotoxin to promote hepatic steatosis, inflammation, and activation of the acute phase response. Feeding rumen-protected OA/PB may reduce intestinal permeability to minimize these outcomes. Therefore, dietary OA/PB supplementation may be a practical approach to improve gut and liver health in dairy cattle.
Objective 2: Define the gut-liver axis in lactating dairy cows experiencing leaky gut. Rationale: The gut-liver axis involves the complex interplay between the gut microbiota, intestinal permeability, systemic endotoxemia, and liver health. In non-ruminants, microbial dysbiosis promotes leaky gut and compromises health, and heat stress modulates the gut microbiome in broilers. Therefore, heat stress likely changes the bovine gut microbiome in dairy cows experiencing heat stress and leaky gut.
Heat stress negatively impacts health and well-being of dairy cattle and represents a major concern for dairy production systems (West, 2003). The physiological response to heat stress is characterized by decreased feed intake, increased sweating and respiration rates, and elevated body temperature (Collier et al., 1982). These changes contribute to increases in maintenance energy costs that can range from 25 to 30% (Fox and Tylutki, 1998). Redirection of blood supply from the visceral organs (i.e., intestines) towards the body periphery also occurs (Hall et al., 1999). This provokes paracellular permeability and tight junction opening (Lambert, 2009), which may promote intestinal permeability and leakage of bacteria and their endotoxin into the circulation to stimulate local and systemic immune responses (Ghosh et al., 2020). Specifically, enhanced uptake of endotoxin by the liver promotes triglyceride accumulation, inflammation, and activates the acute phase response, which is a component of the innate immune system. We hypothesize that heat stress promotes dysbiosis of the dairy cow gut microbiota while enhancing intestinal permeability, endotoxemia, and liver injury. Moreover, we consider the possibility that rumen-protected OA/PB supplementation may restore the intestinal barrier, lower circulating endotoxin, and improve hepatic health in cows challenged by hyperthermia, which combined could be translated in improved health and performance. To test our hypotheses, we enrolled 45 multiparous pregnant and lactating Holstein dairy cows in a complete randomized design trial. Cows were housed in environmentally controlled chambers, which allowed us to create thermoneutral or heat stress conditions. Our experimental design also included a pair-fed control housed in thermoneutral conditions with intake matched to cows experiencing heat stress. Such an approach accounted for effects associated with changes in intake. Plasma and serum metabolites (i.e., fatty acids, glucose, triglycerides, cholesterol, serum amyloid A) were quantified to evaluate metabolic and health status. Regarding other important measurements, we will utilize a contemporary omics approach to characterize the fecal microbiome. Although leaky gut predominantly develops in the small intestine, we emphasize that the fecal microbiota is an adequate reflection of the intestinal microbiome (Barko et al., 2018). To measure intestinal permeability, we will quantify circulating levels of chromium (Cr) after an in vivo challenge using Cr-EDTA which can be used as a proxy of intestinal barrier dysfunction. Collectively, these approaches will allow us the ability to characterize the gut-liver axis during heat stress and OA/B supplementation.
Research
Experimental design and treatments
This study was conducted at the Cornell Large Animal Research and Teaching Unit (Ithaca, NY). All experimental procedures were approved by the Cornell University Institutional Animal Care and Use Committee (protocol #2018-0110). In blocks of 8, a total of forty-five multiparous pregnant and lactating Holstein cows (3.0 ± 0.5 lactation, 208 ± 4.6 DIM, 122 ± 4.9 DCC, 31.4 ± 0.73 kg MY) were enrolled in a complete randomized design trial. Cows were transported from the Cornell University Dairy Research Center (Hartford, NY) directly to the Cornell Large Animal Research and Teaching Unit for facility acclimation in thermoneutrality for 7-d (22.2 ± 0.25 °C; 44.9 ± 5.97 % relative humidity [RH], 68 ± 0.32 temperature humidity index [THI]). Following acclimation, cows were randomly assigned and subjected to environmental condition as follows: thermoneutrality and unsupplemented (TN-Con, n = 12), heat stressed and unsupplemented (HS-Con, n = 11), thermoneutrality and unsupplemented but pair-fed to match the intake of the heat-stressed and unsupplemented cows (TN-PF, n = 11), and heat-stressed and supplemented with organic acids and plant botanicals (75 mg/kg of BW; HS-OAPB, n = 10) for 14 d. The OA/PB supplement was composed of 25% citric acid, 16.7% sorbic acid, 1.7% thymol, 1.0% vanillin, and 55.6% triglyceride (AviPlus R®; Vetagro SpA, Italy). All cows including those on control treatment received an equivalent amount of triglyceride (vegetable lipid encapsulate; Vetagro SpA, Italy). Temperatures for each environment were monitored using HOBO® loggers (model lMX2300; Onset Computer Corporation, Bourne, MA). For thermoneutral conditions, daily ambient temperature was kept at ~22 to 23 °C. For heat stress conditions, ambient temperature increased at 0600 h from 28 to 37°C and decreased at 1800 h from 37 to 28°C. The goal was to maintain a THI of ≤ 68.0 in thermoneutrality and 73 (night) to 83 (day) for heat stress conditioning. Cows were housed in individual tie-stalls bedded with sawdust and provided fresh diets at 0800 h daily. The total mixed ration provided 1.70 Mcal/kg of dry matter and possessed a nutrient composition of 43.9% dry matter, 15.7% crude protein, 65.3 ADF, and 4.7% ether extract. Cows were milked twice daily at 0600 and 1600h.
