Final report for GNE20-226
Project Information
Beekeepers are experiencing over $1 million in annual losses when pollinating NJ blueberries. Fungicide use over the last decade has increased during bloom which correlates with colony declines. Therefore, investigations on the effects of fungicides are needed.
Initial work showed that bees may prefer fungicide laced food sources, while other research has shown bee attractiveness to some fungicides. As part of my dissertation, I found 36 pesticides returned to the hive in pollen, including numerous fungicides. New papers point to the negative impact of fungicides on different bee species, including honey bees. My initial tests showed that some high fungicide rates caused larval mortality. Therefore, I needed to determine the influence of blueberry fungicide use on foraging behavior as well as impacts on brood development.
My objectives were: 1) determine the influence of commonly used blueberry fungicides on honey bee foraging behavior, 2) determine if this behavior influences residues being introduced to the colony, and 3) determine the impacts of in-hive residues on brood development.
Research components included: Field studies that recorded honey bee forager visitations in number and consumption rate as influenced by plain syrup vs syrup with various fungicides; field studies that recorded honey bee forager visitations to blueberry bushes sprayed with water or fungicide; pollen analyses for pesticide residues in hives, and in vitro feeding tests with fungicide amended diet to examine resulting effects on brood and maturation.
Outreach included extension newsletters, web-based blogs, grower winter and spring update meetings, and beekeeper meetings. This work was used to update the pollinator protection chapter in the NJ Blueberry Production Guide.
Results: Foragers showed preference for pure sugar syrup over syrup laced with fungicides. Foragers did not show preference to bushes when sprayed with Quash, Pristine, and Switch. Foragers showed avoidance behavior to bushes when Abound was applied and allowed to dry. Foragers showed attraction behavior to bushes when Ziram was applied (wet and dry). Bushes treated with fungicide at night had less diseased berries than bushes treated during the day. All pollen collected during blueberry pollination contained blueberry fungicide residues, with the active ingredients in Pristine and Switch found in highest concentration. Ziram was not included in the pollen residual analysis. Using pollen traps and supplemental pollen patties in attempts to reduce pesticide exposure had a more negative impact on colony health than colonies which were allowed to bring in natural pollen during blueberry pollination. Ziram was the most toxic (LC50 of 18 ppm) when to honey bee larvae in an artificial diet and Switch had the lowest larval toxicity (LC50 of 6,111 ppm).
Impacts: These findings are being used by growers and beekeepers to reduce pesticide exposure to honey bee colonies and increase blueberry yields through effective pollination and disease management. Ziram is no longer recommended for use during pollination when bees are present as it attracts foragers and is toxic when fed to honey bee larvae. Abound is not recommended for use when good pollination weather is expected as it could reduce pollination visits. Growers are fitting their sprayers with lights to apply fungicides at night (reduces fungicide exposure to bees and provides better disease control).
This project extends the work being done on an existing SARE grant, now coming to a conclusion in its 3rd and final season, but having opened additional questions relative to bee behavior and fungicide toxicity. The proposed work is designed to address those questions not addressed in the original SARE project.
Objective 1. Determine the influence of commonly used blueberry fungicides on honey bee foraging behavior.
Objective 2. Determine fungicide levels in honey bee hives, and when they are most likely to enter the hive in relation to fungicide spray programs.
Objective 3. Determine if there is any impact on honey bee brood development and resulting colony health by fungicide levels found in the hive as influenced by objectives 1 and 2.
The purpose of this project was to identify the behavioral and developmental effects of fungicide use on honey bee colonies used for blueberry pollination. I examined the impacts of fungicide use during bloom on bee forager behavior, resulting hive contamination, and effects on brood development. Declines in brood development during and shortly after pollination services have left beekeepers with severe colony losses resulting in up to 90% hive mortality. Thus, threatening the sustainability of commercialized blueberry pollination. The NJ blueberry industry is valued between $65-70 million annually and is produced on just over 9,000 acres in the southern NJ Pinelands. NJ blueberry growers contract with NJ and regional beekeepers to bring in about 18,000 colonies each year for pollination.
