Final report for GNE20-246
Project Information
Dermo disease, caused by the parasite Perkinsus marinus, is a prevalent problem in northeastern oyster stocks. Oysters living intertidally in the Southeast are regularly exposed to temperatures exceeding the thermal tolerance of P. marinus—but not that of the oysters. We hypothesized that holding oysters in the temperature window between the points at which the parasites would die, but the oysters would not, might limit the proliferation of the parasite in the surviving oysters. Because oysters have a higher thermal tolerance, such exposures may reduce parasite loads without harming the oysters. However, elevated temperatures could also disrupt the microbiome, potentially creating human health risks if pathogenic bacteria increase. We attempted to exploit known temperature vulnerabilities via thermal shock treatments (a novel approach) to reduce disease loss in Northeastern oysters by exposing them to thermal regimens more similar to what oysters experience in the Southeastern US.
Laboratory and field trials were used to test the effectiveness of thermal treatment regimens to reduce P. marinus loads and biofouling, while bacterial safety was also monitored. Temperature can be readily elevated during low tide by temporarily covering oysters with clear vinyl. We designed thermal treatments that involed monitoring the temperature of oysters held under clear vinyl at a therapeutic temperature for one hour. Groups of oysters were treated with different frequencies (0-3 times) over the course of the growing season, with different seasonal combinations of treatments. At each timepoint, the number of survivors was quantified, and a subset of oysters was sampled for dermo disease and microbiome analysis. The microbial composition of oyster the tissues was profiled using novel DNA sequencing techniques leveraging in-house expertise in nanopore technology.
The vinyl covering was effective to induce the desired temperatures. While days with higher temperatures were prioritized, the thermal treatments likely would have been easier to conduct if avoidance of cloudy and windy conditions had held equal weight. Seasonality of treatment was the most consequential in the spring, as oysters that were subjected to the earliest treatment event experienced very high mortalities. The bluetooth monitors were not as helpful as we had hoped, as the margin of error and lag time were too great for this particular application.
Reducing biofouling was hypothesized to be a secondary benefit of this treatment, but our results were inconclusive because the baseline infestation of mudworms was negligible for this location in 2021.
To understand the bacterial community dynamics that may be modulated along with the parasites during thermal treatment. Oysters that were treated during the latter two treatments did have slightly lower dermo disease, but this difference was not significant. No dermo disease prevention benefit was observed that could outweigh the mortalities caused by the heat treatment. While seasonal shifts in microbiome composition were observed, there was no apparent differentiation based on whether oysters had been subjected to thermal treatments or not, with the exception of the timepoint in the middle of the season and treatments. There was no longterm impact of the thermal treatments on the microbiome, as all treatment groups were indistinguishable.
These results, in combination with anecdotal evidence from other researchers, point to an early-season susceptibility of oysters to heat shock. This would be worth pursuing with further research to advise farmers that extra care should be taken to avoid early-season exposure to unseasonably warm conditions. Although our experiments did not successfully reduce dermo disease, we are not fully satisfied that the hypothesis was rejected since there is ample room for improvement of the treatment methods. The microbiome data at least shows that there may not be short- or long-term consequence of further experimentation.
Assessed the effect of heat treatment frequency and seasonality in a farm-like context on:
-
- Dermo infection intensity
- Biofouling
- The bacterial microbiome, including pathogens of human concern
- Oyster growth, condition, and mortality
Testing both the frequency and seasonality of treatment was meant to reveal if either factor had a particularly strong impact on Dermo infection intensity since P. marinus abundance changes seasonally, increasing exponentially from Spring to Fall as temperatures warm. Similarly, biofouling changes seasonally, as does the microbiome, which is defined in part by the bacterial community in the surrounding waters.
The purpose of this project was to develop a low-cost, low-tech method that reduced the impact of Dermo disease on oyster farms via a regimen of heat treatments conducted throughout the growing season. The intensity and frequency of treatments was optimized for testing in the context of a real oyster farm. Changes to human pathogens in the context of the full bacterial community (microbiome) were profiled to assess unintentional risks to human health.