Data and sample collection
Clinical assessments were performed thrice daily. During these assessments, rectal and skin temperatures, and respiration rates were recorded at 0700, 1200, and 1700 h. Rectal temperatures were measured using a large animal digital rectal thermometer (model GLA M900; GLA Agricultural Electronics, San Luis Obispo, CA). Skin temperatures were measured using a non-contact infrared temperature gun (model 586; Fluke Corp., Everett, WA) on a shaved area on the left flank. Respiration rates were determined by counting flank movements for a 15-s period, then multiplied by 4 to obtain movements per min. Body weights were recorded weekly. Blood was collected in the morning prior to feeding and at the peak of heat in the afternoon (days -2 [baseline sample], 1, 2, 3, 7, 14) by coccygeal venipuncture into evacuated blood tubes, which contained potassium EDTA as an anticoagulant when plasma was collected. Intestinal barrier function was evaluated in vivo using the paracellular permeability marker Chromium (Cr)-EDTA as previously described (Wood et al., 2015; Horst et al., 2020). Beginning at 0700 h (after milking, before feeding) on days 3 and 10 of environmental conditioning a 180 mM solution of Cr-EDTA (1.5 L) was pulse dosed into the rumen. Blood samples were collected at 0, 1, 2, 4, 8, 12, 18, and 24 h relative to Cr-EDTA administration into a royal blue top tube containing K2EDTA (BD, Franklin Lakes, NJ; plasma for trace element analysis). Both plasma and serum samples collected during regular morning and afternoon bleeding regimen and Cr-EDTA challenge were separated using centrifugation (3,200 × g for 20 min). Separated plasma or serum samples were initially stored at -20°C and then transferred to -80°C for long-term storage within 2-wk of collection. At the end of the 14-d period, a subset of cows (n = 6 per treatment) was subjected to a standing laparotomy in which cecal, and duodenal content were collected as well as a duodenal biopsy was performed.
Sample analyses
Feed samples were analyzed by for DM (Goering and Van Soest, 1970; AOAC, 2000), CP (AOAC, 2000), NDF (Van Soest et al., 1991), indigestible NDF (Goering and Van Soest, 1970), ADF (AOAC, 2000), and EE (Thiex, 2008) by Cumberland Valley Analytical Services Inc.
Plasma total fatty acids (FA; HR series NEFA-HR #999-34691, 995-34791, 991-34891, and 993-35191; Wako chemicals USA Inc., Richmond, VA), glucose (Autokit Glucose #997-03001; Wako Chemicals USA, Inc., Richmond, VA), triglycerides (#P803-T7532-01 Triglyceride GPO; Pointe Scientific, Inc., Canton, MI), total and free cholesterol (Cholesterol E #999-02601 and Free Cholesterol E #993-02501; Wako chemicals USA Inc., Richmond, VA), and serum amyloid A (SAA; #TP-802; Tridelta Development Limited Ireland Co., Maynooth, Kildare) were quantified according to manufacturer’s instructions. All spectrophotometric measurements were conducted using a SpectraMax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA).
