Final report for GNE21-247
Project Information
Acalymma vittatum - striped cucumber beetle – is a common and destructive insect herbivore pest. Adult beetles are especially destructive because they can vector a variety of plant diseases in addition to damaging plants. One solution could be biological control, but our understanding of natural enemies of adult beetles and their application is insufficient. Therefore, to address this knowledge gap, we set out to study the most common and most abundant natural enemy of adult striped cucumber beetles, Celatoria setosa, a parasitoid tachinid fly. We asked three key questions; one, what factors influence parasitoid abundance and preferences, two, how do preferences relate to offspring performance, and three, how do beetles respond to parasitism? To answer these questions, we conducted field and lab experiments. In the field, we surveyed hundreds of cultivated plants to determine what attracted parasitoids and influenced which beetles were attacked. In the lab, we monitored the survival of parasitoids that emerged from wild-caught adult beetles and cross-referenced our field data to determine the relationship between parasitoid preference and offspring parasitoid performance. Finally, in the lab as well, we manipulated parasitism in adult beetles to understand how infection affects their survival, behavior, and fitness. In summary, we found that C. setosa parasitoids were highly effective at reducing beetle survival, leaf consumption, female oviposition, and male pheromone emission. We also found that beetle abundance, specifically male beetle abundance, was most strongly correlated with parasitoid abundance suggesting that male beetles may drive parasitoid attraction in the field. Our findings are some of the first published work investigating the relationship between striped cucumber beetle and C. setosa, specifically detailing multiple mechanisms for how these natural enemies can benefit the agricultural community. Furthermore, our findings also suggest that the use or facilitation of C. setosa as a biological control agent is still complex and may require additional research.
Project Objectives
- Determine what host beetle factors influence the preference of C. setosa parasitoids.
a. How does the presence of male A. vittatum adults influence parasitism?
b. How does the presence of female A. vittatum adults influence parasitism?
c. How do host mass, sex, and sequestered defenses influence parasitism?
2. Determine what host beetle factors influence the performance of offspring C. setosa parasitoids.
a. How does A. vittatum mass relate to offspring parasitoid performance?
b. How does A. vittatum sex relate to offspring parasitoid performance?
c. How do plant defenses sequestered by A. vittatum relate to offspring parasitoid performance?
3. Determine the effects of parasitism on A. vittatum performance.
a. How does parasitism affect A. vittatum survival?
b. How does parasitism affect A. vittatum fecundity?
c. How does parasitism affect A. vittatum consumption?
d. How does parasitism affect A. vittatum pheromone production?
Acalymma vittatum - striped cucumber beetle – is a common but serious insect herbivore pest of cultivated cucurbit crops in the northeastern United States. Adult beetles are particularly destructive due to their unique biology which requires them to aggregate in high densities on individual plants for reproduction (Houser and Balduf 1925; Gould 1940; Symth et al. 2002; Smyth and Hoffman 2003). This group behavior, referred to as colonization, is driven by the release of vittatalactone, the key chemical component of their aggregation pheromone, released exclusively by males (Smyth and Hoffman 2003; Morris et al. 2005). Vittatalactone then serves an as important signal, recruiting dozens of additional beetles, where they feed, mate, and lay offspring eggs in the soil. Consequently, colonized plants can suffer immediate damage to leaves, blossoms, and fruits, and subsequent damage to roots by offspring beetle larvae (Houser and Balduf 1925; Gould 1940; Symth et al. 2002; Reed et al. 1986; Smyth and Hoffman 2003; Ellerns-Kirk et al. 2000). Furthermore, adults can vector a variety of plant diseases during these dense feeding events, including bacterial wilt, which is untreatable and quickly kills the plant (reviewed in Saalua Rojas et al. 2015).