Perkinsus marinus, the agent of Dermo disease, is a protistan parasite infecting the oyster as it feeds. Once ingested, P. marinus targets hemocytes—immune cells in the oyster hemolymph—which become infected and are destroyed as the parasite proliferates. The cascading effects of infection include compromised immunity, reduction in reproductive capacity, slowed growth, and increased mortality. The emaciation of the oyster meat degrades the quality of the oyster and mortality reduces farm production. Although P. marinus can be found along most of the Atlantic coast of the US, its impacts vary for reasons that are not fully understood, including climate, salinity, circulation, host resistance, and parasite virulence. In the Southeast, intertidal oysters are routinely exposed to temperatures that exceed the thermal tolerance of P. marinus (> 35 ºC [1]) yet the oysters survive. This thermal exposure may curtail the proliferation of P. marinus thereby reducing the impact of Dermo disease. We tested the effectiveness of thermal treatments that mimic the conditions experienced by intertidal oysters in the Southeastern US to reduce Dermo-induced mortality (Figure 3).
Heat treatment may also reduce biofouling as such temperatures may exceed thermal tolerances for these organisms. Two mud worms are particularly problematic: 1) Polydora websterii causes mud blisters within the oysters reducing quality, and 2) Polydora cornuta encases oysters and gear in mud, restricting water flow and potentially smothering oysters if not frequently removed. Farmers must invest significant portions of their time to keep oysters and farm gear free of these fouling organisms, therefore any treatment that reduces fouling will increase efficiency and improve marketability.
Although P. marinus is harmless to humans, attempts to thermally control the impact that Dermo disease has on aquaculture output may have unintended effects on the oyster microbiome, including bacteria of concern to human health. Warm temperatures, including those experienced on some intertidal farms can elevate levels of pathogenic vibrios [2, 3]. The effect of higher heat treatments may, however, be positive – V. parahaemolyticus, for example, has a low heat tolerance [4] and can be readily eliminated with heat as demonstrated on shrimp exoskeletons exposed to 50 ºC [5]. The effect of the thermal treatment may be similar to dipping shellfish in boiling water—a method already being used to control biofouling [6]. The microbiome can be sensitive to disruptions such as heat shock, but this has mostly been studied in the context of naturally-occurring heat waves [7, 8], not the acute exposures that were used in the course of this experiment.
Research
Field Experiments
Large pieces of 20 gauge clear marine vinyl (www.MarineVinylFabric.com) were cut to temporarily cover oyster bags raised above an intertidal flat while exposed around mid-day by a strong low tide. For each replicate of the treated group, an MX2202 HOBO® Pendant MX Temperature/Light was positioned between the vinyl and the oyster bags. Once the threshold temperature of 40 °C was crossed, an hour timer was set and temperature was continuously monitored via phone app connection to the bluetooth-capable HOBO devices. 50 °C was the target temperature, so if the treatment approached 55 °C then the vinyl was vented (adjusted to allow more airflow or completely removed temporarily) to bring the temperature back down. At the end of the hour, the vinyl was removed and the bags secured to their racks.