Plasma samples collected during the Cr-EDTA challenge were submitted to the USDA-ARS Robert W. Holley Center (Ithaca, NY) for analysis of total chromium, using inductively coupled plasma mass spectrometry (ICP-MS; Agilent G 3272 A, Agilent Technologies, Santa Clara, CA). The USDA-ARS lab is currently finishing the ICP-MS analysis.
Ruminal, intestinal, and fecal samples will be analyzed using the shotgun metagenomics in collaboration with Dr. Ilana Brito at the Biomedical Engineering Department at Cornell University. The target completion for these analyses is December 2021.
Calculations and statistical analysis
The THI was calculated according to the equation reported by Kendall et al. (2008): THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T − 26)].
The yields of 4% fat corrected milk (FCM), energy corrected milk (ECM), and milk components were calculated using milk yield and component concentrations for each milking, summed for a daily total, and averaged for each collection period. The efficiency for milk yield, FCM and ECM production were calculated as the ratio of milk yield, FCM or ECM in relation to DMI. Somatic cell score was calculated from SCC for statistical analysis using a logarithmic transformation, where Log2 (SCC/100,000) + 3 (Ali and Shook, 1980).
Statistical analyses were carried out using the mixed model procedure of SAS (v9.4, SAS Institute Inc., Cary, NC, USA) according to the following model:
Yijklmnopq = µ + Ci + Dj + Tk + Rl + Blockm + DIMn + DCCo + LACp + pVarq + eijklmnopq
Where is Yijklmnopq = dependent variable; µ = overall mean effect for the measure; Ci = random effect of cow (i = 1 to 45); Dj = fixed effect of day (j = 1 to 14); Tk = fixed effect of time (k = 1 to 2); Rl = fixed effect of treatment (l = 1 to 4); Blockm = fixed effect of block (m = 1 to 6); DIMn = fixed effect of days in milk; DCCo = fixed effect of days carrying calf; LACp = fixed effect of lactation; pVarq = baseline measurement value (last 4-d of acclimation period) for each response variable used as a covariate; and eijklmnopq = the residual error. The covariance structures used to test fit statistics included variance components, compound symmetry, autoregressive one, unstructured, and ante-dependence one. Smaller fit values (BIC) were always selected.
Observations were deemed as outliers if Studentized residuals > 3.0 or < −3.0, and the effect of their removal was evaluated (≤ 1 per variable). Normality of the residuals was checked with normal probability and box plots and homogeneity of variances with plots of residuals versus predicted values in order ensure no violation of model assumptions. The least square means comparisons were performed using the Tukey-Kramer test. Linear contrasts were performed to evaluate treatment responses between HS-Con vs. TN-Con, HS-Con vs. PF-Con, HS-Con vs. HS-OAPB, and TN-Con vs. TN-PF. Main effects were declared significant at P ≤ 0.05 and trending towards significance at 0.05 < P ≤ 0.15. Interactions were declared significant at P ≤ 0.10, and tendencies were declared at P ≤ 0.15. Results are expressed as least squares means ± SEM, unless otherwise noted.
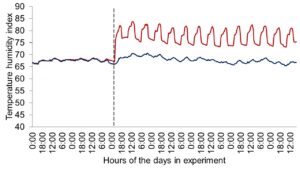
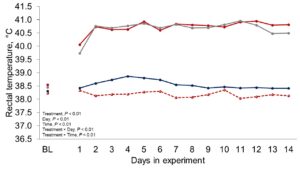
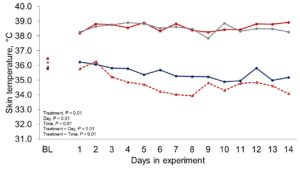
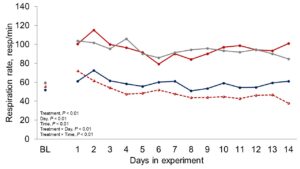
Figure 1 depicts the hourly THI index achieved during the entire trial. Temperature and THI were maintained at 22 °C and 68 during acclimation and thermoneutral conditions and peaked at 37.2°C and 82 for heat stress conditions. The RH fluctuated from 40 to 44% in thermoneutral conditions, and from 25 to 34% in heat stress conditions. Clinical responses of rectal temperatures and respiration rates are depicted in Figures 2-4. Housing lactating dairy cows in heat stress conditions for 14 d markedly increased rectal (40.3, 40.2 vs. 38.4 and 38.1°C; P < 0.01) and skin (38.2, 38.0 vs. 35.2 and 34.6°C; P < 0.01) temperatures, as well as respiration rates (92, 90 vs. 58 and 48 resp/min; P < 0.01) of cows grouped in heat stress conditions (HS-Con, HS-OAPB, respectively) compared to cows housed in thermoneutrality (TN-Con and TN-PF, respectively). These results were expected, because increased respiratory rates and elevated body temperatures are classical physiological adaptations caused by heat exposure in mammals (Robertshaw, 1985). Furthermore, these may serve as a proof of concept for our heat stress model.
