Final report for GNE18-181
Project Information
The two main objectives of this study were 1. to examine the in vitro germination percentage of cold hardy wild American hazelnut pollen, as well as determine incompatibility alleles (S-alleles) for the 19 wild American hazelnuts in this study, and 2. to determine fruit set percentages of American hazelnut pollen crossed with European hazelnut female flowers and evaluate the resulting nut characteristics. This project is one of very few on the subject of American hazelnut pollen and its potential for use as a potential pollenizer to commercially valuable European hazelnuts in colder regions. Some key results are that pollen in-vitro germination percent is not correlated with higher fruit set (R2=0.0066), higher fruit set is likely correlated with higher nut weight (R2=0.48), and that the previous year and this year's fruit set results are correlated with each other (R2=0.56). Predictability is valuable in this situation. The overall findings of this report will likely be important to the agricultural community, especially in colder regions, to expand the growing region for a great, low maintenance crop like European hazelnut. More research will need to be done in this area, however, as the results were not conclusive in all areas.
1. Examine the in vitro germination percentage of cold hardy wild American hazelnut pollen, as well as determine incompatibility alleles (S-alleles) for the 19 wild American hazelnuts in this study.
2. Determine fruit set percentage of American hazelnut pollen with European hazelnut female flowers from controlled crosses and evaluate the resulting nut characteristics from successful crosses.
The purpose of this project was to evaluate cold hardy native American hazelnut pollenizers for their utility as pollenizers for disease resistant European hazelnut cultivars slated for release from Rutgers University in 2019, which could substantially increase their productive range. These commercial-quality European hazelnuts are resistant to the disease eastern filbert blight (EFB), which historically prevented their production in eastern North America. However, frost damage to their male flowers (catkins) can limit their usefulness in colder parts of the region. Hazelnuts are self-incompatible, which necessitates the planting of additional cultivars in hazelnut orchards as pollen parents know as pollenizers (Mehlenbacher et al, 1997, Olsen et al, 2000; Thompson, 1979). The pollenizers that are suggested for these Rutgers selections are adequate for growing climates like New Jersey and areas with milder winters but expanding farther north would risk the catkins becoming frost damaged in some years and limiting pollen shed, therefore decreasing yields.
Hazelnut has many qualities that makes it a sustainable crop and a worthwhile addition to any farms: low input requirements, high yields, high value (and ability for value-add with confections and oils), only four years from seed to first crop, mechanically harvested, and high nutritional content. It is often used by those practicing permaculture for these reasons. This work contributes to the development and establishment of this new crop in the northeast US region.
The release of EFB-resistant Rutgers breeding selections provides farmers with a crop that was previously not able to be grown in the eastern US due to EFB. With EFB no longer the main concern of growing hazelnuts in the region, the limiting factor is cold hardiness of the male flowers. Cold hardy pollenizers are necessary to protect yields in colder regions, and from highly variable weather, where warm spells followed by cold spells can damage catkins that begin to break dormancy. The growing zone for the Rutgers breeding selections can be expanded significantly northward if American hazelnuts are found to be suitable as pollenizers from this study. Aside from this fundamental research, very little has been done on the use of American hazelnuts as pollenizers in European orchards, despite the numerous benefits this would have in expanding the growing range northward.
Cooperators
Research
Objective 1: Examine the in vitro germination percentage of cold hardy wild American hazelnut pollen, as well as determine incompatibility alleles (S-alleles) for the 19 wild American hazelnuts used in this study.
Pollen collection
Phenology ratings were recorded twice weekly to determine the ideal time to collect pollen from extended catkins as described in Capik and Molnar (2014). When pollen is in peak shed catkins were collected from the field. Pollen was sifted through a filter onto a large sheet of paper, and then transferred to a 1.4mL micro centrifuge tube. Pollen was frozen until female flowers became receptive.

The American pollenizers for this study included pollen from early and mid pollen shedding plants supported by phenology data from 2017-2019. The C. americana plants were sourced from Pennsylvania (PA), Wisconsin (WI), Illinois (IL), New Jersey (NJ), Ohio (OH), Missouri (MO), Michigan (MI), and Minnesota (MN). The original nuts to grow these trees were collected with the assistance of the Arbor Day Foundation and planted at the Rutgers Fruit and Ornamental Research Center in Cream Ridge, NJ in 2010-2011. In addition to C. americana, pollen from a mix of C. avellana cultivars will be collected from known compatible pollenizers available on site as controls.
