Final report for GNE18-188
Project Information
Wild pollinators provide crucial pollination support and resiliency to orchards and other fruit crops in the Northeastern US. Although wild pollinators have consistently been found to be more diverse and abundant in orchards near forested areas, woodlots, and with hedgerows, the ways in which bees interact with forests and forest trees are poorly understood. This is particularly important and interesting given that forests are the dominant historical and current landcover in the Northeastern US. We used a newly adapted canopy-sampling method, paired with understory and adjacent sampling, to compare bee captures in these habitats across the spring field season, and then identified over 340,000 pollen grains to the lowest possible resolution in order to understand how bees' diets were structured by their habitat and the time of year. We found high bee activity in forest canopies, and that bees move from forests to orchards. Male bees eat more pollen than previously expected, including canopy pollen, and rely heavily on forest habitats; they were barely caught in orchards relative. Supporting forests for this usually-overlooked half of their life cycle may be crucial for conservation. Finally, we find that bees caught in canopies had consumed much more canopy pollen than those caught in the understory or orchard, suggesting that prior sampling that did not catch in the canopies underestimates the importance of forests as a food resource. These results provide increased clarity into the mechanisms by which forests support important agricultural pollinators, and suggest that tree diversity, ecological forest management for wildlife, and general availability of forest habitat, can all support pollinators and maintain longterm pollination resiliency and vibrancy.
In spring 2017, 2018, and 2019, I employed novel canopy-sampling methods using elevated “bee bowl” traps at 11 sites in NY state. I caught wild bee pollinators in the canopy and understory of woodlots adjacent to pollination-dependent fruit trees. Woodlots were dominated by wind-pollinated tree species, and more than half of captured bees were known apple pollinators (Russo et al. 2015, Blitzer et al. 2016). Canopy bees were highly abundant during tree bloom, and rapidly declined after, supporting the idea that bees were foraging.
I completed several research objectives investigating (1) bee abundance and activity in forest canopies, with analysis of the evolutionary relationships and life history diversity of the forest bees, (2) the relationship between forest and orchard habitats for male and female bees at different times of year, highlighting the importance of male bees, (3) the amount of pollen consumed by the males of important orchard pollinating mining bees, opening new avenues for natural history understanding, and (4) the importance of wind-pollinated and other canopy pollens for spring bees, with an eye to how availability both on the landscape and from year to year (e.g. mast bloom) influences these consumption patterns.
(1) I used taxonomic guides, expert support, and DNA barcoding to identify the wild bee species found in the canopy, understory, and adjacent orchards. Many commonly found forest bees are poorly known and thus hard to identify due to underdeveloped taxonomic resources. This was especially true for groups such as mining bee males; the females of this group of bees are highly effective pollinators, so understanding the behavior and foraging choices of males is crucial.
For the remaining objectives, I used microscopy to identify the pollen from guts of trap-caught wild pollinators. Traps demonstrated unprecedented diversity of canopy-active bees, but traps alone cannot demonstrate the behavior or foraging preferences of those canopy-active bees. Pollen identification through microscopy provided detailed tree pollen use by the wild pollinating bees, in the canopies, understories, and adjacent apple orchards.
(2) I asked if bees of the same species are moving from forests to orchards over the spring season. I asked if the canopy is occupied similarly or differently to the understory in this phenological transfer. I also hypothesized that pollen preferences of bees in the forest strata (understory vs. canopy) vary over space, time, and availability of resources.
(3) I mentored an undergraduate researcher in a project to quantify the pollen volumes consumed by male bees of Andrena, a highly effective orchard pollinator. If male bees are consuming more pollen than we previously thought, this might help us understand new pathways to conserving these important wild pollinators.
(4) I synthesize detailed phenological records and the previously described extensive pollen dataset to ask how availability both on the landscape and from year to year (e.g. mast bloom) influences these consumption patterns.
The purpose of this project was to identify the forest, woodlot, and hedgerow tree species that are most important for supporting fruit tree pollinators. While much research has focused on herbaceous wildflower plantings for pollination services in agricultural crops (Blaauw and Isaacs 2012, 2014, Grass et al. 2016), almost nothing is known about how forest and shrub resources support pollinator populations and effect pollination services. This is a surprising gap in knowledge since forests and forest fragments commonly surround orchards in the Northeastern US, and the resources they provide may be critical for robust pollinator populations. For example, our own data from flower counts taken in spring 2017 found the number of flowers on a single canopy maple or oak tree often exceed 600,000 or even 700,000 blooms/tree. A single male red maple can present up to 1350 flowers/m 3 of the outer 2 meters of canopy, which translates to an estimated 1.35 ml nectar/m 3 (Batra 1985). This exceptional resource likely contributes to findings that natural forest habitat and plant diversity support thriving pollinator populations in apple orchards in the northeast (Park et al. 2015, Kammerer et al. 2016), yet these patterns have never been measured directly, nor is the contribution of specific tree and shrub resources known.