Table 1 and Table 2 summarize the least square means for production performance and contrasts of interest, respectively. The exposure to heat stress and pair-feeding decreased body weight in comparison to cows maintained in thermoneutrality and fed ad libitum. However, heat stressed cows supplemented with OA/PB lost less body weight when compared to both HS-Con and TN-PF cows. As expected, heat stress and pair feeding reduced DMI. This is an expected result, as one of the main coping strategies that cows undergo during periods of heat exposure is the reduction in feed consumption (Baumgard and Rhoads, 2013). Overall, heat exposure negatively impacted lactating performance. However, it is interesting to note that OA/PB supplementation during heat exposure seemed to improve performance when compared to the unsupplemented cows (HS-Con). Cows supplemented with OA/PB tended to express an increased DMI (14.8 vs 13.4 kg/d, P = 0.14). This response was previously reported in dairy calves exposed to chronic heat stress (Fontoura et al., 2020). The concept of feeding acidifiers, such as OA/PB, to improve gut health and growth has been explored in non-ruminants (Sofos et al., 1985; Roth and Kirchgessner, 1998; Partanen and Mroz, 1999). Their benefits and applications involve improving nutrient digestibility, enhancing immune function, exerting antimicrobial effects against pathogenic bacteria, and increasing growth performance (Pearlin et al., 2020). This becomes of great interest as a nutritional therapy aimed at improving gut health during heat stress exposure in dairy cattle. Furthermore, OA/PB supplemented cows also tended to express higher milk yield and 4% fat corrected milk (P = 0.12 and P = 0.10, respectively), and significantly higher energy corrected milk (P = 0.05) when compared to unsupplemented cows. The observed increased water consumption for these cows may have been one of the supporting mechanisms in which HS-OAPB cows sustained higher milk production opposed to HS-Con cows. Relative to milk solids, OA/PB supplemented cows yielded more lactose and milk protein daily; milk composition was also modified, as HS-OAPB cows tended to have less somatic cells in milk (1.92 vs. 2.32 cells/mL, P = 0.12), which can be used as a proxy for inflammation of the mammary gland (Schultz, 1977). Lastly, HS-OAPB cows had less milk-urea nitrogen (13.5 vs. 15.9 mg/dL, P < 0.01), indicating that nitrogen efficiency was improved when compared to HS-Con cows.
Table 3 and Table 4 summarize the least square means for blood markers and contrasts of interest, respectively. Compared to TN-Con cows, heat stress increased free fatty acid mobilization (P < 0.01). However, it is important to note that the highest mobilization of FA occurred in the TN-PF group and that these levels were greater than HS-Con (459 vs. 349 μmol/L, P = 0.02). This is important because one of the hallmarks of heat stress adaptations in dairy cattle is the restrained capability for body fat mobilization (Baumgard and Rhoads, 2013). Observations of increased concentration of plasma fatty acids in pair-fed controls relative to their heat stressed counterparts have been widely reported in lactating cows (Baumgard and Rhoads, 2013; Wang 2020). Importantly, these changes in plasma fatty acid concentrations come in conjunction with a decrease or tendency for a reduction in glucose levels of HS animals (O’Brien et al., 2010; Yazdi et al., 2016). This is likely a result from glucose sparring mechanism in which TN-PF animals can mobilize body fat to obtain the energy required for basal metabolic functions and lactation. In terms of cholesterol metabolism, heat stress appears to increase the circulation of free cholesterol. This is of great importance, as cholesterol accumulation in the liver may be a contributing factor to fatty liver disease (Kim et al., 2014). Importantly, cholesterol toxicity is mostly associated with esterified cholesterol (Iglesias et al., 1996), and TN-PF cows had remarkable higher levels of this metabolite when compared to all other cows. This is suggestive that feed restriction is as undesirable as heat stress. Regarding OA/PB supplementation, there were no differences in terms of cholesterol circulation. In addition, it is also important to consider that cholesterol serves as a backbone for building bile acids as bile acids aid in cholesterol secretion. Thus, this increase may affect bile acid metabolism and signaling. This is one area in which we will explore at a later stage, as the gut microbiome play an important role in the metabolism of bile acids and may affect host metabolism and health (Li and Chiang, 2020). Surprisingly, heat stress did not change the concentration of serum amyloid A, a positive acute phase protein. This contrasts with previous studies, which showed an increase in this protein during heat stress in murine, swine and cattle models (Leon et al., 2013; Abuajamieh, 2015). In this regard, our study may have imposed some confounding factors that could have triggered an inflammatory process prior to heat stress (i.e., transportation, change from 3X to 2X milking, liver biopsy and anti-inflammatory therapy). To further explore this, we will analyze the levels of lipopolysaccharide-binding protein (LBP) and in conjunction with the results from the Cr-EDTA challenge, we will be able to tease that apart and verify if heat stress alone can increase pro-inflammatory markers (LBP) but also if it induces intestinal permeability in vivo (i.e., leaky gut).
