Final report for GNE19-213
Project Information
Results of the study demonstrate the feasibility of using LAB as an antimicrobial hurdle to promote the microbial safety of whole and cut apples. Specifically, we demonstrate the LAB spray application can be used along the processing continuum to reduce pathogen survival on apples under commercial refrigerated storage in the packing house and ambient storage in retail and home settings. Further, LAB strains used in the study can also be applied to cut apples prior to ambient/refrigerated to storage as a mean to reduce Listeria survival on cut fruit. Also, our results demonstrate that these LAB strains can survive in significant numbers on apples/cut apples under refrigerated and ambient storage conditions thereby providing a sustained antimicrobial effect. Overall, given our data, it is anticipated that these lactic acid bacteria cultures could be used as part of a multi-hurdle approach to provide sustained antimicrobial activity against foodborne pathogens on fruits including apples.
The overall objective of the proposed study is to determine the antimicrobial efficacy of select lactic acid bacteria for controlling L. monocytogenes at high (5 log CFU/apple) and low inoculation (3 log CFU/apple) levels on whole apples. A high inoculum level (5 log CFU/apple) will be used to enable measurement of several log reductions in pathogen counts during the study. Additionally, this study will incorporate a low level of inoculum (3 log CFU/apple) in order to simulate low levels of pathogen contamination that are likely to occur under normal postharvest handling and storage conditions. In order to simulate commercial conditions, Listeria control on apples will be specifically evaluated following: 1. Spray application of LAB and fruit storage under conditions simulating cold storage at the packing house. 2. Spray application of LAB and apple storage under conditions simulating room temperature storage in retail and home settings. 3. Spray application of LAB on fresh-cut apples under conditions simulating storage at the retail outlet and at home.
To date, there are two multistate foodborne outbreaks linked to Listeria monocytogenes with the consumption of apples, indicating that contaminated whole apples may serve as a vehicle for foodborne listeriosis. Apples can get contaminated pre-harvest in the field, post-harvest in the processing plants and during storage and shipping. Listeria monocytogenes can survive on apples under refrigeration and ambient storage conditions. Therefore, there is a critical need for effective antimicrobial strategies to control pathogens on apples. Lactic acid bacteria (LAB) can serve as excellent candidates to reduce pathogen levels on apples.
Research
Apples: Gala apples were purchased from local grocery store and stored at 4°C. A Day before the experiment, the required number of fruits were transferred to room temperature (22°C) for tempering prior to use (Penteado et al., 2004).
Bacterial strains and culture conditions: A five-strain cocktail of L. monocytogenes (580-072, 583-060, LM1, LM2, LM3 – caramel apple and apple isolates) were used in the study. All L. monocytogenes strains were induced for resistance to nalidixic acid (NA; 50 µg/ml) to facilitate selective enumeration of the inoculated pathogen (Danyluk et al., 2014). Each strain was cultured separately in 10 ml of sterile tryptic soy broth (TSB) containing NA (50 µg/ml) at 37°C for 16-18 h. The bacterial cells were then centrifuged at 3500 g for 10 min and washed twice with sterile phosphate buffered saline (PBS, pH7.0). Equal portions from each of the five strains were combined to make the pathogen cocktail. The bacterial population in the five-strain mix were determined by plating appropriate dilutions on Modified Oxford agar with NA (MOX-NA) followed by incubation at 37°C for 24-48 h (Danyluk et al., 2014; Ukuku et al., 2012). Appropriate dilutions of the five-strain mixture in PBS were used to obtain the desired level of inoculum (5 and 3 log CFU/ fruit). Five strains of lactic acid bacteria including Lactococcus lactis B-23802 (LL23802), L. lactisB-23804 (LL23804), Lactobacillus plantarum B-4496 (LP4496) and L. rhamnosus B-442 (LR442), all obtained from the ARS Culture Collection (NRRL) were grown aerobically in 10 ml of sterile de Man Rogosa and Sharpe (MRS) broth (Oxoid) at 37°C for 16-18 h. Following incubation, the cells were washed and resuspended in PBS to obtain the desired inoculum (9 log CFU/apple). The bacterial population in the LAB inoculum were determined as previously described (Carey et al., 2008; Muyyarikkandy and Amalaradjou, 2017).