To reduce the risk of damage and disease, most growers rely heavily on chemical insecticides (reviewed in Saalua Rojas et al. 2015). Other common management options include applying row cover to prevent colonization on young plants or applying black plastic mulch (Diver and Hinman 2008), and or entomopathogenic nematodes to suppress egg and larvae populations (Reed et al. 1986; Ellerns-Kirk et al. 2000; Hladun and Adler 2009). While these options are effective, row cover is temporary, and the other two primarily target juvenile life stages of A. vittatum rather than adults which again pose the most significant threat (reviewed in Saalua Rojas et al. 2015). There are, however, dozens of other organisms that have been found to prey on or be parasitic on A. vittatum adults including various species of arthropods, entomopathogenic nematodes, entomopathogenic fungi, and a few protists (reviewed in Toepfer et al. 2009; Coco et al. 2020), but our understanding of these adult natural enemies and their application is insufficient. For example, Celatoria setosa is a common parasitoid natural enemy of adult beetles and has been found to infect over 40% of wild populations at a given time (reviewed in Barrett et al. 2021), and yet, the handful of studies that have investigated this species have never gone beyond monitoring abundances or parasitism rates, and the few studies that have remained unpublished (Fischer 1981; Fischer 1983; Miller 2005).
What is known about C. setosa may suggest that they could be beneficial as control agents. For example, their range overlaps with A. vittatum can be commonly found in the midwestern, mid-Atlantic, and northeastern regions of the United States (Houser and Balduf 1925; Gould 1940; Fischer 1981; Platt et al. 1999; Miller 2005; Smyth and Hoffman 2010; Coco et al. 2020). We also know that parasitoid abundance and parasitism rates tend to peak twice a season, tailing each A. vittatum adult population peak in June and August (Smyth and Hoffman 2010; Coco et al. 2020), and there is speculation that C. setosa rely on their host’s pheromone as an important chemical cue (reviewed in Barrett et al. 2021). Lastly, we know a few key life history details such as approximately how long each developmental stage is (Fischer 1983) and that offspring parasitoids overwinter inside host beetles (Houser and Balduf 1925).
However, C. setosa is also a specialized koinobiont parasitoid (Fischer 1983) and infected adult beetles can continue to live for weeks (Fisher 1983). Unlike predators and other types of parasitoids, koinobiont parasitoids – parasitoids that keep their hosts active, cause a wide range of effects on their host, some of which can harm the plant (reviewed in Cuny et al. 2021). For example, while some studies have found that herbivores infected with koinobiont parasitoids consume less (Hoballah & Turlings 2001), others have found parasitism can increase it (reviewed in Cuny et al. 2021). Similarly, while some studies have found that parasitism can reduce herbivore fecundity, others have found the opposite, in which infected individuals have more offspring (Kaiser & Heimpel 2016). Furthermore, parasitism in insect herbivores has also been shown to affect their hosts in more complex ways such as changing foraging behavior (Karban & Loeb 1997; Singer et al. 2009), delaying development (Coudron et al. 1990) or altering mating behavior (Zuk et al. 2006). The changes, while broad, are often behavioral changes to ensure offspring parasitoids survive (Stamp 1981; Brodeur & McNeil 1989; Brodeur & Vet 1994; Harvey et al. 2005). In addition to the potential consumptive effects C. setosa may cause in infected striped cucumber beetles, their presence may also induce non-consumptive effects as well. In studies investigating the relationship between predator presence and prey, researchers have found presence alone can also cause herbivores to feed less (Rypstra and Buddle 2013) or shift their reproductive strategy, having more offspring than fewer (Xiong et al. 2015). Some studies investigating parasitoid presence have found comparable results in that the presence of parasitoids can decrease herbivory or alter fecundity as well (Ingerslew and Finke 2017).