Of the 420 species of wild bees in New York State, over 120 species have been found visiting apple orchards (Russo et al. 2015, Blitzer et al. 2016) . Wild bees emerge after spring thaw immediately requiring pollen and nectar, and forests are among the only available forage resources (Chambers 1968, Russo et al. 2013) . Since the orchards they pollinate will not bloom for several weeks, bees require other resources to stay active until crops bloom, and to amass sufficient resources to reproduce to maintain healthy populations each year (Schellhorn et al. 2015) . While spring ephemerals certainly provide pollen and nectar and are the focus of much research (e.g. Motten 1986, Williams and Winfree 2013, Parker et al. 2016, Austen Emily J. et al. 2018) , they pale in comparison to the sheer number of flowers in the canopy. Many scattered literature observations suggest wind-pollinated trees are visited by diverse bee species, including by Osmia and Andrena, two highly effective genera of orchard pollinators (e.g. Kraemer & Favi 2005, Yourstone et al 2021, Saunders et al 2018, Chambers 1968, Raw 1974, Russo and Danforth 2017) .
A healthy wild bee population reduces the need to rent honey bee hives and provides pollination services in poor weather and unpredictable climates (Blitzer et al. 2016). Biodiverse pollinator populations are insurance against climate change, pests, and diseases, as bees with different traits respond differently to climate pressures (Bartomeus et al. 2013) , so species diversity supports economic security in the form of a successful crop each year (Aizen and Harder 2009, Garibaldi et al. 2013) . It will thus directly inform land management for fruit tree pollination services and thus crop viability to identify the preferred tree species and the quantity and quality of pollen they produce for a maximally diverse bee population.
The value of pollination services in NY State is estimated at $500 million/year for crops including apples, pumpkins, and strawberries (New York State Departments of Environmental Conservation and Agriculture & Markets. 2016) . Pollination is crucial for human diets, including a high proportion of nutrient dense foods (Eilers et al. 2011) . Yet, the average loss rate for honey bee hives in the U.S. in 2014-2015 was 49.0% (Seitz et al. 2015) . Orchardists are struggling to find honey bee hives to rent for pollination services, (personal communication to Scott McArt from Jeff Morris, Glenora Farms, Dundee NY), and rentals have risen to $140/hive on average in recent years (Wheeler et al. 2018). Wild bees increase pollination services regardless of honey bee abundance (Winfree et al. 2007, Garibaldi et al. 2013) , and are important pollinators in New York apple orchards (Blitzer et al. 2016) . There is also widespread cultural interest and knowledge around managing for wild bees. A survey of over 600 orchardists in New York and Pennsylvania found that 93% of respondents highly valued wild pollinators, and sought to actively manage their land for wild bee conservation (Park et al. 2018). Some orchardists are now choosing to rely entirely on wild bee services (Dunn 2018) .
Wild bees often suffer from nesting habitat and forage loss associated with land-use change and agricultural intensification (Steffan-Dewenter Ingolf et al. 2002, Carvell et al. 2006) . Wildflower plantings, a frequently suggested solution, can increase wild bee abundance, diversity, and pollination services (Blaauw and Isaacs 2014) , yet such strips require expensive establishment and are often overtaken by weeds requiring herbicide management (Landis and Savoie 2018) . In contrast, once established, perennial trees and shrubs provide resources to bees in addition to long-term benefits such as soil stabilization, wind breaks, carbon sequestration, timber, firewood, shade for animals, and even maple syrup or nuts (Farming the Woods 2014, Graham and Nassauer 2017) . Thus, identifying the forest and hedgerow species which provide food to bees could successfully maximize ecological services and increase not just fruit pollination but whole-farm ecological and economic sustainability (Bommarco et al. 2013)(Potts et al. 2016) .
There is a growing call for basic research into insect use of wind-pollinated resources, a large portion of which are early-blooming trees (Saunders 2018) . Their pollen-releasing catkins are not showy or colorful, so not traditionally understood as attractive to bees and thus ignored in forest resource calculations. Yet several “wind-pollinated” species are in fact used extensively by bees and partially pollinated by insects, including willow (Peeters and Totland 1999) , several maples (Sullivan 1983, Batra 1985) , linden (Anderson 1976) , and others. Scattered historic data suggest that bees frequently collect from wind-pollinated oak, ash, beech, and birch (Chambers 1968, Raw 1974) . Thus, to understand how to select forest and hedgerow trees to support pollinators, we must re-evaluate the importance of these less-showy resources. Therefore, I propose here to calculate the use patterns and quality of this resource for the broad community of wild bees in order to fill this knowledge gap.