Table 1. Productive performance traits of cows exposed to heat stress or thermoneutral conditions.
|
Variable, unit |
Treatment |
|
P-values |
|||||
|
TN-Con |
TN-PF |
HS-Con |
HS-OAPB |
Treatment |
Day |
Treatment x Day |
||
|
Productive Performance |
|
|
|
|
|
|
|
|
|
Body weight, kg |
684 |
655 |
629 |
640 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Live weight variation, kg/d |
0.23 |
-4.10 |
-5.22 |
-3.83 |
|
< 0.01 |
0.32 |
< 0.01 |
|
DMI, kg/d |
24.8 |
12.8 |
13.4 |
14.8 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Water intake, L/d |
135.2 |
80.6 |
97.2 |
121.6 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Milk yield, kg/d |
34.3 |
25.3 |
22.5 |
25.2 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
4% FCM, kg/d |
39.2 |
30.8 |
26.8 |
29.2 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
ECM, kg/d |
41.0 |
31.9 |
27.4 |
30.2 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Milk solids, kg/d |
|
|
|
|
|
|
|
|
|
Fat |
1.66 |
1.35 |
1.18 |
1.24 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Protein |
1.13 |
0.86 |
0.69 |
0.80 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Lactose |
1.67 |
1.28 |
1.08 |
1.26 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Milk composition, % |
|
|
|
|
|
|
|
|
|
Fat |
4.93 |
5.22 |
5.26 |
4.80 |
|
0.06 |
< 0.01 |
< 0.01 |
|
Protein |
3.02 |
3.06 |
3.29 |
3.23 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
Lactose |
4.86 |
4.78 |
4.79 |
4.84 |
|
0.07 |
< 0.01 |
0.01 |
|
Total solids |
14.1 |
14.1 |
14.0 |
13.6 |
|
0.08 |
< 0.01 |
< 0.01 |
|
Somatic cell count, cells/mL |
2.01 |
2.14 |
2.32 |
1.92 |
|
0.42 |
0.45 |
0.55 |
|
Milk urea nitrogen, mg/dL |
11.5 |
11.7 |
15.9 |
13.5 |
|
< 0.01 |
0.14 |
0.67 |
|
Feed efficiency |
|
|
|
|
|
|
|
|
|
ME, ratio |
1.39 |
1.94 |
1.72 |
1.67 |
|
< 0.01 |
< 0.01 |
< 0.01 |
|
4% FCME, ratio |
2.46 |
2.21 |
1.72 |
1.58 |
|
0.01 |
< 0.01 |
< 0.01 |
|
ECME, ratio |
2.53 |
2.24 |
1.76 |
1.69 |
|
0.01 |
< 0.01 |
< 0.01 |
DMI: dry matter intake, 4% FCM: 4% fat corrected milk, ECM: energy corrected milk, ME: milk efficiency, 4% FCME: 4% fat corrected milk efficiency, ECME: energy corrected milk efficiency.