It is unlikely, however, that A. vittatum beetles lack defenses to counter parasitism by C. setosa. For example, plants within the Cucurbitaceae family, including cultivated species, produce a unique class of secondary metabolites known as cucurbitacins or cucs (reviewed in Metcalf and Lampman 1989). These defensive compounds are toxic and function as a chemical defense against insects and other animal herbivores (reviewed in Metcalf and Lampman 1989). A. vittatum beetles have been found to sequester these chemical defenses and, in some instances, have gained protection against predators (Ferguson and Metcalf 1985). Although the role of cucurbitacins in Diabroticina beetles such as A. vittatum is still debated, cucurbitacins may negatively affect parasitoids (reviewed in Barrett et al. 2021)
While C. setosa could be an effective natural enemy, their biology and life history may reduce A. vittatum populations and damage or may not. Therefore, to better understand this natural enemy and its potential as a biological control agent, we asked three key questions; one, what factors influence parasitoid abundance and preferences, two, how do preferences relate to offspring performance, and three, how do beetles respond to parasitism? To answer our questions, we first conducted a field survey in which we measured beetle and parasitoid abundance, as well as key plant characteristics, weekly for twelve weeks across five common cucurbit varieties. We also sampled 1-10 beetles per plant per week to monitor individuals for parasitism and then reared out all offspring parasitoids. Finally, we then analyzed sequester plant defenses in both organisms using HPLC-QTOF. In a separate experiment, we investigated how the consumptive effects (CEs) of parasitism and the non-consumptive effects (NCEs) of parasitoid presence affect A. vittatum survival and behavior. To measure (CEs), we manipulated parasitism of individual A. vittatum adult females and measured survival, leaf consumption, and fecundity. We also manipulated parasitism of groups of A. vittatum adult males and quantified emitted vittatalactone using volatile headspace and GC-MS techniques. Lastly, we investigated (NCEs) of parasitoids by measuring survival and leaf consumption in female beetles in the presence or absence of C. setosa parasitoids.
Justification of project and application to sustainable agriculture
This project is critical to sustainable agriculture and its future in the northeastern region for three reasons. First, the results of this project will inform stakeholders about the effectiveness of a common natural enemy that attacks a destructive and recurring insect pest, Acalymma vittatum - striped cucumber beetle. Given that insecticides are the most common control strategy (reviewed in Saalua Rojas et al. 2015), alternative methods such as biological control integrate natural systems and may help reduce the reliance on toxic chemicals. Second, emerging research has shown that vittatalactone, the key chemical component of striped cucumber beetle pheromone (Smyth and Hoffman 2003; Morris et al. 2005) is an important chemical cue in the cucurbit agroecosystem, attracting and influencing the behavior of other relevant cucurbit insect pests (Brzozowski et al. 2021; Haber et al. 2021; and Weber et al. 2022). The results of this project may reveal interactions between parasitism and vittatalactone emission, which could impact how future researchers target multiple insect pests simultaneously. Third, adult striped cucumber beetles can vector a variety of plant diseases such as bacterial wilt, which is an untreatable plant disease (reviewed in Saalua Rojas et al. 2015). The results of this project may reveal solutions to reduce adult herbivory and impact the dynamics of plant diseases restricted to the northeastern United States.
Cooperators
Research
Insect and organism care
In this study, we used wild-caught A. vittatum primarily collected from two organic farms in the Finger Lakes region of New York State including Fellenz Family Farm located in Phelps, NY, and West Haven Farm located in Ithaca, NY. All insects were collected from flowering cultivated plants using nets and aspirators between mid-June and early August of 2022. To care for A. vittatum, newly caught adult beetles were sorted into male and female cages and were supplied weekly with fresh-cut plants and flowers [C. pepo (var. Saffron summer squash)]. To care for C. setosa, newly caught parasitoids were sexed and housed individually, in small plastic cups supplied with a small piece of cotton soaked in a 5% honey water solution. We also collected newly emerged C. setosa pupae weekly from our A. vittatum colonies to supplement wild C. setosa colonies. The parasitoid offspring were then housed in large opaque plastic containers on top of damped black gauze. Containers were monitored daily for parasitoid eclosion, sexed, and then housed similarly to wild flies. All organisms were kept in indoor growth chambers in the Entomology Department at Cornell University set to a 16:8 hr. L:D cycle with daytime temperatures ranging between 25-30 degrees Celsius, and night-time temperatures ranging between 18-22 degrees Celsius with humidity constant at 50%.