Hives with greater pesticide exposure show higher rates of death. In some cases multiple pesticides may show additive or even synergistic mortality effects to bees. Most older literature has focused on pesticide effects on adult bees, and most of this was done with insecticides. Only recently have researchers started to look at larval responses to pesticides, with fungicides being the newest frontier. Since fungicides are heavily used in blueberries during the pollination period, several areas were needed to be investigated.
I examined the behavior of adult foragers to commonly used fungicides, and the resulting residue levels brought back to the hive. Additionally, I looked at the developmental effects of fungicide contaminants.
By conducting larval bioassays with fungicide laced diets, I identified fungicides that pose risks to bee development. The identification of any negative impacts of fungicides can be used to support mitigation strategies and improve recommendations by Rutgers research and extension faculty. My findings were presented at apicultural and agricultural meetings across the Eastern states. Such findings are improving honey bee health, impacting pest management practices, reducing beekeeper losses and reducing production costs for fruit growers.
Research
Behavioral tests for adult honey bee forager response to fungicides
This study investigated the effects of 5 fungicides commonly used in blueberries on adult honey bee foragers. The formulated fungicides that were evaluated included Ziram (active ingredient [a.i.] = zinc dimethyldithiocarbamate), Abound (a.i.= azoxystrobin), Quash (a.i. = metconazole), Switch (a.i. = cyprodinil & fludioxonil), and Pristine (a.i. = boscalid & pyraclostrobin). These tests included fungicides from 5 FRAC groups: 3-DMIs, 7-SDHIs, 11-Qols, 9-Anilino-Pyrimidines, and M03-dithiocarbamates.
In 2021, the formulated fungicide products were mixed in sugar (sucrose) syrup and compared to pure sugar (sucrose) syrup alone. I used tunnel enclosures similar to those in Liao et al. (2017). I trained 5-framed hives with similar weights and sister queens, consisting of approximately 3,000 honey bees (one per tunnel) to feed on 2 separate but identical sugar syrup feeders. The feeders consisted of red (non-see-through) inverted plastic containers with approximately fifty holes (1-mm in diameter) evenly dispersed just below the lid of each container (syrup dispenser). They were placed on 2 separate cinder blocks oriented in a straight line on one side of the enclosure (opposite side of the hive). After two days of foraging at each feeder, I began the experiment. Each feeder was filled with the appropriate diet and weighed before and after each run to determine consumption rate. An experimental run consisted of 30-minute visual observations where the number of foragers visiting each feeder was recorded. Visits were recorded when a forager was seen extending its proboscis into the syrup dispenser. Two runs were conducted each day per hive and continued for a total of 5 days. An appropriate diet was replaced and measured as needed. On day 5, colonies were weighed and visually inspected for any adverse effects of the treatment and used for comparison in my behavior response analysis. Each experiment was repeated 4 times (over 4 weeks), and to ensure there was no bias to feeder location, the feeders were placed in a new location each time. Data on consumption rate and larval mortality were analyzed using analysis of variance (ANOVA) among the 5 fungicide treatments.
In 2022, I conducted a study using potted blueberry plants and the same 5 fungicides. For each fungicide, there were two groups: 1) Day Spray, where bushes were sprayed immediately prior to visual observation and 2) Night Spray, where bushes were sprayed 12 hours prior to visual observation. Each colony was presented with two new bushes each day, just prior to beginning a 30-minute visual observation, in individual flight tunnels. One of these bushes was sprayed with fungicide and the other was sprayed with water according to their treatment (sprayed at night or during the day). This study continued for 5 consecutive days using the same colony in each treatment and fungicide group (each colony was only used for 1 week and was in the same treatment group using the same fungicide). At the end of each trial (day 5), new colonies replaced the original colonies and given 2 days to forage from bushes that had not been sprayed. The experiment was repeated 3 times. Blueberry samples were collected from treatment and control bushes when ripe (1 pint per 5 bushes) and allowed to incubate at room temperature (20˚C) for 7 days. After incubation, the presence of disease was recorded and analyzed via ANOVA.