Research
As a brief background on the methods for this project, in 2017, 2018, and 2019 I respectively sampled in 6, 11 and 9 different forest-orchard pairs across the Finger Lakes region of New York State. Prior to receiving the SARE grant, I worked with each of the growers to confirm sampling goals and areas and completed two seasons of bee sampling. To set up, I used a "Big Shot" 7-ft slingshot to launch a weight attached to a thin rope. In five trees per forest, I aimed precisely for a branch or branching fork that would allow for sampling at the edge of the canopy tree.  Once positioned correctly, I then reeled a bee trap into the canopy, and installed a paired trap at ground level. These traps were "bee bowl" cups (also called "pan traps") in standardized fluorescent yellow, white and blue, each color of which is known to attract a different suite of bee species. Together the three bowls comprise a standard bee sampling method. Five similar tri-color traps were deployed in adjacent orchards in the branches of apple trees. Bees were collected weekly from each trap at all sites and placed directly into small Whirlpak bags with 95% Ethanol for later processing. Sampling began each spring immediately after winter thaw and continuing one week after apples finished blooming across all sites (each year, roughly end of March / first week of April-first week of June).
Once positioned correctly, I then reeled a bee trap into the canopy, and installed a paired trap at ground level. These traps were "bee bowl" cups (also called "pan traps") in standardized fluorescent yellow, white and blue, each color of which is known to attract a different suite of bee species. Together the three bowls comprise a standard bee sampling method. Five similar tri-color traps were deployed in adjacent orchards in the branches of apple trees. Bees were collected weekly from each trap at all sites and placed directly into small Whirlpak bags with 95% Ethanol for later processing. Sampling began each spring immediately after winter thaw and continuing one week after apples finished blooming across all sites (each year, roughly end of March / first week of April-first week of June).
All specimens were pinned and databased using Biota software with unique identifiers and associated metadata (Colwell 1994). Specimens have been accessioned to the Cornell University Insect Collection. Specimens were identified to species or nearest possible morphospecies using online guides and existing taxonomic keys (Laberge 1971, LaBerge 1973, 1980, 1985, Gibbs 2011, Gibbs et al. 2013, discoverlife.org, and Andrena subgeneric keys by Mike Arduser, unpublished). Due to unresolved taxonomy and lack of available keys, specimens of Nomada (n=61), Hylaeus (n= 3), and Sphecodes (n=3) were only determined to the genus level; some individuals were determined to the generic or subgeneric level due to damage in bee bowls (n= 35). Other difficult taxa were resolved in two different ways: with the help of taxonomic experts (Jason Gibbs assisted with Lasioglossum (Dialictus) and Prof. Andrew D. Young identified the flower flies in family Syrphidae), or using DNA barcoding for a subset of unidentified Andrena (see below).
We extracted DNA from 130 Andrena bees using a CTAB protocol available on the Danforth lab website (http://www.danforthlab.entomology.cornell.edu/research/resources/). These bees were selected as a subset of the full dataset either (1) to confirm a tentative morphological identification, or (2) because the specimen was damaged or exhibited traits that could not be categorized clearly with the existing keys. DNA was extracted from 3 legs which allowed us to retain the remaining, nearly complete, specimen for further study and as a pinned voucher. We used primers developed by Creedy et al. (2020) for barcoding British bees: BeeF (5’-TWY TCW ACW AAY CAT AAA GAT ATT GG-3’) and BeeR (5’-TAW ACT TCW GGR TGW CCA AAA AAT CA-3’). These bee-specific primers worked consistently across nearly all of our samples and rarely yielded non-target sequences. For PCR, we used 35 cycles of 94°C for 45 seconds, 52°C for 45 seconds, and 72°C for 1min. All PCR fragments were sequenced in both directions. After trimming primers, our fragments were 658bp in length. Sequences were edited and aligned in Geneious (Geneious Prime® 2019.0.4; Build 2018-11-27 02:05; Java Version 11+28 (64 bit), available at https://www.geneious.com).
Objective (1) was first to finalize bee abundance and activity in forest canopies, with analysis of trait and life history diversity of bees active in the forest.
This work has now been published, and is available on the Forest Ecology & Management website (see citation and discussion of results below).