Greenhouse and planting
All plants used in organism care and lab-based experiments were grown in greenhouse conditions managed at Cornell University, Ithaca, NY. C. pepo (var. Saffron Summer Squash) seeds obtained from W. Atlee Burpee & Co were planted weekly in 72-cell seed trays using a Lambart 111 premix soil. Greenhouse conditions were set to 16:8 hr. L:D cycle with daytime temperatures between 25-30 degrees Celsius and nighttime temperatures between 18-22 degrees Celsius with ambient humidity.
Objective 1 - Determining what host beetle factors influence the preference of C. setosa parasitoids
Overview
In our first objective, we were interested in the natural history of C. setosa adult parasitoids and specifically wanted to determine what host beetle factors were associated with their presence, abundance, and host choice. To accomplish our objective we conducted a survey, with a repeated measures design, on an unmanipulated organically managed farm.
Experimental Design
Between May 30, 2022, and August 15, 2022, we monitored 150 individual plants across five popular varieties including C.pepo (var. Desert Zucchini), and C.pepo (var. Zephyr Summer Squash, C.pepo (var. New England Pie), C. sativus (var. Marketmore), and C. lanatus (var. Crimson Sweet) at Fellenz Family Farm, located in Phelps, New York, USA. We used a stratified sampling technique in which 30 plants (~10% of the total crop) were selected within each variety [strata]. Sampling began about two weeks after transplanting. Each week, we recorded total counts of A. vittatum adult and Celatoria setosa adults per plant. We also recorded details of plant ontogeny such as the number of leaves, flowers, and fruits, estimated percent beetle damage as well as the severity of disease using a ranked scale from 0 - 5 [0 = healthy, 5 = severely diseased]. Lastly, we collected between 1-10 A. vittatum adults haphazardly per plant per week using aspirators to estimate the ratio of male and female beetles and percent parasitism. These estimates were determined by processing newly collected batches of beetles, in which each batch was sorted into individuals, and each beetle was given a unique identification code, weighed, sexed, and then placed in a 1 oz plastic cup and left for fourteen days to monitor for parasitoid emergence.
Statistical analysis
To analyze our survey data, we summed all ten measurements of each plant together and then used Spearman’s correlation test and generalized linear models (GLMs) to determine what factors were associated with total parasitoid abundance and total parasitism rates. We used total counts rather than raw counts due to overdispersion and zero inflation in both the number of parasitoids observed and the number of beetles observed.
To analyze our sampled beetles and determine how mass, sex, or sequestered defenses influenced parasitoids, we used Pearson's Chi-squared Test to test the proportions of male and female beetles, a Kruskal-Wallis Rank Sum Test to test differences in mass, and lastly Wilcoxon Rank Sum Tests and generalized linear models (GLMs) to test differences in sequestered defenses.
We used R and RStudio (Ver. 4.2.1; R Core Team, 2022) with the following packages: tidyverse, ggplot, and MASS, to run our models and generate figures.
Objective 2 - Determining what host beetle factors influence the performance of offspring C. setosa parasitoids
Overview
In our second objective, we were interested in the relationship between adult parasitoid preference and offspring parasitoid performance. To accomplish this objective, we conducted a post hoc analysis of beetles collected from the field survey described in the first objective, in which we monitored hundreds of beetles for parasitism and then monitored offspring parasitoid development into adult flies. We then compared parasitoid fitness to key host beetle characteristics such as host mass, sex, and concentration of sequestered plant defenses.