Fungicide residues in pollen from commercial blueberry farms
Based on the behavioral response of foragers to fungicides, honey bee hives were placed in the center of commercial fields and monitored for fungicide residues in pollen. I coordinated residue sampling with commercial grower participants so that a total residue load in the colony’s food stores could be calculated. I had previously determined fungicide residues that are present in pollen at the peak of bloom. However, this ‘1 shot’ sampling does not give us information over time or enable a correlation with fresh fungicide applications with the developmental stage of the bees. For this, I collaborated with 4 blueberry growers who each have individual farm sites of over 200 acres. Four hives were placed in the centers of each of these 4 farms so that the foraging ability of the bees was mostly restricted to those fields, and any fungicide residues found were primarily a reflection of the growers’ spray programs. At each farm site, 2 hives were fitted with pollen traps to prevent the entry of foraged pollen. Each of these 2 hives (treatment hives) on each farm were provided substitute pollen patties (Mann Lake) as a protein source. The other 2 hives (standard) on each farm did not have pollen traps and were freely exposed to all pollen returned to the hive. This gave an experimental design with 2 treatments and 4 replicate farms. After each fungicide application (within 8 to 24 hours after application, depending on application method), pollen was sampled from 1 of the 2 pollen trapped hives. Pollen was collected every 24 hours for 7 days, or until the next application, where the process started again. The other pollen trapped hive was not sampled, but all 4 hives were examined for parameters of hive health. These measurements include hive weight, number of frames with brood, percent brood coverage, egg and queen presence, and disease incidence. At the end of pollination (~3.5–4 weeks), hives received a final sampling for hive health and residue samples. Data on hive parameters and residues were analyzed using ANOVA.
Fungicide impact on honey bee brood
Using the fungicides found to be attractive and those found at high residue levels in pollen (based on previous studies), I examined those fungicides in in-vitro feeding tests. Each residue was tested in its pure active ingredient form. The concentrations of each were evaluated on a gradient of 3, 6, 12, 24, and 48 ppm. A control of pure diet and acetone control (solvent) were included. Laboratory procedures were adapted from Schmehl et al. (2016). I established eight 5-framed honey bee hives with newly mated sister queens. Inside each hive there was a caged frame on empty drawn comb and the queen, this frame was swapped out every week (four days prior to larvae extraction) to ensure all larvae were of the same exact age. First instars were grafted to 48 well-plates and fed fungicide laced diets, with 48 larvae per treatment and each treatment was replicated 3 times. Larval mortality was recorded daily until the sixth day. Dosing of fungicide active ingredients in diet occurred on day 3–6. Mortality was recorded at 48, 72, 96, and 120 experimental hours. All grafting, feeding, and data collection were conducted inside a positive laminar flow hood which was sterilized for 30-minutes before and after each procedure via UV light and by wiping down with 70% ethanol. Data on larval mortality were analyzed using ANOVA among treatment rates and probit analysis for the effects of pesticide concentration on larval mortality.
