Final report for GNE18-172
Project Information
Apart from regulatory criteria, very few siting and harvesting recommendations are available to support kelp farmers considering new ventures in Casco Bay, Maine. Grebe et al. sought to address this lack of information by characterizing and exploring the relationship between cultivated Saccharina latissima growth and the ambient salinity, nitrate, and water temperature at three existing kelp farms in the Bay. Their results showed high variation in kelp growth and ambient water conditions between the sites and no direct correlations between the measured water conditions and the cultivated S. latissima morphology or yields. This was an unexpected outcome, but it highlighted the need for site-specific pilot studies prior to the siting of a large farming array.
Our project objectives and strategies were:
1) Conduct a spatiotemporal analysis of nutrient availability and crop characteristics for kelp farms in Casco Bay
a. Characterize relationships between observed nitrogen availability and the morphological characteristics of kelp on existing kelp farms in Casco Bay
b. Model spatial and temporal variability in bioavailable nitrogen throughout Casco Bay
2) Generate siting and harvesting recommendations for current and prospective kelp farmers
a. Explain how nitrogen incorporated into kelp tissue over a growing season compares to nitrogen dynamics both observed at specific sites and projected throughout the bay
b. Summarize observations about environmental conditions that support peak biomass or other desirable characteristics of kelp
Despite its popularity in eastern Asia, kelp farming is relatively new to the United States. In 2009 the first kelp farm was launched in the coastal waters of southern Maine. During the past 12 years, the number of kelp farmers in the state has risen from a handful to dozens, but decisions regarding where to site kelp farms are still somewhat haphazard. Farmers currently take into account parameters like access to the coast, potential user conflicts, benthic composition, depth, and wave exposure, but very little information about nutrient dynamics is readily available.
Within the state of Maine, Casco Bay has the highest density of aquaculture leases approved for growing kelp. We sought to provide Casco Bay's existing and prospective kelp farmers operating with more knowledge about how nitrogen in the bay varies in space and time during the kelp farming season (Oct - May), and what impact that variation could have on the morphology and composition of kelp cultivated in the bay.
Cooperators
- (Educator and Researcher)
Research
Environmental Data
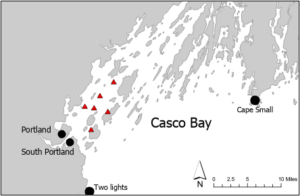
We collected new environmental data from 5 locations in Casco Bay during the Spring 2018 and 2019: Peaks Island, Long Island, Clapboard Island, Brothers Island, and Cow Ledge (Figure 1). Mean monthly water temperatures at each site were calculated from continuous measurements made by suspended loggers (Hobo Pendant Temperature/Light Loggers; UA-002-08). Mean monthly salinity and nitrate concentrations were characterized using grab samples collected from 2 m depth with a Niskin bottle. All samples were stored in Whirlpak bags prior to processing. Salinity of the water was assessed using a refractometer (Cole-Parmer RSA-BR90A; 0 – 90%). Nitrate-N was quantified spectrophotometrically using Hach Nitrate TNTplus Low Range Vial Tests and a Hach DR3900 VIS spectrophotometer.
Kelp Cultivation and Sampling
Saccharina latissima (sugar kelp) was cultivated at the Brothers Island, Cow Ledge, and Long Island locations in collaboration with partner farmers. The kelp sporelings were produced using techniques described in Redmond et al. (2014) and a density of 6,000 - 8,000 spores per mL in the inoculation water. Outplanted kelp was maintained at 2m depth throughout the cultivation cycle and the kelp longlines at each farm were oriented parallel to the prevailing currents (N-S). Longline density varied between 1 - 5 longlines depending on the site. Spacing between longlines at the same site was ≥ 6m.
We collected samples from the growing kelp from January until its harvest in mid to late May (Figure 2). Our target sampling frequency was 14 days, but it varied slightly with boat availability and water conditions. During each sampling event, we removed 20 kelp thalli from a randomly-determined location on the kelp longline to be analyzed back at the laboratory. The samples were stored in plastic bags in a cooler during transport and then transferred to a refrigerator until they could be processed.

Assessing Morphology and Growth
In the laboratory, we measured and recorded the length, three width measurements (at 25%, 50%, and 75% of total length), and total wet weight of each blade (Figure 3). Length, diameter, and total wet weight were recorded for each stipe. Notes regarding the presence/absence of sorus tissue, degree of fouling, and general condition of the specimen were also recorded.

The meristematic portion (site of active growth) of 5 randomly selected thalli from the sample were also prepared for carbon and nitrogen analysis. These samples were rinsed with deionized water and dried in an oven at 50°C for 24 hours. Once dry, each sample was ground and homogenized into a fine powder. A known quantity, 2 - 5mg, of this powder was encapsulated to prepare three replicates. The samples were shipped to the Stable Isotope Facility at UC Davis. At this facility the samples were analyzed for total nitrogen and total carbon using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer. The sample’s preliminary nitrogen and carbon content was measured relative to reference gases analyzed with each sample. This measurement of total nitrogen or carbon content was divided by the sample weight to obtain %C or %N for the dry kelp.