Experimental Design
Following the emergence of parasitoids from field-collected beetles, offspring C. setosa pupae were collected and placed into new 2mL centrifuge tubes, sealed, and monitored for up to three weeks for eclosion into adult flies. After emergence, each individual was given a unique identification code, weighed, sexed, and evaluated for deformations. Next, A. vittatum adult beetles, both parasitized and non-parasitized from the field, as well as eclosed and non-eclosed offspring C. setosa, were prepped for chemical analysis of sequestered cucurbitacins. For analysis of beetles, we removed the elytra from parasitized and non-parasitized adults using fine-tip dissecting forceps and then stored them in 2 mL centrifuge tubes aliquoted with 500uL of methanol at -80 degrees Celsius until further processing. For parasitoids, whole-body emerged and non-emerged parasitoids were placed in 2 mL centrifuge tubes aliquoted with 300uL of methanol and stored at -80 degrees Celsius.
In the spring of 2023, samples of both beetles and parasitoids were prepped for analysis of sequestered cucurbitacins. Samples were first homogenized in solution using an Omni Bead Ruptor Elite ® and then centrifuged for 5 min at 10K RPM at 0 degrees Celsius. Next, we transferred the supernatants of each sample into clean 0.5mL microcentrifuge tubes and centrifuged again for another 5 min at 10K RPM at 0 degrees Celsius. Next, 150µL of each sample was transferred to 2mL Agilent ® glass vials fitted with 250µL Agilent ® glass inserts. We then used HPLC-Q-TOF technique to measure concentrations of sequestered cucurbitacins in beetle and parasitoid tissue. Concentrations and retention times were determined using Mass Hunter ® software from Agilent and pure cucurbitacin B, D, E, and I obtained from Sigma-Aldrich. All samples were prepared and analyzed at Cornell University, Ithaca NY, and Cornell AgriTech, Geneva, NY.
Statistical analysis
To analyze adult parasitoid preference and offspring parasitoid performance we used logistic regressions. In adult preference models, we set the condition of “parasitized” as a binary outcome in our collected beetles (1 = parasitized, 0 = not parasitized) and then evaluated the effect of key variables such as beetle abundance, host beetle mass, host beetle sex ratio, or cucurbitacin concentration, as well as other co-variables, such as plant genus, plant species, or time at which the beetle was sampled. For offspring performance, we set the condition of “eclosion” as a binary outcome in our collected parasitoids (1 = eclosed, 0 = not eclosed) and then evaluated the same variables and co-variables as with adult preference. We used R and RStudio (Ver. 4.2.1; R Core Team, 2022) with the following packages: tidyverse and ggplot, to run our models and generate figures.
Objective 3 - Determine the effects of parasitism on A. vittatum performance
Overview
In our third and final objective, we were interested in determining the consumptive effects of parasitism on A. vittatum adult behavior and performance. Specifically, we wanted to determine how direct parasitism and parasitoids affected survival, leaf consumption, and fitness. To achieve this objective, conducted a series of lab-based experiments in which we manipulated parasitism and measured survival, leaf consumption, and oviposition in A. vittatum females and the concentration of emitted male aggregation pheromone – vittatalactone in groups of A. vittatum males using headspace volatile collection and GC/MS techniques. Finally, we conducted an additional experiment in which we investigated the non-consumptive effects of parasitoids by measuring survival and leaf consumption in female beetles in the presence or absence of C. setosa parasitoids
Experimental Design
Manipulation of parasitism
To manipulate parasitism for lab-based experiments, newly collected beetles were first quarantined for about a week and a half to reduce the chance of selecting individuals with prior infection. After this time, surviving adults were sorted into male and female cages for later use. When ready, selected individual beetles were enclosed with one gravid parasitoid in a 0.5 oz plastic cup for about one minute. Parasitoids would typically oviposit only after coming into direct contact with enclosed beetles. After oviposition was visually observed, we assumed that individual beetles had been parasitized. In instances where oviposition did not occur, parasitoids were removed, and a fresh parasitoid was introduced. Each parasitoid was used only once and then returned to its rearing cups. For beetles selected as controls, individuals were treated similarly except wild-caught gravid parasitoids were swapped for non-gravid lab-reared parasitoids.