Behavioral tests for adult honey bee forager response to fungicides
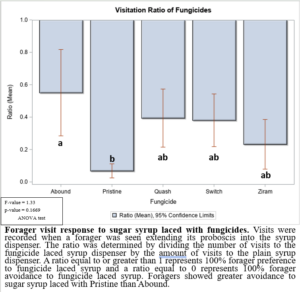
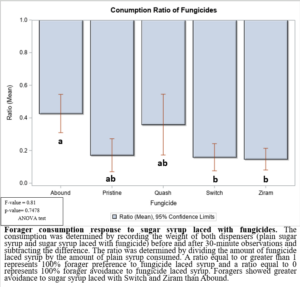
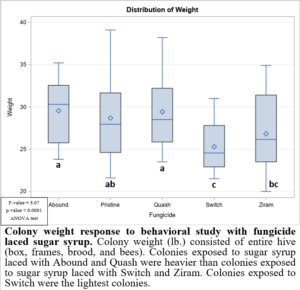
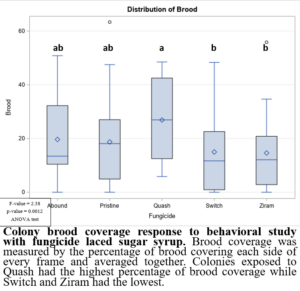
In the 2021 forager behavioral study, all foragers showed preference for dispensers with pure sugar syrup, i.e., I found lower visits and consumption rates to sugar syrup laced with fungicides (Figure 1). Despite foragers preferring plain sugar syrup, foragers consumed more sugar syrup laced with Abound than sugar syrup laced with Switch or Ziram (Figure 2). Visit ratios were slightly different than consumption ratios. While Abound showed the least amount of avoidance (forager visits), Pristine had the greatest amount of avoidance (forager visits), and Quash, Switch, and Ziram were not significantly different from any of the fungicides tested. Colonies exposed to sugar syrup laced with Abound and Quash were heavier than colonies exposed to sugar syrup laced with Switch and Ziram (Figure 3). Colonies exposed to Switch were the lightest colonies. These results indicate differences in adult honey bee foragers avoidance to fungicide-treated food. Colonies exposed to Quash had the highest percentage of brood coverage while Switch and Ziram had the lowest (Figure 4). This indicates that in addition to being consumed the least, Switch and Ziram have a greater negative impact on brood production.
Figure 1: Forager visit response to sugar syrup laced with fungicides
Figure 2: Forager consumption response to sugar syrup laced with fungicides.
Figure 3: Colony weight response to behavioral study with fungicide laced sugar syrup.
Figure 4: Colony brood coverage response to behavioral study with fungicide laced sugar syrup.
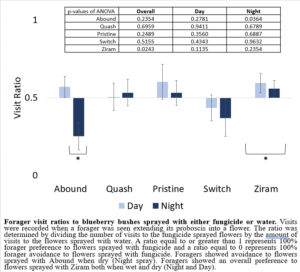
My 2022 results using potted blueberry bushes sprayed with fungicides were different from those in 2021. While foragers did not show a preference between a bush sprayed with water and a bush sprayed with either Quash, Pristine, or Switch, foragers showed a strong preference in the treatments where bushes were sprayed with Ziram or Abound (Figure 5). Ziram-treated bushes had higher forager visits overall than the bushes sprayed with water in both treatment groups (day spray and night spray), suggesting that foragers preferred Ziram-treated bushes over bushes sprayed with water. Abound-treated bushes had less forager visits than bushes sprayed with water in the night spray treatment group, suggesting that Abound, when dry, acts as a forager repellent.
Figure 5: Forager visit ratios to blueberry bushes sprayed with either fungicide or water.
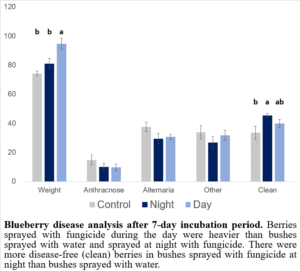
There were no differences in disease incidence among treatments; however, bushes treated with fungicides at night had more clean, disease-free blueberries than bushes that were sprayed with water while there was no difference in the number of clean berries from bushes sprayed with fungicides during the day (Figure 6). This finding (applying fungicides at night) could be used to reduce fungicide exposure to pollinators and reduce disease incidence in blueberries. Interestingly, however, berries in the treatment with fungicides sprayed during the day were the heaviest, suggesting a potential tradeoff between disease prevention and berry size.
Figure 6: Blueberry disease analyses after 7-day incubation period.