Table 1: Summary of tide and meteorological information for all treatment (both successful and failed attempts)
|
Attempt |
Treatment |
Date 2021 |
Low tide time |
Low tide height (feet) |
Wind speed (knots) |
Ambient Temp (F) |
Barometric Pressure (mb) |
Weather |
|
1 |
Failed attempt |
6/21 |
12:45 PM |
-0.13 |
8, gusts of 12 |
74.3 |
1008.5 |
Partly cloudy |
|
2 |
A- Spring |
6/23 |
2:33 PM |
-0.33 |
7.39, gusts of 13.61 |
68.7 |
1022.9 |
Sunny |
|
3 |
B - Summer |
7/20 |
12:23 PM |
0.05 |
2.92, gusts of 4.08
|
76.3 |
1014.4 |
Sunny |
|
4 |
Failed attempt |
8/24 |
5:16 PM |
0.23 |
7, gusts of 7.8 |
80.2 |
1014.2 |
Sunny |
|
5 |
C- Fall |
9/7 |
4:12 PM |
0.10 |
4.28, gusts of 5.05 |
75.4
|
1016.1 |
Sunny |
Meteorological observation (Cape May, NJ): https://tidesandcurrents.noaa.gov/met.html?bdate=20210823&edate=20210825&units=standard&timezone=GMT&id=8536110&interval=h
Tide predictions (Bidwell Creek Entrance) : https://tidesandcurrents.noaa.gov/noaatidepredictions.html?id=8536581&units=standard&bdate=20210823&edate=20210825&timezone=LST/LDT&clock=12hour&datum=MLLW&interval=hilo&action=dailychart
Figure 1: Treatment in progress as bags of oysters are covered (and sometimes wrapped, if windy) with vinyl. Photo from 6/23 treatment.
Figure 2: NSF REU intern Grace Jackson (left) monitors the treatments in real-time from a dashboard on a phone that connect to the Bluetooth-capable temperature and light-loggers (right).
Figure 3: Approximately one week after treatment oysters were removed from bags to count those that were alive or dead, as well as to collect a subsample to be sacrificed for molecular analysis.
Figure 4: At the final timepoint, 30 oysters from each condition were sacrificed and a sample taken from the mantle and rectal tissue for analysis using RFTM to assess Dermo disease prevalence and intensity of infection.
Molecular Methods to Study the Microbiome Composition
DNA extraction was optimized for these samples using the E.Z.N.A. Mollusc DNA Kit (Omega Bio-tek, Norcross GA). The majority of samples have had mantle tissue dissected (the remaining oyster is stored at –80C), DNA extracted, amplified, and sequencing using the Oxford Nanopore MinION is currently underway using R10 sequencing chemistry. Water samples collected at the high tide prior to each date of oyster sampling was filtered (0.2 micron) and the filter paper is undergoing phenol-chloroform extraction for subsequent amplification and sequencing by the same means as the mantle tissue. Following sequencing and bioinformatic analysis, these data will show if any microbiome differences persisted between groups a week after each treatment, and if there was any long-term change to the microbiome composition at the end of the season. Water samples also provide a point of comparison showing if any treatment groups diverged more significantly from the ambient bacterioplankton assemblage. If no differences are observed between treatment groups, then these data will at least show the seasonal trend for the microbiome of oysters grown at the Rutgers Cape Shore facility.
Oven Experiments
Anticipating some difficulty performing consistent treatments on the farm, we ran a set of concurrent experiments to try thermal treatments of oysters in an oven. An initial test was performed to observe the mortality that resulted from a single exposure. In case mortalities may have been delayed, the oysters were maintained off of the dock at the Haskin Shellfish Research Laboratory in Port Norris, NJ for periodic checks.
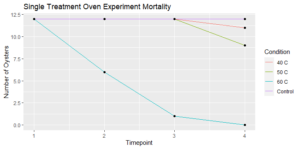
Figure 5: 8/28/21 check for the oyster mortalities treated in the oven a single time at 40, 50, and 60 C.
As part of an NSF REU program hosted at Rutgers, we engaged an undergraduate student (Grace Jackson from the University of Dayton), to perform a set of experiments summarized in a poster.
Her results from experiment 1 in which pure cultures of Perkinsus Marinus were subjected to the thermal treatment reaffirmed our foundational concept that the treatment should nearly eliminate the P. marinus population. However, we have yet to definitively show this in oysters in vivo. Experiment 2, in which oysters were treated daily over the course of a week, found the resulting dermo levels to be insignificant between the treated and untreated group. The team then wondered if the mortalities that occurred before the endpoint in this and experiment #3 may have been “culling” more highly infected oysters from group, resulting in an artificially low dermo result (as treated oysters seemed less infected, but not significantly so in experiment #3).