Table 2. Contrasts of interest for productive performance traits of cows exposed to heat stress or thermoneutral conditions.
|
Variable, unit |
HS-Con vs. TN-Con |
HS-Con vs. TN-PF |
HS-Con vs. HS-OAPB |
TN-Con vs. TN-PF |
|
Productive Performance |
||||
|
Body weight, kg |
< 0.01 |
< 0.01 |
0.17 |
< 0.01 |
|
Live weight variation, kg/d |
< 0.01 |
0.22 |
0.15 |
< 0.01 |
|
DMI, kg/d |
< 0.01 |
0.54 |
0.14 |
< 0.01 |
|
Water intake, L/d |
< 0.01 |
0.05 |
< 0.01 |
< 0.01 |
|
Milk yield, kg/d |
< 0.01 |
0.09 |
0.12 |
< 0.01 |
|
4% FCM, kg/d |
< 0.01 |
0.01 |
0.10 |
< 0.01 |
|
ECM, kg/d |
< 0.01 |
< 0.01 |
0.05 |
< 0.01 |
|
Milk solids, kg/d |
||||
|
Fat |
< 0.01 |
0.01 |
0.34 |
< 0.01 |
|
Protein |
< 0.01 |
< 0.01 |
0.01 |
< 0.01 |
|
Lactose |
< 0.01 |
< 0.01 |
0.01 |
< 0.01 |
|
Milk composition, % |
||||
|
Fat |
0.07 |
0.82 |
0.02 |
0.11 |
|
Protein |
< 0.01 |
< 0.01 |
0.53 |
0.28 |
|
Lactose |
0.04 |
0.75 |
0.11 |
0.02 |
|
Total solids |
0.64 |
0.52 |
0.08 |
0.88 |
|
Somatic cell count, cells/mL |
0.20 |
0.44 |
0.12 |
0.58 |
|
Milk urea nitrogen, mg/dL |
< 0.01 |
< 0.01 |
< 0.01 |
0.81 |
|
Feed efficiency |
||||
|
ME, ratio |
< 0.01 |
< 0.01 |
0.51 |
< 0.01 |
|
4% FCME, ratio |
0.02 |
0.09 |
0.65 |
0.42 |
|
ECME, ratio |
0.01 |
0.08 |
0.81 |
0.33 |
DMI: dry matter intake, 4% FCM: 4% fat corrected milk, ECM: energy corrected milk, ME: milk efficiency, 4% FCME: 4% fat corrected milk efficiency, ECME: energy corrected milk efficiency.
Table 3. Blood markers of cows exposed to heat stress or thermoneutral conditions.
|
Variable, unit |
Treatment |
|
P-values |
||||||||
|
TN-Con |
TN-PF |
HS-Con |
HS-OAPB |
|
Treatment |
Day |
Time |
Treatment x Day |
Treatment x Time |
|
|
|
Free fatty acids, μmol/L |
147 |
459 |
349 |
325 |
|
< 0.01 |
< 0.01 |
0.81 |
< 0.01 |
< 0.01 |
|
|
Glucose, mg/dL |
69.5 |
64.6 |
62.4 |
63.5 |
|
0.07 |
0.02 |
0.54 |
0.01 |
0.02 |
|
|
Triglycerides, mg/dL |
11.0 |
12.0 |
13.0 |
12.0 |
|
0.07 |
< 0.01 |
0.42 |
< 0.01 |
0.01 |
|
|
Total cholesterol, mg/dL |
186 |
212 |
184 |
182 |
|
0.10 |
< 0.01 |
0.75 |
< 0.01 |
0.73 |
|
|
Free cholesterol, mg/dL |
39.2 |
45.3 |
45.3 |
43.9 |
|
0.16 |
< 0.01 |
0.40 |
< 0.01 |
0.15 |
|
|
Esterified cholesterol, mg/dL |
145 |
166 |
138 |
138 |
|
0.04 |
< 0.01 |
0.90 |
< 0.01 |
0.42 |
|
|
Serum amyloid A, ug/mL |
226 |
140 |
192 |
186 |
|
0.14 |
< 0.01 |
- |
0.34 |
- |
|
Table 4. Contrasts of interest for blood markers of cows exposed to heat stress or thermoneutral conditions.