Fungicide residues in pollen from commercial blueberry farms
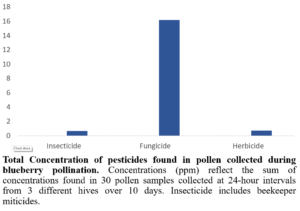
Fungicide residues in all pollen samples collected during blueberry bloom contained high levels of the active ingredients found in Pristine and Switch (Figure 7). Note that the analytical lab could not analyze the residues for Ziram so concentrations of this fungicide in pollen remain unknown. It is worth mentioning that Switch and Pristine, the two fungicides found in highest concentration in pollen, are applied to blueberry bushes at field rate concentrations of 1,310 ppm and 1,309 ppm, respectively. Ziram is applied at a field rate concentration of 7,284 ppm; therefore, further investigation of Ziram residues in pollen is needed to effectively assess its toxicity risk to honey bees.
Figure 7: Total concentration of pesticides found in pollen collected during blueberry pollination.
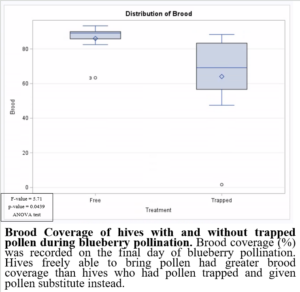
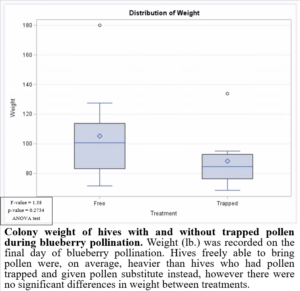
Preventing pollen from entering the hive while providing supplemental pollen during blueberry pollination had negative impacts on brood production when compared to colonies which were able to freely forager for pollen (Figure 8). This may have been due to the limited nutrients provided by the supplemental pollen. Based on these results, it would not be beneficial to trap pollen in pollination settings. Trapped pollen had high residues of fungicides, suggesting potential fungicide exposure to colonies. However, the colonies exposed to fungicides in pollen were, on average, heavier (Figure 9) and produced more brood (Figure 8). These results highlight the importance of honey bee nutrition and require further investigation.
Figure 8: Brood coverage of hives with and without trapped pollen during blueberry pollination.
Figure 9: Colony weight of hives with and without trapped pollen during blueberry pollination.
Fungicide impact on honey bee brood
Larval toxicity bioassays showed different LC50s for all 5 fungicide active ingredients (azoxystrobin, Abound; metconazole, Quash; boscalid & pyraclostrobin, Pristine; cyprodinil & fludioxonil, Switch; zinc dimethyldithiocarbamate, Ziram) (Table 1). Ziram was shown to be the most toxic when fed to honey bee larvae, with an LC50 of 18 ppm followed by Abound with an LC50 of 145 ppm. Switch showed to have the lowest larval toxicity with an LC50 of 6,111 ppm, followed by Pristine (LC50 of 2,027 ppm) and Quash (LC50 of 834 ppm).
These findings could assist blueberry growers in making fungicide management decisions. Based on these results, Abound is one of the safest fungicide to use because it repels foragers. By using a fungicide that acts as a repellent, honey bee forager exposure to fungicides may be reduced. Alternatively, using a fungicide, such as Ziram, which is attractive to foragers and toxic when fed to larvae, is less desirable.
Table 1: Calculated LD50 when active ingredients were fed to larvae in artificial diet and compared with compared with concentration of active ingredients when applied in commercial settings at maximum field rates and residues found in pollen.
High concentrations of fungicides were found in pollen collected during blueberry pollination, with Switch and Pristine found in highest concentration. The fungicide residues found in pollen could be reduced if growers spray at night when bees are not actively foraging.
Colonies allowed to freely forage for pollen were healthier than colonies that were not able to bring in fresh pollen and provided supplemental pollen instead. Honey bee nutrition may play a role in reducing negative impacts of fungicides on colony health. Supplemental pollen patties will not be recommended to reduce colony declines and instead additional foraging resources are recommended to be planted in farrow fields, along irrigation canals, and/or the perimeter of the farm.