To address the above question, as well as the concern that the temperature threshold was likely not being maintained when treatment was solely based on time, since adding oysters into the oven caused a precipitous drop in temperature, a second iteration of Experiment #2 was performed by the grantee graduate student Heidi Yeh. In this experiment, oysters were treated semi-daily over the course of a week in an oven that was pre-heated with a large mass of rocks to help maintain thermal inertia. Additionally, the treatment clock was not started until the oven with oysters had returned to the target temperature. For this iteration, oysters were then maintained in a raceway tank so that mortalities could be observed within hours and the oysters removed for RFTM sampling.
Figure 12: Oysters were maintained in a raceway tank on a mesh platform that made daily transfer to the oven easier.
In vitro experiments
The original plan to test oyster homogenate response to thermal treatment was modified to use just extra-pallial fluid (EPF) at the recommendation of Heidi’s dissertation committee. The EPF was then spread on bacterial plates, and samples preserved for DNA extraction and subsequent sequencing with the MinION to observe composition changes that may have occurred as a result of treatment.
Field Experiment
Treatment Key
1,2, and 3 refer to the number of treatments
A = spring (6/23/21)
B = summer (7/20/21)
C = fall (8/24/21)
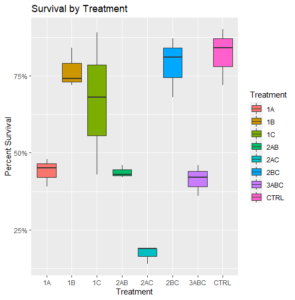
There was a clear negative impact on survival observed in all bags that were treated as part of the first round of fever in June (Figure 1).
Figure 1: Final survival of oysters by treatment (N=3)
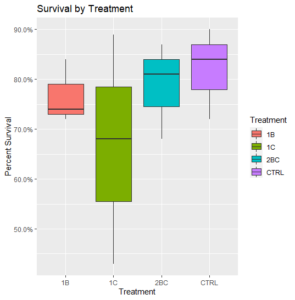
Removing this potentially problematic round of treatment, survival rates were generally high, but all treatments had a deleterious effect on survival relative to the control (Figure 2).
Figure 2: Final survival of oysters (N=3) with certain treatments omitted.
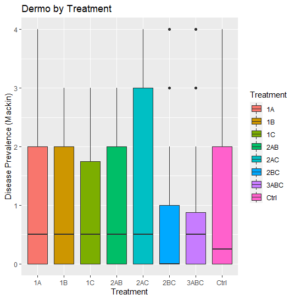
Across treatments, final dermo levels were low to moderate, with a median of 0.5 or lower for all (Figure 3). There is no clear difference in the dermo outcomes between all of the treatment groups, despite the large disparity in their survival rates (Figure 1).
Figure 3: All Dermo values by treatment
Biofouling:
There was no substantial difference in mud buildup on the oysters, and infestation with mud worms was non-existent to light across treatments
Figure 4: Cleaned shells from the control group show that mud worm infestation was not a baseline problem for the oysters grown at this site this year, so there is no biofouling problem for the heat treatment to have potentially addressed.
Oven Experiments
Following a single oven treatment, all oysters that experienced a 60 °C heat shock eventually died, whereas oysters from the other treatments (40 °C, 50 °C, and the control) experienced minimal mortalities (Figure 5).
Figure 5: Surviving oysters at each timepoint (1 = 8/24/21, 2= 8/25/21, 3= 8/28/21, 4=11/9/21).
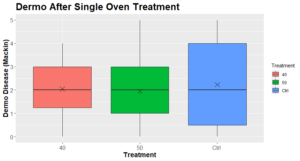
The Dermo levels observed at the end point were not significantly different between the conditions (Figure 6):
Figure 6: Dermo disease intensity (mackin) 2+ months after a single oven treatment (N=9-13).