Objective (2-4) all relied on pollen analyses to characterize the relationship between forest and orchard habitats for male and female bees at different times of year and, and to do so I am in the process of identifying the pollen collected by each bee species. This will allow us to understand which tree species in the early spring forest are most important for wild bee pollinators. In the first step towards this answer, an undergraduate mentee (Xavier Carroll) and I dissected each of the bees collected in 2018, which had been collected into 95% Ethanol. Bees were surface sterilized so no contaminant pollen was collected. Dissection was done carefully to remove only the internal guts, but not damage the structure of the bee's external integument, so that they might still be identified to species under the microscope. All guts were immediately placed into individually labeled tubes and placed in the -80C freezer, to maintain them for future identification via molecular (or microscopy) methods. The original method for this pollen identification was to use pollen meta-barcoding, a molecular approach to identify the full community of pollen in a sample (in this case, in a wild bee's digestive tract). However, since the time of funding, several new papers have demonstrated discrepancies in the ability of this method to accurately quantify the amount of different pollens, and bias depending on the relative abundances in the original sample. This could make it impossible for me to know if bees were actually using significant amounts of certain species of trees that might amplify in their gut samples, or if there were simply contamination or otherwise negligible amounts of those pollens in their digestive tracts. Thus, upon careful reflection, my advisors and I have adjusted my allocation of funds to ensure that I am accurately & thoroughly able to answer the ecological questions here funded. Using the DNA barcoding instead for the wild bee identification allows for a highly accurate and cutting-edge approach to characterizing this relatively-unknown insect community (methods described above). For pollen, using microscopy instead from meta-barcoding allows for accurate insight into relative resource use. This is very important, as I am seeking to particularly identify resources that have been overlooked in the past and might be dismissed as contamination unless we can clearly demonstrate amount of use.
For morphological analysis, we identified up to 300 grains (or ten unique transects per slide, whichever came first); slides were marked as “no pollen” if there were fewer than 30 grains of pollen on the entire slide (Harmon‐Threatt and Kremen 2015, McArt et al. 2017). We initiated a transect along the margin of the slide with a random number generator, then identified each grain along that transect to one of 31 family, genus, or morphogroup categories representing the lowest possible resolution identity. Multiple categories of pollens that did not match any of our reference samples were grouped as “other.” In both sampling years, we collected pollen samples from all blooming plants and maintained detailed phenological records at each farm and forest site. Only the site name and date were known during identification to allow for phenologically-informed identification, but all other metadata was hidden to avoid bias. Finally, we took multiple voucher images for each slide to allow for validation of consistency throughout the identification process, e.g. we iteratively checked all voucher photos within a category to catch any misidentifications.
Pollen categories were further grouped for some analyses as “canopy,” “understory,” “Rosaceae,” and “other.” We note that Black Cherry, a canopy species, would be grouped as Rosaceae, not canopy, despite being canopy tree. This was unavoidable with the difficulties of differentiating rosaceous plants below family level, but we emphasize that this only makes our estimates of canopy resource use more conservative. We further note that although in our orchard and orchard-adjacent study system, rosaceae is heavily dominated by orchard tree pollens, but may also include serviceberry (Amelanchier sp.), wild rasberries (Rubus sp.), wild strawberry (Fragaria sp.), and other common local rosaceous species. Because all of these except Black cherry are “edge” or orchard species, rather than understory or canopy, however, we still use this category as a broad proxy for orchard-collected resources. Finally, willow may take many growth forms, including coppiced hedges, shrubs, and trees, but was never growing in any of our woodlots and only rarely present on-farm, so was not included as a “canopy tree.”
Background Data & Important Ecological Correlates
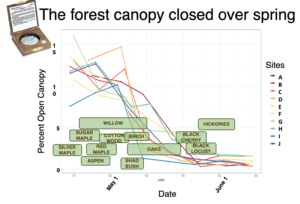
Descriptive graphs characterizing the forest composition (measured with variable radius plots using an angle gage, BAF 10) and light levels (measured with a hand-held Densiometer; superimposed bloom windows estimated to represent dates compiled across sites).
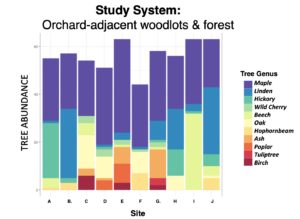 .
. 
OBJECTIVE (1) Forest bee community characteristics
Since last winter's report, I have published my first peer-reviewed journal article, describing the vibrant and diverse wild bee pollinators found in early spring forest canopies.
citation: K. R. Urban-Mead, P. Muniz, J. Gillung, A. Espinoza*, R. Fordyce, M. Van Dyke1, S. H. McArt1, B. N. Danforth. “Bees in the trees: Diverse wild bee communities in temperate forest edge canopies” Forest Ecology & Management (2021 In Press).