Experiment 1 - Investigating the effects of parasitism on survival and consumption
Newly parasitized and non-parasitized (control) females were first kept without food for four hours, weighed, and then placed in 16oz plastic cups containing a single leaf taken from a plant at the two-true leave stage. Leaf stems were cut using a sterilized razor and then placed in 1oz plastic cups and filled with water to prevent wilting. Beetles were then left to feed in cups for two weeks (the maximum time of C. setosa larval development [Fisher 1983]). During this time, we monitored beetle survival and parasitoid emergence daily. We recorded changes in mass and leaf area consumed by imaging leaves and traced them in ImageJ every two to three days, replacing leaves if necessary. Lastly, we recorded the presence or absence of beetle eggs, given that some adult females oviposited eggs during the trial. After the experiment, all beetles were returned to their respective rearing cages. In total, 105 A. vittatum adult females were selected and assessed over two trials. Seven of those selected beetles did happen to be infected with wild C. setosa parasitism (the parasitoid emerged within the first few days of the trial) and were excluded from our analysis.
Experiment 2 - Investigating the effect of parasitism on pheromone emission
The release of A. vittatum male aggregation pheromone, vittatalactone, is a presumed group behavior, emitted exclusively by groups of males while actively damaging a plant to attract females (Smyth et al. 2003). Therefore, to investigate how parasitism may affect the emission of vittatalactone, we collected headspace volatiles of groups of parasitized and non-parasitized (control) males. To collect volatiles, parasitized and non-parasitized (control) males in groups of six (n = 6 (for a total of 36 parasitoids) and n = 23 (138 parasitoids) respectively) were placed into 1-gallon glass jars seventy-two hours post parasitism. Jars were supplied with a plant at the two-true leaf stage and left to feed for three more days under ambient temperature conditions and full-spectrum lighting set to a 16:8 hr. L:D cycle. Lab benchtop air was pushed through activated charcoal to filter, distilled water to humidify, and then delivered into the glass containers at 0.5mL/min. Outflow air was then pushed through an Orbo® 6×75mm active charcoal filter. After the allotted time, volatile filter traps were eluted with 1000µL of MeOAc into 2mL screw cap Agilent glass vials and then stored at 0 degrees Celsius. Volatile chemicals were then analyzed using Agilent GC-MS and Agilent MassHunter ® software.
Experiment 3 - Investigating how the presence of parasitoids affects survival and consumption
Selected females were first starved for four hours, weighed, and then placed in 16oz plastic cups containing a single leaf taken from a plant at the two-true leave stage. Leaf stems were cut using a sterilized razor placed in 1oz plastic cups and filled with water to prevent wilting. Females were then assigned to either one of two treatments, the presence, or the absence of parasitoids, in which cups were either loaded with a parasitoid or no parasitoid (Note: To reduce contact and prevent parasitism, we used large containers. From experience working with C. setosa, parasitism of individual beetles was unsuccessful in larger, more open containers. Beetles were then left to feed for twenty-four hours, afterwards, organisms were removed, weighed, and returned to rearing cages. We then imaged leaves and traced calculated removed areas using ImageJ. In total, 60 A. vittatum adult females were selected and assessed over two trials. Two of those selected beetles did happen to be infected with wild C. setosa parasitism and were excluded from our analysis.
Statistical analysis
In Experiment 1, we ran a Kaplan-Meier survival analysis paired with a log-rank test to compare differences in survival between parasitized and non-parasitized females. We then used generalized-linear regression models (GLMs) to compare differences in leaf consumption relative to individual female size. Lastly, we used Pearson's X2 Tests to compare differences in oviposition occurrences set to the observed frequency of controls. In Experiment 2, we also compared differences in the frequency of pheromone emission between groups of parasitized and non-parasitized males. We used Pearson's X2 Tests set to the observed frequency of controls. We then used (GLMs) to compare differences in the concentrations of vittatalactone emitted by groups. Within our analysis of concentration, two samples, one parasitized group, and one non-parasitized (control) group were found to be outliers and were omitted from our analysis of concentration. Finally, in Experiment 3, we compared differences in survival and leaf consumption relative to individual female size. We used generalized-linear regression models (GLMs).