Ziram (LC50 of 18 ppm) had the highest mortality rate out of all 5 tested fungicides when fed to larvae, followed by Abound (LC50 of 145 ppm), Quash (LC50 of 834 ppm), Pristine (LC50 of 2,027 ppm), and Switch (LC50 of 6,111 ppm). Based on these results, Ziram should not be recommended for use while honey bees are foraging because it is applied at high enough concentrations to kill larvae.
Ziram treated bushes had the highest visitation rate of foragers when applied to potted blueberry bushes. Suggesting forager preference to bushes sprayed with Ziram over bushes sprayed with water. This result reinforces the recommendation that Ziram should not be used while honey bees are present because it attracts foragers and kills larvae.
Abound treated bushes had the lowest visitation rate of foragers when applied to potted blueberry bushes. This suggests forager avoidance to bushes sprayed with Abound over bushes sprayed water. Therefore, the use of Abound could negatively impact yields by reducing pollination visits. Based on these results, Abound should not be recommended for use prior to good pollination weather and should instead be used when poor pollination weather is expected (temperatures below 50F and rain).
These results are changing blueberry disease management practices by providing growers with knowledge of the negative impacts that some fungicides have and could improve colony health during and shortly after blueberry pollination. Growers are now eliminating Ziram from their practices or applying it to their crops prior to honey bee arrival. Additionally, many growers are now applying fungicides at night when honey bees are not actively foraging. This knowledge has provided growers and beekeepers with knowledge to have meaningful discussions to improve honey bee safety and protection while also repairing the relationship growers have with their beekeepers.
Education & Outreach Activities and Participation Summary
Participation Summary:
Outreach activities were to be divided into 1) Extension based to blueberry growers and to commercial and hobbyist beekeepers; and 2) To professional journals and the commercial beekeeping industry.
Extension based information to growers and beekeepers: I presented my work at 2 annual blueberry grower meetings reaching at least 70 growers and 1 statewide NJ Beekeepers Association (NJBA) meeting each year reaching about 150 beekeepers (total 3 per year). These meetings include the blueberry session at the Atlantic City NJ Agricultural Convention each January, the annual Blueberry Open House in Hammonton, NJ in late February, and twilight fruit grower update meetings in Hammonton during the spring season. I will work with commercial beekeepers throughout the year, communicate by email and phone, make annual reports available to them and hold 1 or 2 informal meetings per year, likely during the winter. I have contributed efforts to the Rutgers Blueberry Production Guide, so that bee safety information is updated.
Consultations included one-on-one phone conversations with both growers and beekeepers across the country and several farm and bee-yard visits.
On farm demonstrations were given to growers, beekeepers, and 4H members where individuals were educated about pesticide exposure to honey bees and colony health through hands on experience with honey bee colonies.
Honey bee integrated pest management and pollination education was delivered in 2 Rutgers blueberry newsletters.
Tours of Rutgers bee hives, honey bee lab, and honey house were given to growers, beekeepers, and 4H members.
1 Field trip for agricultural vocational high school students was given at a commercial beekeepers farm and during time students were educated about the importance of pollinations and pesticide safety.
7 workshops were provided to 4H members (2), Rutgers cooperative extension agents (1), beekeepers (2), and growers (2).
Other educational activities were invited lectures given to groups interested in honey bee safety and pollination.
Professional journals and commercial beekeeping industry: in 2024 I will target articles for the American Bee Journal, and on a more scientific level, The Journal of Apiculture Research. I will present my work at the Eastern Branch of the Entomological Society of America (ESA), as well as the National ESA meetings, and at the regional Cumberland-Shenandoah Fruit Workers Meeting (95-100 extension, researchers, industry and graduate students from the mid-Atlantic to New England states). I will produce 2 honey bee factsheets under Rutgers Cooperative Extension.