Fruit inoculation: In order to facilitate LAB enumeration, apples were sanitized prior to inoculation by spraying with 70% ethanol and wiping with a sterile paper (Baskaran et al., 2013). Five strain mix of L. monocytogenes were prepared as described previously. Fruits were inoculated by applying inoculum (5 and 3 log CFU/ fruit) to the stem end (Glass et al., 2015). Briefly, in order to simulate pathogen contaminated apples, 100 µl of the L. monocytogenescocktail (5 and 3 log CFU/ fruit) were placed into the stem end of the apple. After inoculation, fruits were held for 1.5 h at room temperature in a biosafety hood for the inoculum to dry (Baskaran et al., 2013). Staggered inoculation of the fruits was performed to maintain consistent drying time for all fruits under each objective (Parnell and Harris, 2003). Following drying, pathogen populations on inoculated fruits from each experiment were enumerated to ascertain initial bacterial load. Microbiological analysis was performed as described below.
Spray application of LAB and fruit storage under conditions simulating cold storage at the packinghouse: Overnight LAB cultures (LL1/LL2/LP/LR) were washed and resuspended in PBS to obtain the desired inoculum (10 log CFU/ml). Each LAB culture was sprayed on to the inoculated apples in a biosafety cabinet using gravity-feed dual action air-nozzle sprayer (Adaskaveg and Forster, 2003). After treatment, the apples were dried, fruit were packed in sterile containers and stored at 2.5-3.5°C and RH-90-95% for one week. Surviving L. monocytogenes and LAB population (n = 6 fruits/treatment/time/inoculation level) were enumerated at different times over a period of 3 weeks (day 0, 1, 5 and 7) as described below.
Spray application of LAB and fruit storage under conditions simulating storage at the retail outlet and at home: Inoculated fruit were sprayed with LAB (LL1/LL2/LP/LR) as previously described. Fruits were dried in the biosafety cabinet and were transferred to sterile containers and stored at 22-24°C and 40-50% relative humidity for 3 weeks to simulate fruit storage at the retail and at home. Surviving L. monocytogenes and LAB population on the apples (n = 6 fruits/treatment/time/inoculation level) were enumerated at different times over the 3-week storage period (day 0, 1, 5, 7, 14 and 21) as described below.
Spray application of LAB on fresh-cut apples under conditions simulating storage at the retail outlet and at home: Whole apples were cored using a corer to generate apple plugs (2 cm diameter) for the apple plug study. This study using apple plugs helped obtain initial data for the cut-apple experiments. To generate cut-apples, the stem end of the apple was cut and used to evaluate Listeria populations during storage. Fruits will be spot inoculated with the bacterial cocktail (6 log CFU/plug or apple half) by placing 50 µl of a five-strain mix around the stem end/ on the plug. After inoculation, fruits will be held for 2 h at room temperature in a biosafety hood for the inoculum to dry. For the antimicrobial treatment, appropriate dilutions of the overnight LAB cultures (LL1/LL2/LP/LR) were sprayed on to the inoculated apples using the air-nozzle sprayer and held in the biosafety cabinet for an additional hour to allow for drying. They were then placed in sterile containers and stored at 22 or 4°C for 7-14 days. Surviving L. monocytogenesand LAB population on the apples were enumerated at different times over the 14-day storage period (day 0, 1, 3, 5, 7, 14) as described below.
Microbiological analyses: At each sampling time, apples/apple plugs/half-apples were individually transferred to sterile stomacher bags containing 20 ml of BPW. Each sample was hand rubbed for 2 min, and 0.1% buffered peptone water (BPW) were used to sample L. monocytogenes and LAB populations. Briefly, BPW from the stomacher bags containing apples were serially diluted in BPW and duplicate 0.1 ml of the suspensions were plated on MOX-NA for L. monocytogenes. LAB populations: In addition to sampling for pathogen populations, the dilution series was also be plated on MRS agar to enumerate surviving LAB populations (Carey et al., 2008; Muyyarikkandy and Amalaradjou, 2017). For L. monocytogenes, in addition to enumeration, BPW samples were enriched in Buffered Listeria Enrichment Broth BLEB at 37°C for 24 h. A 1-ml aliquot of the BPW sample were added to 9 ml of BLEB and incubated at 37°C for 24 h (Ukuku et al., 2012; Glass et al., 2015). When counts for the respective samples were negative by direct plating, enrichment broths were streaked on MOX-NA and incubated at 37°C for 48 h. In this study, BPW were used in place of Buffered Listeria Enrichment Broth (BLEB) to enable simultaneous enumeration of pathogen and LAB populations.