All statistical analyses were conducted using R and RStudio (Ver. 4.2.1; R Core Team, 2022) with the following packages: tidyverse, survminer, and MASS. All figures were created in RStudio using the following packages: ggplot2 and ggstatsplot, and then edited in Microsoft Office ® and Adobe Photoshop ®.
Objective 1 - Determining what host beetle factors influence the preference of C. setosa parasitoids
Aim 1 and Aim 2
How does the presence of male A. vittatum adults influence parasitism?
How does the presence of female A. vittatum adults influence parasitism?
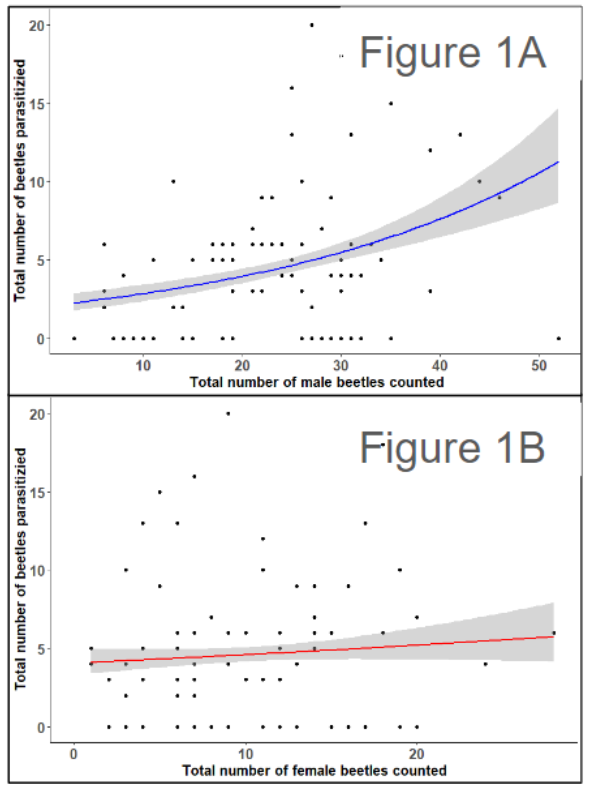
From a preliminary analysis of our field survey, we found that beetle abundance was most strongly correlated with parasitoid abundance (n = 150, rho = 0.31, p < 0.001) and (n = 150, rho = 0.31, p < 0.001). We also found that male beetle abundance was specifically responsible for this pattern and that female abundance was not correlated with parasitoid abundance (n = 85, z = 2.175, p < 0.001) (Figure 1A) and (n = 85, z = 0.665, p < 0.001) (Figure 1B).
Together these initial findings suggest that beetle density specifically male beetle density is associated with higher parasitoid abundance and rates of parasitism.
Aim 3
How do host mass, sex, and sequestered defenses influence parasitism?