I summarize the major results of this paper here:
- Despite equal species richness among strata, woodlots had vertically stratified bee communities
- We found higher overall diversity and more female bees in the canopy
- The traits of nesting habitat and sociality were strongly predictive of strata occupancy
- Canopy cover (leaf-out) was negatively associated with understory but not canopy bee abundance
- Regional amount of coarse woody debris correlated with understory abundance of some trait groups
OBJECTIVE (2-4) Background information on gut pollen dissections & Pollen Identification for diet characterization.
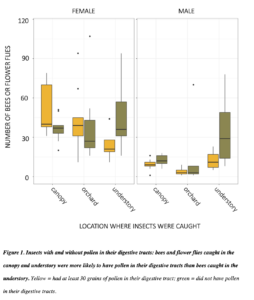
In 2018 and 2019 respectively, we caught 499 and 621 Andrena (male bees 59 and 53%), the important pollinators commonly called mining bees. Following gut dissections in both 2018 and 2019, I found that male mining bees were more abundant in the forest than in the orchard, and females were most abundant in the canopy, followed by the orchard (all contrasts p = <0.001). Male and female adult bees caught in apple orchards and canopies were more likely to have pollen meals in their digestive tracts (p = <0.001). This suggests that the canopy catches may indeed be due to foraging in those treetops. In 2019, 158 out of 328 (48%) of male Andrena had pollen in their digestive tracts.
Overall, we dissected 2885 bees and flower flies, of which 1310 (45.4%) had at least 30 grains of pollen. A higher proportion of all females than males had pollen (49.9% relative to 31.9%). There was pollen in the guts of 59.1% flower flies (n = 93). Among bee genera, 62.5% of Bombus had some pollen (n = 24), 58.1% of Andrena (n=931), 44.6 of Lasioglossum (n= 1166), 44.1% of Augochlora (n = 186), 38.9% of Augochlorella (n = 108), 33.3% of Halictus (n = 21), 15.3% of Ceratina (n = 144), and 12.6% of Osmia (n=127). All other genera were represented by fewer than 20 individuals. Bees in different families were carried different proportions of pollen. Female bees were more likely to have pollen in their guts than males, with log odds ratio of 2.08 relative to males (SE 0.19, p<0.001). Relative to insects caught in the understory, bees caught in the canopy were 2.6 more likely to have pollen (SE 0.24, p<0.001), and those caught in the orchard were 2.02 times more likely (SE 0.20, p<0.001). Bee and flies caught in the canopy were only 1.29 more likely to have pollen than bees caught in the orchard (SE 0.13, p<0.03). These patterns were not driven by male bees, and were more significant with only female bees.
A surprising finding that warrants further study was that of all male Andrena with pollen in their guts, 25% of those with pollen had gymnosperm-type pollen (that is, coniferous). These distinctive grains were highly abundant, suggesting that bees were either consuming them intentionally or otherwise spending sufficient time in conifers that accidental consumption was common. This pattern warrants further investigation in future research.
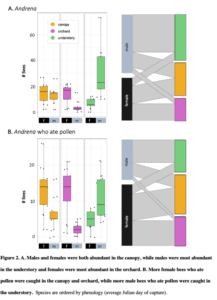
Objective 2: Orchard-pollinating wild bees move from understory to canopy to orchard
This work from a manuscript (Urban-Mead et al in prep) shows that male and female bee abundance in the understory peaks in in the early spring (~Julian day 122), in the understory about a week later (~Julian day 130), and in the orchard three weeks after that (~Julian day 157 ; all day peaks of maximum peak calculated using predict() based on a GLMM model with a Poisson distribution).
[click image to zoom]
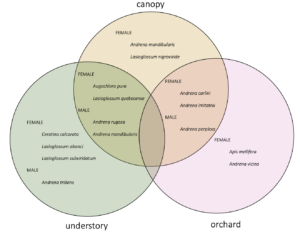
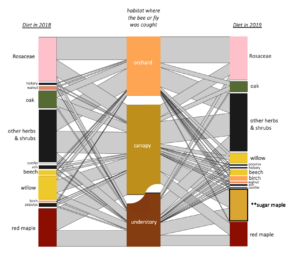
Male Andrena were mostly constrained to the understory and canopy, while females of the same species used the canopy and the orchard. This suggests that in order to conserve these important wild-pollinating orchard female bees, forest habitats must be nearby in order to support the males (to allow for their complete life cycle).
[click image to zoom].
When I calculated indicator species for each habitat (canopy, understory, and orchard) as well as for each pair of habitats, I identified indicator species for each habitat and pair except that no species were indicators for orchard-understory.