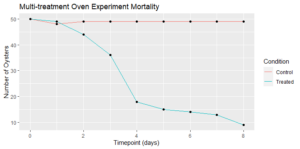
Although exposure to a single heat treatment had been previously established to not have a deleterious effect on the oysters, mortalities quickly ramped up after a few days, and we decided to stop short of our goal of a week of treatments to preserve some survivors for the final sampling to occur a week later (Figure 7). The resulting record of mortality and associated levels of dermo infection are presented in the following figures:
Timeline of the experiment (dates refeering to 2021):
- 1 week of acclimation to wet lab
- 5 days of oven treatments (8/24 (Heidi), 8/25 (Jenn), 8/26 (Jenn); 8/28 (Dave?), 8/29 (Heidi?)
- 5 days to recover/purge
- Final sacrifice on 9/3
Figure 7: Surviving oysters at each timepoint (consecutive days between August 24 and September 1, 2021).
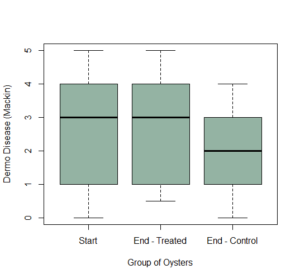
At the starting point, there was a 90% Dermo prevalence among the oysters, with an average mackin rating of 2.4. There may have been a slight reduction in dermo held under the wet lab conditions (Figure 8), but this was not statistically significant.
Figure 9: Dermo disease intensity at the start of the experiment (“Start”), compared to the final Mackin score for both Treated (“End-Treated”) and Control (“End-Control”) oysters.
Following the "control" samples collected at every timepoint, we can learn about the baseline microbiome dynamics that are occuring in intertidally farmed oysters at the Rutgers Cape Shore farm (left) to compare to the microbiome that results after the treatments occuring during different months.
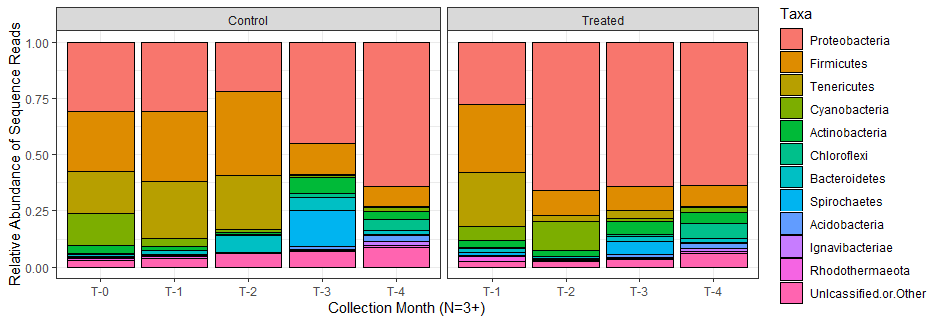
Figure 10: Stacked bar plot shows the relative abundance of sequence reads for oysters sampled during different months. Each column represents the average for the triplicate samples that were collected during a given month.
Proteobacteria were dominant across the dataset, with an increasing influence as the growing season progressed. The dominance of this phyla seems to be exacerbated by the thermal treatment, as this group comprises over half of the relative microbiome composition of oysters treated at most timepoints. Firmicutes were more abundant early in the growing season, with a waning relative abundace later in the season. Similarly, Tenericutes is almost completely absent after the summer months passed. Despite more obvious differences between control and treated oysters at timepoints 2 and 3, both groups seem to coalesce or recover to a shared microbiome profile by T4.