Statistical analysis: Six samples for each treatment at each sampling time were included in each experiment and two independent trials were conducted. The experiments were set up as a completely randomized design. Pooled data were analyzed using ANOVA in R. Differences among the means were detected at p ≤ 0.05 using the Fisher’s least significance difference test with appropriate corrections for multiple comparisons.
Results and discussion:
- Spray application of LAB and fruit storage under conditions simulating cold storage at the packing house.
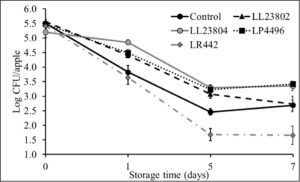
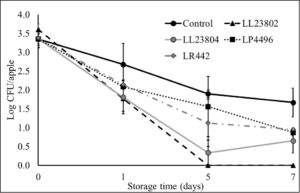
This experiment was performed to investigate the antimicrobial effect of probiotic spray application on apples stored under packing house storage conditions (2.5-3.5ºC, 90-95% relative humidity) on Listeria survival on apples. To obtain a high inoculum load (5 log CFU/apple), apples were spot inoculated with ~9 log CFU/ml. Following inoculation, the apples were held at room temperature in the biosafety cabinet for 1.5 h to allow for the pathogen to attach to the fruit surface. At the end of this period, approximately 5.5 log CFU/apple was recovered from the fruits. The fruits were then subjected to probiotic spray treatment or PBS spray (control), allowed to dry, and stored for 7 days. Spraying by itself (control samples) was not found to significantly reduce pathogen populations when compared to pathogen counts on unsprayed apples. However, LAB spray application was found to significantly reduce Listeria populations on apples throughout the storage period (P<0.05). Specifically, spray application of LR 442 was found to reduce Listeria populations to ~1.6 log by day 5 of storage. However, ~ 2.5-2.7 log CFU/apple was recovered from the control apples at the end of the study. It is also important to note here that we observed a steady reduction in pathogen populations in all the groups over the storage period (Fig. 1). Similarly, at the low inoculum level (3 log CFU/apple), the pathogen population in the control reduced from 3.3 log CFU on day 0 to 1.67 log on day 7 of storage. Further, as previously observed, LAB application significantly reduced pathogen populations when compared to the control. Among the different treatments, LL23804, LP4496 and LR 442 reduced Listeria populations to below detection limits by day 5 of storage (enrichment positive). On the hand, we were unable to recover Listeria (enrichment negative) from LL23802 treated samples by day 5 of storage (Fig. 2). Overall, spray application of LAB to apples prior to cold storage was found to significantly reduce pathogen population on the apples at high and low pathogen load (P<0.05). In addition to pathogen population, we also enumerated surviving LAB populations on the apples during refrigerated storage. Approximately 8 log CFU of the LAB was recovered from the apples at the start of the trial. Although we observed a steady reduction in their population during storage, significant numbers were recovered at the end of the storage period. Specifically, we recovered ~ 6 -7 log CFU of the different LAB strains by 7 days of cold storage (data not shown). This indicates that the LAB strains used in this study survived in significant numbers on the fruit surface under refrigerated storage thereby exerting a sustained effect against pathogen populations.
Fig. 1. Listeria survival on LAB treated apples under refrigerated storage at high pathogen load
Fig. 2. Listeria survival on LAB treated apples under refrigerated storage at low pathogen load
- Spray application of LAB and fruit storage under conditions simulating storage at the retail outlet and at home:
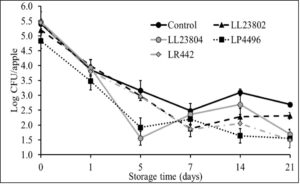
Besides refrigerated storage, apples are commonly held at room/ambient temperature during retail display and at home. Hence, in addition to controlling pathogen populations under refrigeration conditions, it is equally important to reduce Listeria population during ambient storage to improve the overall microbial safety of whole apples. Therefore, the second objective in this study evaluated the efficacy of the LAB strains in reducing Listeria population on apples stored under ambient conditions (22-24ºC, 40-50% relative humidity for 3 weeks). This study was performed using the high inoculation level. Following fruit inoculation and drying, approximately 5.5 log CFU/apple was recovered from the fruit. As previously observed, spray application of PBS (control) was not found to significantly reduce pathogen population on the apples. However, spray application of the different LAB significantly reduced Listeria survival on apples stored under ambient conditions (P<0.05). Specifically, in the control samples, ~5.4 and 2.6 log CFU of Listeriawas recovered from the fruit on day 0 and 21 of storage, respectively. On the other hand, application of LP4496 was seen to significantly reduce pathogen populations throughout storage (4.8 log on day 0 to 1.5 log CFU on day 21). Similarly, treatment with LL23804 and LR 442 also resulted in significant reduction in pathogen population by day 14 of storage when compared to the control (Fig. 3). As observed under refrigerated conditions, LAB strains were found to survive in significant numbers on the apples stored under ambient conditions throughout the study period (data not shown). This again implies that LAB can be used as a spray application to control Listeria populations and promote the microbial safety of apples.