|
Variable, unit |
HS-Con vs. TN-Con |
HS-Con vs. TN-PF |
HS-Con vs. HS-OAPB |
TN-Con vs. TN-PF |
|
Free fatty acids, μmol/L |
<.0001 |
0.02 |
0.62 |
<.0001 |
|
Glucose, mg/dL |
0.02 |
0.41 |
0.69 |
0.10 |
|
Triglycerides, mg/dL |
0.01 |
0.17 |
0.19 |
0.17 |
|
Total cholesterol, mg/dL |
0.89 |
0.03 |
0.89 |
0.06 |
|
Free cholesterol, mg/dL |
0.05 |
0.99 |
0.65 |
0.05 |
|
Esterified cholesterol, mg/dL |
0.49 |
0.01 |
0.98 |
0.06 |
|
Serum amyloid A, ug/mL |
0.35 |
0.13 |
0.85 |
0.02 |
In our study, we were able to test and demonstrate that a supplementation strategy with organic acids and plant botanicals can restore important production losses experienced during periods of exposure to heat stress. The improvement in lactation performance alone is extremely important because it restores significantly valuable traits such as milk fat and protein. Thus, farmers will be able to utilize this supplementation strategy. Combined with heat abatement strategies, this supplementation approach has the potential to improve lactation performance and improve animal welfare. The lack of major changes in terms of metabolic markers of cows supplemented with OA/PB suggests that the homeorhetic adaptations in metabolism are still effective and overdrive the OA/PB supplementation. However, the changes in lactation performance are encouraging and might be related to the microbiome modulation and improvement of beneficial microflora in cows supplemented with OA/PB. While we are still investigating the effects exerted in the fecal microbiome of cows exposed to heat stress, we are confident this work will be vital for our better understanding of the interplay between the host, microbes, and metabolism of dairy cows experiencing heat stress.
Education & Outreach Activities and Participation Summary
Participation Summary:
PUBLICATIONS
Peer-reviewed: Full Articles
- A. B. P. Fontoura, A. Javaid, V. Sáinz de la Maza-Escolà, N. S. Salandy, S. L. Fubini, E. Grilli, and J. W. McFadden. Heat stress develops with increased total tract gut permeability and dietary organic acid and pure botanical supplementation partially restores lactation performance in Holstein dairy cows. Submitted to Journal of Dairy Science on January 14, 2022.
- A. B. P. Fontoura, A. Javaid, V. Sáinz de la Maza-Escolà, S. L. Fubini, E. Grilli, and J. W. McFadden. Effects of chronic environmental hyperthermia on the gut liver axis and gastrointestinal tract microbiome in Holstein dairy cows. In preparation, to be submitted to the Journal of Dairy Science, Spring 2022.
Conference Proceedings and Industry Communications
- A. B. P. Fontoura, A. Javaid, V. Sáinz de la Maza-Escolà, N. S. Salandy, S. L. Fubini, E. Grilli, and J. W. McFadden. Effects of dietary organic acid and plant botanical supplementation on lactation performance in Holstein cows challenged by heat stress. To be presented at the Annual Meeting of the American Dairy Science Association, June 19 - 22, 2022. Kansas City, MO.
- A. B. P. Fontoura, A. Javaid, V. Sáinz de la Maza-Escolà, N. S. Salandy, S. L. Fubini, E. Grilli, and J. W. McFadden. Effects of heat exposure and dietary organic acid and plant botanical supplementation on gastrointestinal permeability in heat-stressed Holstein cows. To be presented at the Annual Meeting of the American Dairy Science Association, June 19 - 22, 2022. Kansas City, MO.
- A. B. P. Fontoura. Climate change and Dairy Farming: Beating the Heat. Foundation for Food and Agriculture Research Newsletter. https://foundationfar.org/impact/insights/climate-change-and-dairy-farming-beating-the-heat-4/. August 2020.
- J. W. McFadden and A. B. P. Fontoura. Help dairy cows beat the heat: Diet matters. Progressive Dairy: https://www.progressivedairy.com/topics/feed-nutrition/help-dairy-cows-beat-the-heat-diet-matters. July 2020.
Project Outcomes
At this stage, our project provides a basis for a novel nutritional strategy to help dairy cows overcome periods of heat stress. Based on our present findings, the supplementation strategy with organic acids and plant botanicals (OA/PB) is beneficial and restores some of the decreases in milk production, which in turn, has the potential to economically benefit dairy producers. Future analysis is still warranted to investigate OA/PB effects on health and metabolism.
Our knowledge of sustainable agriculture was contextualized into a very important problem dairy producers face. We are working towards improving dairy cow comfort, health and productive performance during challenging times of a changing climate. My future research plan is to finalize the results pertaining to the gut permeability data as well as the microbiome work for our research project.