A high level of diversity could not be displayed on a plot that only shows 10 phyla, so the growing size of the pink bars at the bottom which served as a catch-all for "unclassified or other" includes the more rare phyla, and indicates that a large amount of the diversity in the later months is not visible in the bar plot. Higher community diversity in the fall months is supported by alpha diversity measurements (See the left-hand panels labeled "control" in the figure below):
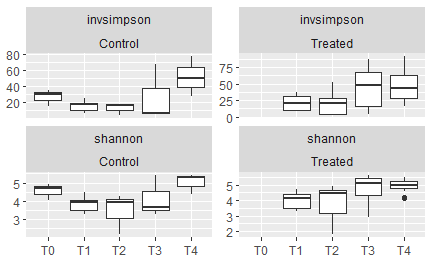
Figure 11: Alpha diversity scores alternately showing the inverse simpson index (top row) or the shannon-weaver index (bottom row), comparing the control oysters on the left to the oysters that were treated at each respective timepoint on the right. T0= June, T1 = July-A, T2 = July-B, T3= September, T4 = November.
A seasonal trend can be observed of lower community diversity in the summer months, which is significantly higher in the fall months. Heat treatment did increase alpha diversity at a few timepoints (this is especially evident in T2 and T3, as measured by the inverse simpson index.
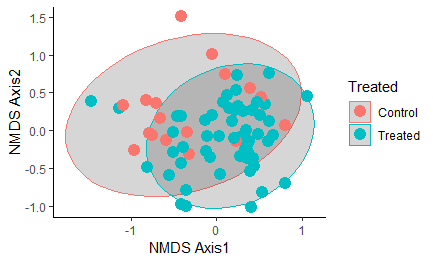
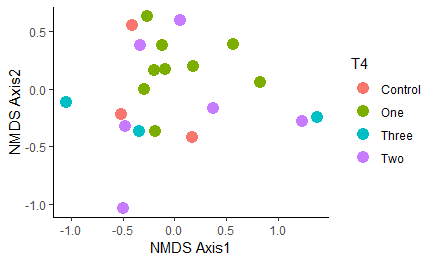
Figure 12: NMDS plot of similarity between samples based on strain-level sequencing data. All samples are considered regardless of timepoint, with all samples heat-treated at any point in time grouped together as the "Treated" group in blue. Ellipse shows the 95% confidence interval.
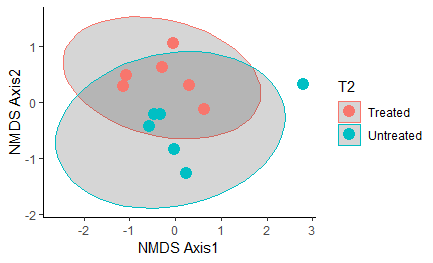
Overall, oysters receiving heat-treatment did not have a microbiome that was significantly different from the controls one week post-treatment, with the exception of T2 when the oysters were treated and sampled in late July:
Figure 13: NMDS plot comparing control oysters to those treated during T2 (one week post-heat-treatment in late July). These groups were significantly different: P-value = 0.02597. Ellipse shows the 95% confidence interval.
Oysters at the final timepoint were not significantly different from each other, so no long-term effects of the different treatments were observed in the microbiome:
Figure 14: NMDS plot of the different treatments grouped by treatment frequency.