Fig. 3. Listeria survival on LAB treated apples stored under ambient conditions
- Spray application of LAB on fresh-cut apples under conditions simulating storage at the retail outlet and at home:
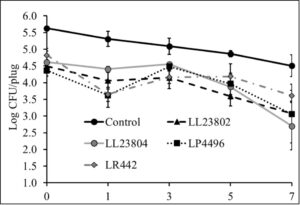
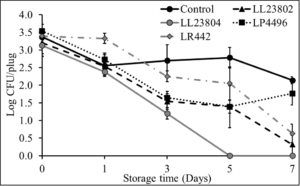
Initial pilot experiments were performed using apple plugs (2 cm diameter). Once cored, the apple plugs were spot inoculated with Listeria followed by spray application of the different probiotic treatments. The effect of LAB spray application on Listeria populations on apple plugs at the high inoculum stored at 4°C is depicted in Fig. 4. As can be seen from the graph, there is a reduction of 0.81-1.26 log CFU/apple plug by all LAB treatment groups on day 0 of the study. In addition, significant reduction in Listeria populations in the LAB treated samples was observed throughout the study (P<0.05). Specifically, LL23802, LL23804, LP4496 and LR442 reduced Listeria populations to 3.08, 2.70, 3.06 and 3.61 log CFU/apple plug at day 7, respectively, while approximately 4.51 log CFU of Listeria was still recovered in the control group at the end of the storage period. At the low inoculum level, we did not observe any significant decrease in pathogen population between the control and treated samples at the start of the study. However, LAB spray treatment led to significant reduction in Listeria survival on the apple plugs during the 7-day refrigerated storage period (P<0.05, Fig. 5). Spray application of LL23802 and LR 442 reduced pathogen populations to below detection limits (enrichment positive) by day 7 of storage. This initial data implies that LAB spray application can be applicable to cut apples to improve their microbial safety. However, to further validate these data subsequent experiments were performed using cut/half apples.
Fig. 4. Listeria survival on LAB treated apple plugs under refrigerated storage at high pathogen load
Fig. 5. Listeria survival on LAB treated apple plugs under refrigerated storage at low pathogen load
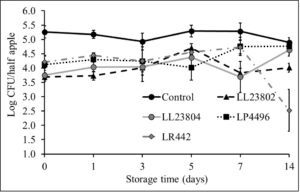
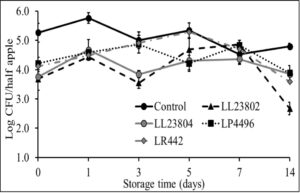
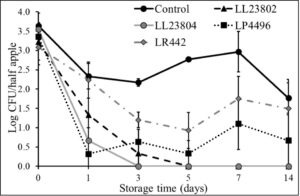
Given the data observed with apple plugs, additional experiments were performed using half-apples to investigate the efficacy of LAB in reducing Listeria survival on cut-apples. The apple was cut into two halves and the stem end of the apple was inoculated with Listeria (high/low inoculum) and sprayed with PBS (control) or the different LAB strains as previously described. The treated cut-apples were then stored at ambient or refrigerated conditions for 14 days. The effect of LAB spray on Listeria populations on half apples at the high inoculum level stored at 4°C and 22°C is shown in Fig. 6 and Fig. 7. Results indicated that there is a 1-1.5 log CFU reduction in Listeria populations in all LAB treatment groups on day 0 (P<0.05). Further, the reduction in pathogen load is sustained from day 0 to day 14 of refrigerated storage. On day 14, LR442 and LL23802 reduced Listeria populations to 2.5 and 4 log CFU, respectively, when compared to the control (4.9 log CFU/half apple). Similarly, when stored under ambient conditions, Listeriapopulations on the cut apple was significantly reduced by LAB treatment (P<0.05). On day 14, in the LL23802 treated group Listeria populations were reduced by ~ 2 log CFU/half apple at 22 °C, while the Listeria populations in the control group was 4.79 log CFU/half apple.