The reason for choosing early and mid pollen-shedding American hazelnut pollenizers was to ensure there is adequate pollen available when the European hazelnut female flowers are receptive. The late-shedding pollen will most often be too late to be useful as a pollenizer for European hazelnuts.
Pollen germination
Pollen collected from C. americana pollenizers was grown onto an agar and sucrose petri dish. The germination solution consisted of agar (10g/L), and sucrose (100g/L), as described in Fattahi et al (2014). The solution was then be microwaved and poured into 20 mL petri dishes. Approximately 100 grains were used to test germination for each replicate of each sample and incubated at 25 Celsius. Pollen was germinated from each wild C. americana used as pollen parents, as well as a mix of European pollen.
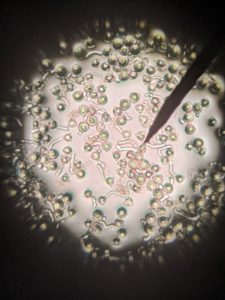
Pollen germination percentage was determined by measuring pollen tube growth with a light microscope at x100 magnification. Germination was considered successful when the pollen tube reaches pollen size, usually 20-30 micrometers. Germination counts were conducted 16 hours after beginning incubation.
Statistical analysis
A linear regression was performed to determine the extent of the correlation between fruit set percentage and in-vitro germination percentage.
Incompatibility (S-allele) testing
Pollen expresses only the dominant S-allele. 33 “tester” pollen parents with known s-alleles are used to pollinate the female flowers for testing. The female flowers express both s-alleles, and the pollination will be incompatible if either s-allele is expressed in the pollen. Once two pollen parents yield incompatible pollinations and all the rest are compatible, the two s-alleles are known for that tree/cultivar. The dominant s-allele can then be determined by looking at the s-allele hierarchy (Mehlenbacher, 1988, 1997a, 2000, 2014).
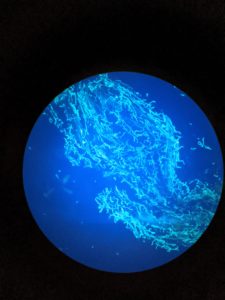
The procedure from Mehlenbacher (1997a) was used to determine the incompatibility alleles for the C. americana pollenizers in this study. Female flowers were collected from two to four branches of each bagged pollenizer when styles are protruding 2-6 mm. These were then placed in petri dishes. Each individual female flower was pollinated with one of the 33 “tester” pollen parents, from clonal material at Oregon State University, and placed in the petri dish overnight. Flowers were stained with aniline blue dye and fluorescent microscopy was used to see pollen tube growth. If the tubes are short and cannot penetrate the stigmatic surface, the test is incompatible. If the pollen tube is long and can penetrate style tissue, the test is compatible.
Objective 2: Determine successful cross percentage (percent fruit set) of American hazelnut pollen with European hazelnut female flowers from controlled crosses, and evaluate the resulting nut qualities and characteristics from harvested crosses.
Controlled Crosses
Phenology ratings were taken two times weekly to determine when female flowers were receptive and ready to be pollinated. Catkins were removed from branches and pollinated from Rutgers breeding selections (and controls) and branches covered with Tyvek HomeWrap® to prevent pollen coming into contact with the female flowers. When receptive, the HomeWrap® was removed, and using a gloved finger, touched the female flower to liberally pollinate. One bag was left on each tree to confirm that bags prevented pollen contamination.

Each of the 19 C. americana pollen parents were crossed with C. avellana female parents and a mix of compatible European pollen which acted as a positive control. The negative control was a bagged branch with no pollination. The female flowers on each branch were counted and used as the maximum number of nut clusters (fruit set) possible. In August, the percentage of successful crosses were determined by comparing the nut clusters to the number of female flowers attempted to be pollinated from February/March.
Nut characteristics
The following characteristics were measured using the average of a sample size of five nuts: Nut length, nut depth, nut height, sphericity, geometric diameter, and nut weight as described in Pliestic et al (2006). The geometric diameter is (L*W*D)1/3 (length, width, depth) and the sphericity is (Geometric Diameter) / (Length). Nuts with defects were not measured and were not common enough to include in the analysis.