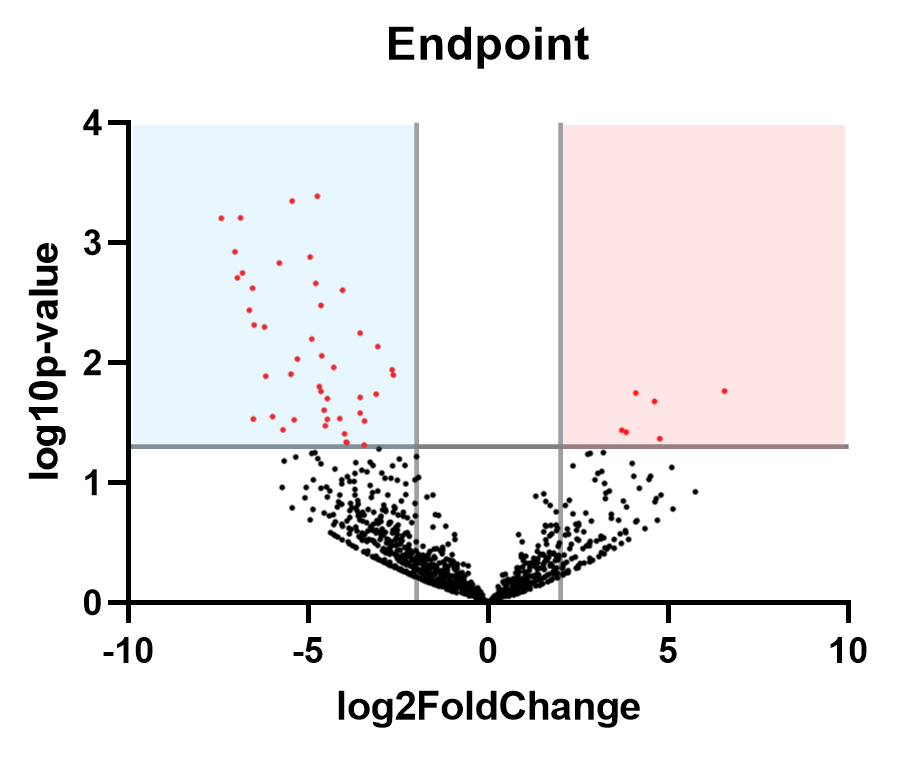
The overall microbiome composition of final samples taken at T4 were not significantly different based on Dermo disease status alone, but DESeq analysis revealed that 6 strains were enriched in oysters with a moderate Dermo infection (Mackin score of 3-4) relative to disease-free oysters:
Figure 15: Volcano plot showing the fold-change observed in the different oyster conditions, and the significance of each (a y-value of 1.3 corresponds to a p-value of 0.05, so all dots above this threshold are statistically significant). Strains significantly enriched in oysters with moderate infections are shown in the red quadrant, with strains associated with healthy oysters shown in the blue quadrant.
Table 1: Bacteria strains enriched in oysters with moderate dermo infections (Mackin score of 3-4) at the experiment endpoint. Listed in descending order of fold-change.
| Exiguobacterium_marinum_DSM_16307_P400 |
| Exiguobacterium_sp._AT1b |
| Sulfitobacter_sp._D7 |
| Synechococcus_sp._GFB01 |
| Rhodospirillaceae_bacterium_RKSG073 |
| Cognatiyoonia_koreensis_strain_DSM_17925 |
Table 2: Top 20 Bacteria strains enriched in health oysters (no Dermo infection) at the experiment endpoint. Listed in descending order of fold-change.
| Endosymbiont_of_unidentified_scaly_snail_strain_Monju |
| Paraclostridium_benzoelyticum_strain_JC272 |
| Desulfobacter_hydrogenophilus_strain_AcRS1 |
| Levilinea_saccharolytica_strain_KIBI-1 |
| Marichromatium_gracile_strain_DSM_203 |
| Prolixibacteraceae_bacterium_WC007 |
| Desulfosarcina_cetonica_JCM_12296 |
| Achromobacter_sp._DMS1 |
| Bacterium_UASB14 |
| Chloroflexi_bacterium__ADurb.Bin120 |
| Ahrensia_sp._R2A130 |
| Sphingobium_sp._RSMS_ |
| Desulfosarcina_ovata_subsp._ovata_strain_oXyS1 |
| Geovibrio_thiophilus_strain_DSM_11263 |
| Clostridiaceae_bacterium_isolate_MGYG-HGUT-00073 |
| Pseudomonas_aeruginosa_strain_UMB2738 |
| Altererythrobacter_epoxidivorans_strain_CGMCC_1.7731 |
| Paraclostridium_bifermentans_strain_Cbm |
| Dethiosulfatarculus_sandiegensis_strain_SPR |
| Brevefilum_fermentans_strain_CAMBI-1 |
- Effectiveness of this heat treatment method for reducing biofouling was unclear, as baseline biofouling was not strong enough among control oysters grown at this site.