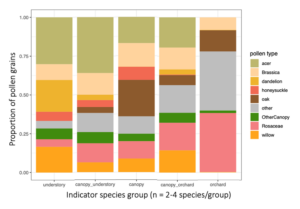
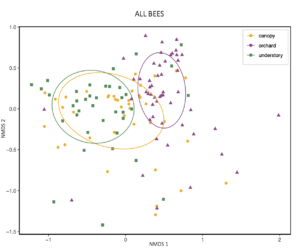
For the indicator species pairs, I then calculated their pollen diets (figure below). Moving from left to right, are the diets of the indicator species for: Understory, Canopy-Understory pair, Canopy, Orchard-canopy pair, and then Orchard. Similarly, as we move from left to right, we see an overall decreaes in maple (light green), willow pollen, and to some degree in "other canopy" pollen consumption (dark green), and an increase in Rosaceous (pink) and "other" pollen (gray). Oak pollen, which is co-blooming temporally with rosaceous pollen, was most eaten by indicator species of caught in the canopy and orchard.
Generally, two main features emerge from this figure: indicator species of forests eat more forest pollen, and across the continuum to the orchard, eat less forest pollen and more orchard pollen. Also, despite this big picture trend, pollen diets also reveal that bees in all habitats are clearly moving across strata. So, all habitats are needed but there is also free movement and combined resource usage across locations.
[Click to zoom]
An NMDS visualization also suggests that the diets of bees caught in the canopy are "transitional" between understory and orchard populations' diets.
[click to zoom]
Objective 3: Male Andrena consume more pollen than expected, but still less pollen than female bees
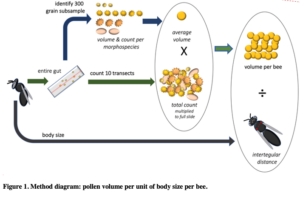
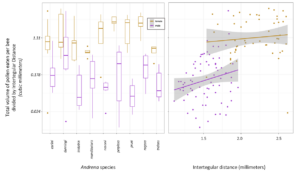
In a new manuscript with an undergraduate mentee, Edward Walter, (Urban-Mead KR, Walter E. et al in prep), we ask if male bees of the important crop-pollinating genus Andrena consume more pollen than previously expected, as we encountered many male Andrena who had eaten pollen during dissections. The generally accepted assumption is that male bees eat no pollen, except incidentally while getting nectar. To calculate this, we first counted the total number of pollen grains found in Andrenid digestive tracts and multiplied those by the weighted average of the pollen volumes of the identified morphotypes from dietary analysis. We then divided the total pollen by the bee's intertegular distances (a standard proxy for body size) in order to get a body-size adjusted volume of pollen consumption for each of 9 Andrena species for each sex.
[click image to zoom]
Ultimately, we found that 86.98% ± 0.08 of female bees had eaten pollen, while 46.3% ± 0.16 of male bees had. Female bees had eaten 9.33 times more pollen than males (calculated using emmeans). Female bees had more pollen in their guts than male bees (Wilcox Rank Sum test: p =5.52e-16). In one case a male Andrena dunningi male bee had mores occasionally had pollen volumes that exceeded the average female pollen gut volume. In our GLMM, we found significant differences between Andrena species (p = 0.008), and that sex was strongly significant (p<0.001). We found a trend between body size (ITD) in males (p = 0.06) and no relationship in females (p = 0.47).
[click image to zoom]
We suggest that male bees may eat more pollen than usually understood, and that future work should investigate male pollen preferences and possible implications for conservation and fitness or reproduction.
Objective 4: Influence of pollen availability and mast bloom patterns on pollen consumption patterns
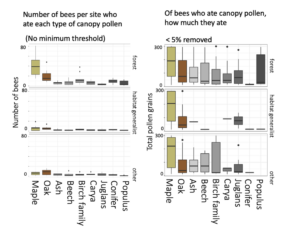
Bees caught in the canopy ate more pollen overall than bees in the understory, comparably to those in orchards, suggesting that canopy and orchard sampling were catching a higher proportion of foraging bees. Bees primarily ate maple and oak pollen -- which were also the most abundant tree genera in the woodlots. But, even if they were not widely eaten, canopy pollens were often a large portion of the individual bee's diet once they ate it.
[click to zoom]
The three panels in each of the above figures indicate whether Smith et al 2021 Biological Conservation (link) had categorized these bees as "forest-associated," as "habitat generalists," or as "other bees." Based on this exploratory visualization, it certainly seems as thought forest-associated bees consumed more canopy pollens, including of wind-pollinated trees, than did habitat generalists.