Statistical analysis
Linear regression was used to determine correlation between fruit set and in-vitro germination, and fruit set year over year. An additional linear regression was used to discover the relationship between nut weight and fruit set.
Objective 1: in vitro germination percentage of cold hardy wild American hazelnut pollen, as well as incompatibility allele discovery
S-alleles for seven of the American hazelnut pollen parents were tested at Oregon State University in February 2019. Only seven were analyzed due to availability of female flowers at the time of travel. Of the seven, only four exhibited conclusive results. Interestingly, none of the S-alleles were shared among the four pollen parents, implying a high level of genetic diversity. The resultant S-alleles were also relatively uncommon, meaning incompatibility with known cultivars should not be an issue, increasing the utility of the plants as potential pollenizers.
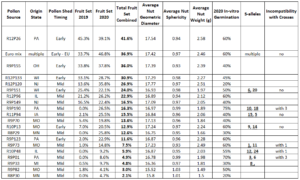
The most common incompatibility alleles are S2, S1, S10, S5, S3, S4, and S6. From Table 1, the S-alleles that were confirmed from this study are: S1 (1), S3 (1), S5 (1), S6 (2), S8 (1), S9 (1), S10 (1), S11 (1), S12 (1), S14 (1), S15 (1), S18 (1), S20 (1), and S24 (1). The fact that there were so few duplicates attests to the diversity and potential for these plants to be used as pollenizers. The S-alleles of the European hazelnut trees were S1 (1), S3 (3), S10 (3), and S12 (1).
Two pollen sources were incompatible with three of the four “mother” trees. That included R9P01 (4.9% fruit set), and R9P190 (16.3% fruit set). It is unclear why R9P190 was able to have a relatively average fruit set percentage considering the incompatibility. Further investigation is required to determine the cause of this unlikely result. The average fruit set was 17.3% for the combined 2019 and 2020 harvests.
In-vitro germination percentage was not able to be determined in 2019 due to a technical issue. The pollen degraded in the time it took to perfect the protocol. The protocol was successfully tested on fresh pollen and on pollen for the 2020 crosses.
In 2020, all in-vitro germination percentages were 20% or greater, with all but two above 20% (R9P30 and R12P120). The largest germination percentage was 75% for plant R9P190. R9P01 was a close second with 70%. Table 1 includes the germination data for each pollen source; the average was 47.6%.
Objective 2: Fruit set of American hazelnut pollen on European hazelnut female flowers from controlled crosses and resulting nut measurements.
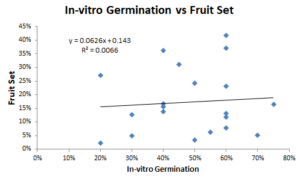
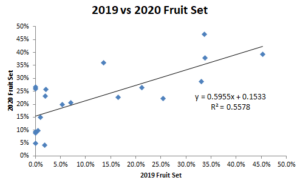
Percent fruit set was determined for all pollen sources used in the study, plus a European pollen mix. The highest percentage total was 41.6%, and the lowest was 2.1%. There was no identifiable correlation between fruit set and in-vitro germination (R2 = 0.007, Figure 1). However, there was a correlation (R2=0.56, Figure 2) between fruit set in 2019 and fruit set in 2020. Predictability is extremely important in agriculture, so this is a positive result.
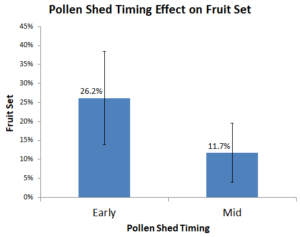
Whether an American hazelnut sheds pollen early or “mid” season might give insight into how well it will act as a pollenizer for multiple reasons. First, early season for American hazelnuts is shifted closer to mid season for the European hazelnuts. Second, according to the data from this study (Figure 3), the early pollen shedding plants had an average of 26.2% (std. 0.12) fruit set compared to 11.7% (std. 0.08) for the mid shedding plants. The p-value associated with this difference is 0.011, which would qualify as statistically significant using an alpha of 0.05.