Fig. 6. Listeria survival on LAB treated cut-apples under refrigerated storage at high pathogen load
Fig. 7. Listeria survival on LAB treated cut-apples under ambient storage at high pathogen load
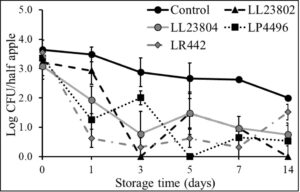
As with the high inoculum, experiments were also performed to evaluate the efficacy of LAB strains to reduce Listeriapopulations on cut apples inoculated at the low inoculum level (3 log CFU/half apple) under refrigerated (Fig. 8) and ambient storage conditions (Fig. 9). At 4°C (Fig. 8), spray application of LL23802, LL23804 and LP4496 reduced Listeria populations to below detection limit by day 7 of storage while there ~ 2.7 log CFU of Listeria was recovered in the control group (P<0.05). Overall, LAB spray application reduced pathogen populations by 2-4 log CFU/half apple by 14 days of refrigerated storage. Similarly, under ambient storage, LAB spray application led to significant reduction in pathogen population when compared to the control (Fig. 9). Specifically, LL23804 reduced Listeria populations to below detection limit by day 1 of storage. LL23802 reduced Listeria to 1.33 log CFU/half apple on day 1, and below detection limit by day 5. Similarly, LP4496 reduced Listeria population from 3.36 log CFU on day 0 to below detection limits on day 14. However, with the control samples, approximately 1.8 log CFU of Listeria was still recovered at the end of the study.
Fig. 8. Listeria survival on LAB treated cut-apples under refrigerated storage at low pathogen load
Fig. 9. Listeria survival on LAB treated cut-apples under ambient storage at low pathogen load
Results of the study demonstrate the feasibility of using LAB as an antimicrobial hurdle to promote the microbial safety of whole and cut apples. Specifically, we demonstrate the LAB spray application can be used along the processing continuum to reduce pathogen survival on apples under commercial refrigerated storage in the packing house and ambient storage in retail and home settings. Further, LAB strains used in the study can also be applied to cut apples prior to ambient/refrigerated to storage as a mean to reduce Listeria survival on cut fruit. Also, our results demonstrate that these LAB strains can survive in significant numbers on apples/cut apples under refrigerated and ambient storage conditions thereby providing a sustained antimicrobial effect. Overall, given our data, it is anticipated that these lactic acid bacteria cultures could be used as part of a multi-hurdle approach to provide sustained antimicrobial activity against foodborne pathogens on fruits including apples.
Education & Outreach Activities and Participation Summary
Participation Summary:
- Poster presented at International Association for Food Protection Annual meeting, Oct 2020 : Use of Lactic Acid Bacteria to Control Listeria monocytogenes on Apples during Simulated Storage Conditions (2020) Deepa Ashwarya Kuttappan, Mairui Gao & Mary Anne Amalaradjou
Project Outcomes
Results of the study demonstrate the feasibility of using LAB as an antimicrobial hurdle to promote the microbial safety of whole and cut apples. Specifically, we demonstrate the LAB spray application can be used along the processing continuum to reduce pathogen survival on apples under commercial refrigerated storage in the packing house and ambient storage in retail and home settings. Further, LAB strains used in the study can also be applied to cut apples prior to ambient/refrigerated to storage as a mean to reduce Listeria survival on cut fruit. Also, our results demonstrate that these LAB strains can survive in significant numbers on apples/cut apples under refrigerated and ambient storage conditions thereby providing a sustained antimicrobial effect. Overall, given our data, it is anticipated that these lactic acid bacteria cultures could be used as part of a multi-hurdle approach to provide sustained antimicrobial activity against foodborne pathogens on fruits including apples.
It was found that spray application of protective cultures used in this study could serve as the antimicrobial strategies to control Listeria monocytogenes on apples and fresh-cut apples. Besides, this strategy could also be applied to control pathogens on other fresh produce.
In the future, I am interested in developing intervention strategies to control foodborne pathogens.