[click image to zoom]
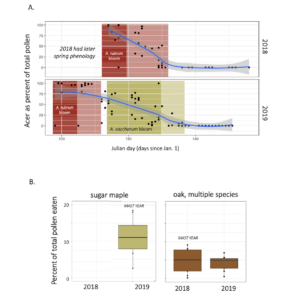
Sugar maple masting strongly influenced pollen diets. Red oak masted in the alternate year to sugar maple, but this was not evident dramatically as it was for sugar maple. We speculate that this is because there were multiple species of oak available on the landscape,
Many canopy trees were present in bees' diets (see network figure below), particularly in bees that were caught in the canopy. Without sampling in the canopy, we miss this aspect.
[click to zoom]
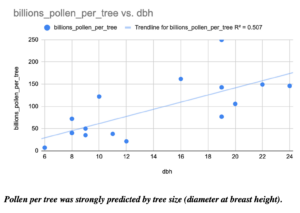
Finally, I counted the amount of pollen produced by many of our most common forest trees. For example, two Red Maple trees had 9,400 and 15,600 flowers, while two canopy red maples respectively had 157,100 and 132,800 flowers. Red maple flowers had an average of 10 anthers per flower, for a range of 94,000 to 1,571,000 anthers per tree of these four counted trees. In a silver maple, two trees had 17,800 and 510,100 flowers per tree, 19 anthers per flower for a range of 338,200 to 9,691,900 anthers per tree, and an average of 2330 pollen grains per anther, for a total estimate of 788,006,000 to 22,582,127,000 (~800 million to ~22 trillion) pollen grains per tree. In sugar maple, as seen below, the diameter at breast height of a tree strongly predicted the number of pollen grains (in billions!) that it produced.
[click image to zoom]
Ultimately, we conclude that wild bees active in orchards begin their springs by actively living and foraging in forests, and forage actively in forest canopies. Mast blooming dynamics influence these patterns, and tree diversity would thus be required in order to allow for resource availability across years. The canopy is a habitat that is dynamic and complex, and should be further studied to understand its nutritional implications for bees, and the relative preferences of bees for canopy pollens under different contexts.
K. R. Urban-Mead, P. Muniz, J. Gillung, A. Espinoza*, R. Fordyce, M. Van Dyke1, S. H. McArt1, B. N. Danforth. “Bees in the trees: Diverse wild bee communities in temperate forest edge canopies” Forest Ecology & Management (2021 In Press).
Generally, we find:
- Despite equal species richness among strata, woodlots had vertically stratified bee communities
- We found higher overall diversity and more female bees in the canopy
- The traits of nesting habitat and sociality were strongly predictive of strata occupancy
- Canopy cover (leaf-out) was negatively associated with understory but not canopy bee abundance
- Regional amount of coarse woody debris correlated with understory abundance of some trait groups
Pollen analysis of nearly 3000 bees and over 340,000 pollen grains further suggests:
- Bee activity peaks in understory a week earlier than their activity in the canopy, which peaks several weeks before they are most active in the orchard.
- Bees move from the forest to the orchard over the course of the spring
- Male bees, who eat more pollen than we previously thought, rely particularly on the forest.
- Male habitat may be a previously-overlooked reason why nearby forest supports diverse and abundant wild pollinators in apple orchards.
- Unless we sample in the canopy, we underestimate the amount of canopy pollen that bees eat.
- Mast blooming patterns by sugar maple are evident in bees' pollen diets. Mast blooming creates a dynamic and unpredictable resource landscape year to year, and is an important reason to have diverse tree communities.
- "Forest-associated" bees (sensu Smith et al 2021) eat more canopy pollen than habitat generalists
Management recommendations for growers include:
- Value nearby forest as pollinator habitat, overwintering habitat, and food resources
- Work with foresters to generate wildlife friendly forest management, particularly prioritizing management for:
- coarse woody debris, snags, and any other sources of deadwood
- species who offer resin, honeydew, nectar, and pollen value
- species diversity at the patch scale
- landscape scale successional diversity
- vertical structure
- Plant and maintain hedgerows
Education & Outreach Activities and Participation Summary
Participation Summary:
August 2021: Thanks to Meri Holm for the invitation to present at the Great Lakes Research Pollinator Task Force meeting! Great discussions and incredible folks.
May 22, 2021: Master Naturalist forest walk “Spring Ephemerals and their Pollinators” -- thanks to Kristi Sullivan at Natural Resources for inviting me to join this marvelous day as a pollinator guide!
April 2021: I had a great time presenting on wild bee ecology & common pitfalls in “bee-ginner” sampling for the FORCES intern program through NYS Parks DEC!
(CANCELED DUE TO COVID-19) June 2 2020: Schuyler County Conservation Field Days — helping with day-long festival outreach
(CANCELED DUE TO COVID-19) July 25, 2020: "Tent of Knowledge" Catskill Forest Festival – Invited presenter (50 minute presentation)
(CANCELED DUE TO COVID-19) September 25, 2020: Cleveland Pollinator & Native Plant Symposium —Invited Speaker
(CANCELED DUE TO COVID-19) September 22, 2020: Volunteer Pollinator Specialist program and ‘Urban Forestry’ career track student visit in Kent, OH;.