Statistical and Spatial Analysis
All data were examined for assumptions of normality and heterogeneity, and outliers were removed. Multivariate analyses of variance (MANOVA) were used to compare the effect of the environmental factors on morphology of the cultivated kelp. If significant effects (p ≤ 0.05) were detected, then separate, one-way analyses of variance were used to analyze each dependent variable. Then Tukey’s Honest Significant Difference tests and Pearson R correlations were measured between ambient nitrate and S. latissima morphology.
Publicly available environmental data were also downloaded from regional datasets and combined with the new environmental data. Salinity and sea surface temperature data for Spring 2018 and 2019 were downloaded from 12 locations routinely sampled by volunteers for Friends of Casco Bay (FOCB). Sea surface temperature data was also downloaded from the Northwest Atlantic Marine Ecoregional Assessment conducted by The Nature Conservancy (TNC). Inverse distance weighting (IDW) was used to spatially interpolate between the environmental data. The interpolation procedure produced separate raster layers for each environmental variable with smoothed predictions between individual sampling points. The IDW interpolation was performed in QGIS (3.10.1).
Relationships between ambient nitrogen and morphological characteristics of farmed S. latissima in Casco Bay
Location of the cultivation had a significant effect on the temperature, salinity, and nitrate measured around the longlines, so we analyzed the results from each site separately (MANOVA). We hypothesized that there would be a relationship between the ambient nitrate measured at sites and the morphology of S. latissima grown at those sites. This hypothesis was not supported (MANOVA). There were no significant relationships between nitrate and S. latissima blade or stipe length, width, and weight.
Spatial and temporal variability in nitrogen throughout Casco Bay
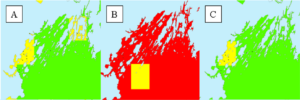
This strategy could not be applied as originally envisioned due to the limited extent of historic nitrogen measurements for the kelp farming season (Oct – May). Spatial interpolation techniques require more points of measurement to generate useful predictive maps. Of all the months in the kelp growing season, the greatest availability of nitrate (and environmental) measurements for Casco Bay were available for May 2019. So, we focused on making a map for this month by calculating the monthly mean for each point and using the IDW interpolation technique (explained above) to produce a map of salinity, nitrate, and temperature. Then, we reclassified these predicted values according to the known optimal and tolerable ranges of each parameter for S. latissima (Table 1).
Table 1. Optimal, tolerable, and stress-inducing levels of ambient salinity, temperature, and nitrate for Saccharina latissima.
|
Classification |
May temperature |
Mean salinity |
Mean nitrate |
|
Optimal |
<10°C |
27 - 35 |
> 10 µM |
|
Tolerable |
>10°C |
<27 |
2 - 10 µM |
|
Stress-inducing |
>15°C |
<15 |
< 2 µM |
|
Growth of S. latissima sporophytes is optimal at 10 – 15°C (Fortes and Lüning 1980). Fouling in WGoM observed at temperatures >9.8°C |
The optimal salinity range for S. latissima is 27–33, and anything lower can exert physiological stress on the kelp (Gerard 1987) |
Growth rate of S. latissima is saturated with 10µm NO3 (Chapman et al. 1978). |
The resulting suitability maps for these three environmental parameters suggest that the mean nitrate in Casco Bay in May 2019 was at tolerable or stress-inducing levels for S. latissima (Figure 4). The May 2019 ambient salinity and temperature in Casco Bay was at optimal or tolerable levels.
Kelp nitrogen content and nitrogen dynamics in Casco Bay
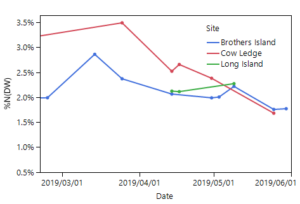
Mean percent nitrogen in the cultivated S. latissima tissue was highest in mid to late March 2019 (2.9 – 3.5% nitrogen in the dry tissue: Figure 5). This percent nitrogen content decreased throughout the spring at the Brothers Island and Cow Ledge sites, reaching the lowest values at harvest (1.7 – 1.8% nitrogen in the dry tissue). For this species, 3% nitrogen or greater is sufficient, 1.9% nitrogen is the limit for maximal growth, and nitrogen levels below this begin to affect growth rates (Chapman et al. 1978; Wheeler and Weidner 1983; Kim et al. 2015).
Environmental conditions supporting S. latissima growth and total biomass production
Saccharina latissima cultivated at our research sites grew well despite the less-than-optimal nitrogen and salinity levels. The total biomass per longline meter at time of harvest in late May (10.4- 13.4 wet kg per meter) was on the higher end of values reported in the scientific literature.