- Covering oysters with vinyl was an effective way to induce heat shock in oysters, but treatments should be planned around the sun/cloud percentage in the forecast, as well as wind-speeds.
- Heat treatment early in the growing season (before July) should not be done to avoid large mortalities before the oysters have apparently become acclimated to the hotter summer temperatures.
- Effectiveness of this heat treatment method for reducing Dermo disease infection prevalence or intensity was inconclusive; however, long-term effects of the treatment on the microbiome have been ruled out.
- Middle- to late-season (T2 and T3 in this study) heat treatments may be worthwhile targets of continued attempts and research.
Education & Outreach Activities and Participation Summary
Participation Summary:
Presentations on the results of this experience and challenges associated with this method were presented at the 2022 Northeast Aquaculture Conference & Exposition (NACE) in Portland, Maine--this resulted in a conversation with someone from the Cape Cod Cooperative Extension who shared their anecdotal experience of also observing high mortality following early-season heat events. A subset of the microbiome results were also presented at the 2022 meeting of the Mid-Atlantic Chapter of the American Fisheries Society in Asbury Park, NJ. A presentation has been prepared for the Rutgers Shellfish Research Symposium being hosted in January 2023 by Rutgers University and the New Jersey Aquaculture Association.
A manuscript of the results is in preparation for submission to scientific journals such as Aquaculture, Microbial Ecology, or the Journal of Invertebrate Pathology. Sequences generated by the project will be deposited in the Sequence Read Archive (SRA) of NCBI: https://www.ncbi.nlm.nih.gov/sra
Project Outcomes
Now we know that temperature abuse does not have long-term imapcts on the microbiome, but that heat shock should especially be avoided early in the growing season as this results in abnormally high mortality that is not induced by the same conditions occuring later in the summer (supported by my data and anecdotal evidence from a conversation after presenting at NACE). Although the effectiveness of this method to reduce Dermo disease was inconclusive, this idea seemed to garner a lot of interest from growers who attended my presentation at NACE. If the early season mortality can be avoided, then this project has shown that the method at least is not harmful to long-term microbiome dynamics or oyster mortality.
Presenting this research to growers and scientists at NACE underscored for me the importance of presenting results at conferences and forums; even when the research feels unsuccessful or is just preliminary data with many questions left unanswered, the conversations that are spurred by this form of public dialogue can help add new perspectives to what would otherwise appear to be dead ends in the research. We don't intend to continue pursuing this particular idea for dermo disease treatment, but this preliminary data on mortality responses to heat shock varying across the growing season may be carried over to the oyster breeding efforts that are ongoing at Rutgers.
Although the bluetooth capability of the temperature loggers was useful, the time lag associated with the use of these devices is not ideal to the thermal volatility that the oysters experienced when we hit good weather to apply the treatment--however, the thermal mass of the oysters that we were working with was small, so less volatility would be expected when applying the treatment on a commercial scale. Treatment days were selected primarily by choosing days with the highest temperatures occuring at low tide, but it seems that at least as much weight should be given to the amount of sunlight vs. clouds to maximize the greenhouse effect, especially if working with a larger thermal mass of oysters. Wind speed was also more consequential than expected, but its impact could be mostly circumvented by more tightly wrapping the oyster bags with vinyl.
Since these oysters were collected at the same time as other samples that I was collecting for another project in Delaware Bay, I have been performing analyses that leverage the controls from this heat shock experiment to provide an intertidal comparison to the subtidal oysters in the managed oyster beds of the Delaware Bay, with an additional level of comparison to oysters in the New York City harbor. This meta-analysis will be included in my dissertation, as well as a manuscript submission for journal publication.