____________________________________________________
May 6, 2020: Catskill Forest Radio —Invited guest. https://catskillforest.org/radio-new/
I had fun chatting with Ryan Trapani & John MacNaught at Catskill Forest Radio. I’m sorry that the in-person event at the Catskill Forest Festival’s Tent of Knowledge was canceled, but look forward to joining in 2021.
____________________________________________________
May 14th, 2020: Oregon Bee Atlas Remote Speaker Series —Invited zoom presentation. (40 min)
As a follow-up to my interview on PolliNation with Andony Melathopolous at the Oregon State Extension Service, Andony and Linc Best invited me as a remote speaker as part of the Oregon Bee Atlas’ weekly speaker series! I stayed up late to give a west coast talk, and I’m glad that I did–144 people tuned in. whew!
The link to the youtube video is here, and the link to the Wild World of Bees full speaker series is here.
____________________________________________________
Staatsburgh State Historic Site March 2020: Video & social media outreach, cellophane bee aggregation
____________________________________________________
New York Forest Owner's Magazine Extension publication Jan 2020: Wild Bees Amidst the Trees
My article “Wild Bees Amidst the Trees” on bee biology and forest management for wild bee conservation in the New York Forest Owner’s Journal.
https://www.nyfoa.org/resources/archives-new-york-forest-owner/2020
____________________________________________________
Project Outcomes
I hope that this project will contribute to future sustainability by directly integrating pollinator conservation into agroforestry, silvopasture, and small-farm woodlot and hedgerow management. This will be particularly relevant for apple orchards, the focal commodity of our lab's research. However, many crops and wild plants benefit from or entirely rely on ecosystem services provided by free-living pollinators. As I begin my work with Xerces, I plan to maintain my relationships with all of the scientists with whom I have collaborated and communicated on forest-bee work over the past 4 years. I believe that continuing to integrate invertebrate and now pollinator perspectives into landscape-scale ecological forest management could be transformational in helping bridge the connections between agricultural and forestry initiatives, and to support carbon-sequestering and multifunctional projects such as agroforestry initiatives. This is particularly full of potential for communication efforts geared at spurring policy and landowner priorities. Although some of my objectives were modified due to COVID restrictions and barriers, the work on this project has only underscored the importance of forest management and generally of woody plants in order to enhance wild pollinator resiliency. Sustainability requires trees for bees!
This project was transformational in terms of my understanding of forests and bees, two important disciplines that have been historically quite siloed. Outside of my lab group, I have been able to work with Prof Mike Ulyshen from Georgia, Kristi Sullivan in Cornell DNR, Dr. DJ McNeil on intersections of forest management and bee and bird habitat, Rachael Winfree's vibrant lab at Rutgers which is increasingly working on forest bees and collaborating with Marlyse DuGuid at Yale Myers Forest, and many other folks. Orchardists, farmers, and others across Tompkins County have welcomed me onto their properties to discuss bees and forests. I am eager to continue working with growers, researchers, and community members to answer these exciting questions about the role of woodlots and forests in wild pollinator conservation. I am committed to sharing the knowledge I gain through careful research and collaboration. This current SARE project is deepening my natural history knowledge in the context of working agricultural lands, and should generate concrete outcomes in terms of being able to recommend woodlot and hedgerow management for pollinator conservation. Given the importance of trees for soil stabilization, wind breaks, carbon sequestration, forage, and numerous other sustainability benefits, I am particularly excited to find myself poised at the cusp of integrating pollinator conservation with these efforts. I will start in January as a pollinator conservation biologist with the Xerces Society and NRCS, and will carry the relationships, collaborations, and knowledge of these agricultural and forested systems directly into my hands-on conservation work going forward. I am incredibly grateful to have been funded and supported by SARE.
Future work should investigate synergies between forest management for wildlife (e.g. birds, mammals) with management for bees. Along these lines, the need for deer control to support regeneration of crucial bee-supporting canopy seedlings as well as understory plants is a strong need for both future research and public and policy communication. Outreach efforts should highlight the importance of all trees, including wind-pollinated trees, for bees. Second, communication should go through community forestry and, private landowner, and public channels to help highlight the importance of active ecological forest management and wildlife-oriented management for diversity of woodlots and forests. I am eager to learn, support, and contribute materials to any related effort, and would love to collaborate with folks and hear their perspectives on initiatives in these directions, or on collaborations I have not yet considered!