Results references
Chapman, A. R. O., Markham, J. W., & Lüning, K. (1978). Effects of nitrate concentration on the growth and physiology of Laminaria saccharina (Phaeophyta) in culture. Journal of phycology, 14(2), 195-198.
Fortes, M.D. & Lüning, K. (1980). Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresun, 34(1):15.
Gerard, V. A. (1987). Hydrodynamic streamlining of Laminaria saccharina Lamour. in response to mechanical stress. Journal of Experimental Marine Biology and Ecology, 107: 237-244.
Kim, J., Kraemer, G., & Yarish, C. (2015). Use of sugar kelp aquaculture in Long Island Sound and the Bronx river estuary for nutrient extraction. Marine Ecology Progress Series 531, 155-166.
Wheeler, W. N., & Weidner, M. (1983). Effects of external inorganic nitrogen concentration on metabolism, growth and activities of key carbon and nitrogen assimilatory enzymes of Laminaria saccharina (Phaeophyceae) in culture. Journal of Phycology, 19, 92-96.
- Observations of ambient conditions in Casco Bay are sparse during the winter and spring. Additional sampling, and the use of data loggers that can continuously record nutrient, temperature, and salinity measurements would greatly assist future research and siting efforts.
- The location of cultivation had a significant effect on the environmental conditions important for kelp farming, and this suggests that site-specific pilot studies are an important step in the site selection process for Casco Bay.
- Stress-inducing nitrate levels, and some less-than-optimal salinity and temperature conditions were measured at the research sites during May 2019, however, the total Saccharina latissima biomass produced at the sites was high.
Education & Outreach Activities and Participation Summary
Participation Summary:
Consultations
I was contacted by 8 prospective kelp farmers or researchers for consultations related to this project.
Field Work
In Spring 2018 and 2019 I collected environmental measurements, water samples, and kelp samples from Casco Bay, ME. This sampling was conducted in partnership with kelp farmers Tollef Olson (Ocean's Balance, LLC), Marci Trains, and Matt Moretti. Funds for the direct costs associated with this sampling effort were provided by National Science Foundation award #IIA-1355457 to Maine EPSCoR at the University of Maine. This SARE award contributed to the costs of researcher time to analyze and synthesize the results of this sampling effort.
Presentations
In January 2019, I gave an oral presentation sharing news of this SARE project with aquatic farmers, researchers, students, and governmental representatives at the Northeast Aquaculture Conference and Exposition in Boston, MA. I also engaged in numerous one-on-one discussions about kelp aquaculture and nitrogen dynamics in the Gulf of Maine. In January 2020 I shared progress on this SARE project with aquatic farmers, researchers, students, and governmental representatives in one-on-one conversations and small-group breakout sessions at the Maine Aquaculture Research and Education Forum in Belfast, ME. In February 2020, I shared project updates as part of an oral presentation at Aquaculture America in Honolulu, HI.
Manuscripts/Journal Articles
We have prepared two manuscripts using analysis enabled by this SARE award. The first is accepted pending revisions and expected to be published in Spring/Summer of 2021. The second is still undergoing internal review and revisions. Both manuscripts are chapters in G. Grebe's dissertation.
Project Outcomes
This project provided additional insight into the complexity of the marine conditions within Casco Bay which highlights the need for kelp farmers to conduct site-specific pilot cultivation to assess the suitability of a site prior to establishing larger-scale seaweed farms. It also exposed a high degree of variation in kelp morphology and composition within the same farm and within the same bay which suggests that there may be opportunities to improve the efficiency of kelp farming activities through strain selection, modifications to seeding density, selective harvesting of larger blades, or intermediate harvests in the early spring.
This project helped me to learn much more about existing kelp farming practices and research. I compared our results to existing extension and peer-reviewed literature from around the world, and in this process, I broadened my understanding of the well-researched and established practices and the research and extension that still needs to be done. I also learned more about the challenges facing kelp farmers in New England and throughout North America. Despite the hard work of many organizations, there is still very little marine nutrient data available at the spatial and temporal scale that could be used to compare multiple sites within the same bay.
My short-term research objectives are very similar to the work completed in this project. I am continuing to study and support the development of seaweed farming in the U.S. and have now expanded my focus to include tropical seaweed species. The same need for more robust measurements of water conditions for farm siting and crop management to increase yield and revenue exists for prospective seaweed farmers in the tropical United States. I am applying much of my broader knowledge gained from this project to planning more effective research and development of seaweed farming beyond Casco Bay.
The acquisition of new marine nutrient data for the S. latissima farming season (Oct - May) proved to be more challenging than originally anticipated. Winter weather and field logistics limited the number of sampling trips that could be completed, and in particular during January and February. The geographic and temporal scope of historical nitrogen data for Casco Bay was also more limited than we understood when proposing this project, presumably for the same reasons that limited our own sampling.