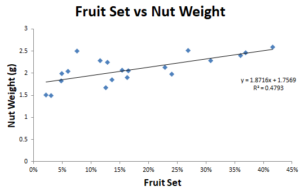
There is a relatively interesting correlation between nut weight and fruit set. High nut weight corresponds with a higher fruit set (R2 = 0.48, Figure 4). In future studies, it would be interesting to understand this in more detail.
It could be that American hazelnuts on the larger side of nut weight could correlate to an increased fruit set, or it could be that the increased fruit set and nut weight are due to the European side of the equation.
The European nut characteristics were towards the high end of the American hazelnut range, but the largest difference in geometric diameter was only 6% compared to the European (excluding two groups with very small sample sizes). The sphericity was also acceptable for all American hazelnut averages compared to the European. The largest and heaviest nut was R12P26, an American hazelnut that also had the highest fruit set % at 41.6%.
- There is likely a relationship between nut weight, fruit set percentage, and time of pollen shed that will need further study.
- American hazelnuts can be successfully used as pollenizers to European hazelnuts, depending on which americanas are chosen and taking incompatibility into consideration. This can dramatically expand the growing range of C. avellana.
- There is not a correlation between in-vitro germination percentage of American pollen and successful fruit set.
To be sustainable, we need to adapt to our environment. As temperatures become extreme, there is more need for safety measures and backup plans. This work was done with the vision of extending the knowledge to farmers that want to grow hazelnuts in a colder than usual region for the crop. This can be done, but there is also a need for current growers of hazelnut to grow some American hazelnuts in the fields just in case there is a severe temperature change that damages the pollen-carrying catkins.
Education & Outreach Activities and Participation Summary
Participation Summary:
Poster: Mayberry, A., J.M. Capik, T.J. Molnar. Establishing a core collection of Corylus americana from wild germplasm for hazelnut breeding and pollenizer efforts. Fourth Annual Northeastern Plant, Pest, and Soils, Conference (NEPPSC). January 10, 2019. Baltimore, MD.
Oral presentation: Mayberry, A., J.M. Capik, S.A. Mehlenbacher, T.J. Molnar. Evaluating wild American hazelnuts as cold hardy pollenizers in European hazelnut orchards. American Society of Horticulture Science (ASHS) Annual Conference. July 23, 2019. Las Vegas, NV.
Oral presentation: Mayberry, A. J.M. Capik, S.A. Mehlenbacher, T.J. Molnar. Benefits and drawbacks of American hazelnut pollenizers to expand the commercial hazelnut production range. American Society of Horticulture Science (ASHS) Annual Conference. August 10, 2020. Virtual Conference.
Project Outcomes
- Hazelnuts are sustainable, low input, low maintenance crops and most growers would benefit financially, economically, and environmentally from growing and selling hazelnuts.
- American hazelnuts can be successfully used as pollenizers to European hazelnuts, depending on which americanas are chosen and taking incompatibility into consideration. This can dramatically expand the growing range of C. avellana.
- There is likely a relationship between nut weight and fruit set %, and time of pollen shed that will need further study.
Research on American hazelnuts is slim, but growing. Thanks to the interest in growing hybrid hazelnuts and/or using cold hardy pollenizers in colder regions has increased demand for understanding how C. americana can be used in agriculture. To my knowledge, there has not been a more comprehensive study on crossing American hazelnut pollen with European hazelnut flowers in a field setting. There seems to be a correlation between nut weight, fruit set, and time of pollen shed. More will need to be done in this area to give us confidence that this is a general trend. This work has also revealed that American hazelnuts are not all created equal. There is a selection process that should take place before relying on the theory that they will work as pollenizers. They should, but maybe not all will.
Some interesting results came from this study. I would like to see this work expanded by investigating the relationship between early vs late pollen shedding and its effect on fruit set and potentially nut weight. Included in that work could be a genetic diversity study to find further correlation or explore new questions.
It is difficult to have access to enough (or large enough) clonal hazelnut trees to account for all the samples, bags, pollen, and branches needed. If that were the case, I would have liked to include more diverse European varieties with different S-alleles and to pinpoint the remaining C. americana S-alleles.
Distinguishing a European from an American or Hybrid is sometimes difficult. The ability to quickly (and relatively inexpensively) use DNA markers to accurately classify each tree is essential to furthering the knowledge of this area.