Project Outcomes
A direct outcome of my project is an ordered pollinator-risk ranking of the top five fungicides used during New Jersey blueberry bloom. I've found one fungicide, Ziram, in particular which exceeds the LC50 (Lethal Concentration which kills 50% of subjects) when fed to larvae at realistic concentrations. That same fungicide was also found to attract foragers. While, Abound is significantly less toxic to larvae and acts as a repellent to foragers. With this knowledge, I have and will continue to discuss the implications of these results with growers and beekeepers alike at conferences, during seminars, at meetings, and with one-on-one discussions between growers, beekeepers, and other extension agents in my field.
As growers implement this new understanding, beehive rental prices will stabilize and beekeepers will experience less colony losses due to blueberry pollination. This will improve the economic sustainability of pollination services for both beekeepers and growers while bringing the two industries closer together with a better understanding of bee health, crop demands, and pollinator safety. As the communication/science gap is closed, this will restore and improve grower and beekeeper relationships as they will work together to ensure the safety of their bees and the success of their crops.
A survey of 44 growers reported an improvement in their knowledge and understanding of pollinator health and safety. At least 6 growers and 5 beekeepers have changed their practices based on the results of this project. Of which, some growers now spray exclusively at night (approximately 500 blueberry acres), another grower has reduced his fungicide applications from 3 to 1 while beehives are present (>200 acres of blueberries), and all 6 growers have stopped using Ziram (the most toxic fungicide) when beehives are present. Beekeepers no longer use the miticide, Apivar, which was found to have significantly negative synergistic impacts on honey bee larvae when applied in conjunction with DMI fungicides. Beekeepers now have the knowledge base to have effective conversations with the growers, whom their bees pollinate for, to ensure reduce colony losses.
This work has led to the collaboration with other blueberry scientists who seek to improve the recently (10-15 years) decreasing blueberry yields via improving soil health. I hypothesize that by improving soil health, we will improve the nutrient availability in blueberry pollen and thus increase blueberry and honey yields in New Jersey blueberry crops. Upon the completion of my PhD program, I would like to repeat this work in cranberry bogs which have also reported recent declines in New Jersey crop yields.
I gained skills in data collection and analysis, communicating science with industries (growers, beekeepers, and pesticide companies), and am beginning to learn how factsheets can be used as a form of extension when scientific results are difficult (or complex) to communicate to industries in an effective manner.
This project has inspired me to continue my work in extension because I have had the opportunity to tackle current problems with science, digest the results, design new experiments to learn how the original results might be used, communicate that knowledge with the industries, and then see how those changes can improve our working relationships, environment, and economy.
I wish to pursue a career in pollinator and fruit IPM extension where I will be in a position to reduce our dependency on environmentally harmful pesticides while improving the economic sustainability of honey and fruit production. My research will continue to investigate the impacts of pesticides on our environment (pollinators, soil health, and yields) and seek to understand how IPM "tools" (forms of pest control) can be used most efficiently to increase yields and improve both human and environmental health.
When I conducted my first forager behavioral study to examine the preference of foragers to plain sugar syrup and sugar syrup laced with fungicides, I found that all 5 fungicides tested acted as a repellent to foragers, meaning foragers preferred to "drink" from plain sugar syrup over fungicide laced syrup. However, this evidence did not support grower reports of bees landing on puddles of freshly sprayed fungicide to "drink". When I improved the experiment in 2022 using freshly sprayed potted blueberry bushes, I found opposing results. While 3 fungicides didn't seem to impact forager visits, 1 fungicide acted as a repellent (Abound), and another acted as an attractant (Ziram). With this knowledge I hope that future efforts in understanding forager preference to pesticides will be conducted on living plants, instead of using sugar syrup in dispensers, as this method was not as reliable.
When it comes to extension, growers and beekeepers have repeatedly expressed their need for "simple" science. While scientists are accustomed to presenting their research in great detail, this information can often be received as an "overload" of information to the people that comprise the industries that we support. I have learned to keep these extension talks, short and sweet. Additionally, when new practices are being recommended, a simple factsheet will assist in the implementation of sustainable practice changes.