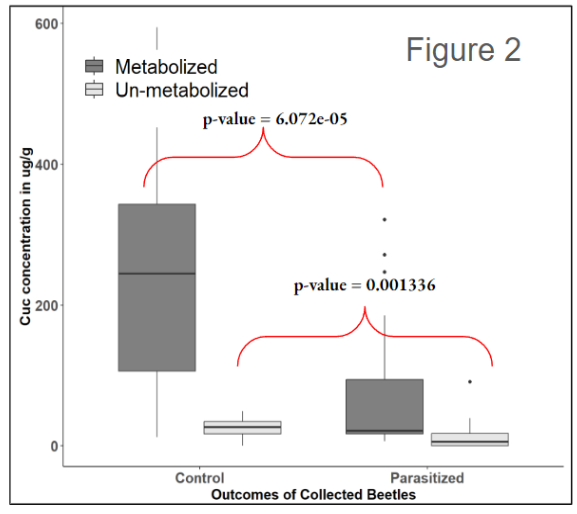
From our field survey, we sampled and processed 1,659 A. vittatum adult beetles. Within this sample, 942 were identified as male, 438 were identified as female, and 279 were not identified due to desiccation before processing, the emergence of parasitoid offspring before processing, or severe body damage such as missing abdomens. Within the subset of sexed beetles (N = 1,380), 1,030 beetles (74.64%) survived isolation and yielded no parasitoid offspring, 159 beetles (11.52%) yielded parasitoid offspring, and 191 beetles (13.84%) died without yielding parasitoid offspring. From a preliminary analysis, we found that we caught about twice as many male beetles as females (n = 1380, X2 = 184.07, df = 1, p < 0.001) and that there was no difference in parasitism rates between male and female beetles when adjusted for our observed sex ratio (n = 159, X2 = 0.06529, df = 1, p= 0.7983). We did however find that more males died during isolation than female beetles when adjusted for our observed sex ratio (n = 191, X2 = 10.257, df = 1, p < 0.01] (Table 1). We found that both parasitized beetles were larger on average than non-parasitized beetles (n = 938, X2 = 34.957, df = 2, p < 0.001]. Lastly, from a preliminary analysis of sequestered defenses, we found beetles infected with parasitoids had less metabolized and non-metabolized cucurbitacins, compared to non-infected beetles (n = 50, w = 501, p < 0.001) and (n = 50, w = 460, p < 0.01) respectively.
Together these initial results suggest that larger beetles may be targeted more than smaller beetles or may be more robust as hosts for offspring parasitoids. We found no effect of sex suggesting that male beetles are not preferred over females despite being more strongly associated with parasitoid abundance. Lastly, there seems to be a pattern associated with parasitism and sequestered chemical defenses. It may be possible that parasitoids prefer less chemically defended host beetles but more in-depth analysis is still needed.
Objective 2 - Determining what host beetle factors influence the performance of offspring C. setosa parasitoids
Aim 1 and Aim 2
How does A. vittatum mass relate to offspring parasitoid performance?
How does A. vittatum sex relate to offspring parasitoid performance?
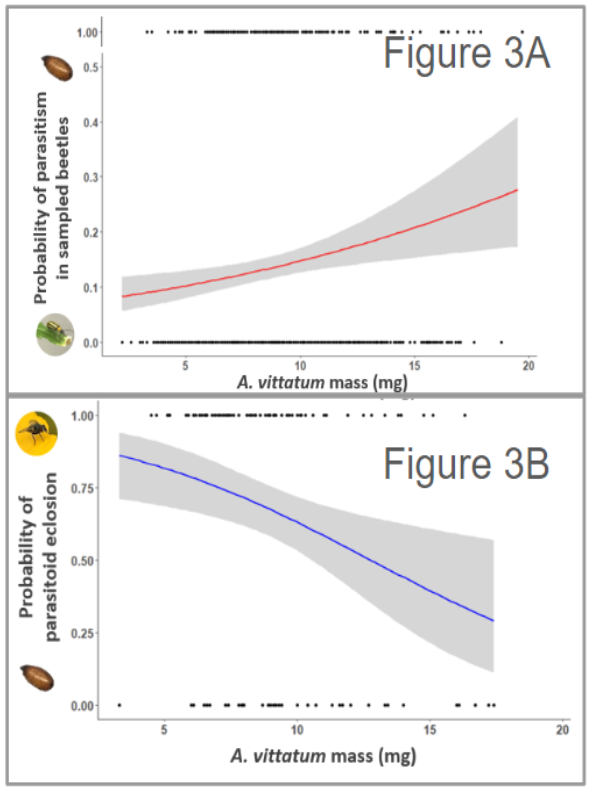
From a preliminary analysis, we found that host mass was most linked to both parasitoid preference and subsequent offspring performance. For example, we found that larger beetles were more likely to be parasitized (n = 1141, t = 1.021, p = < 0.001) (Figure 3A) but were less likely to yield successful offspring parasitoids (n = 119, t = 1.866, p < 0.01) (Figure 3B). We did not find sex to be an important predicting factor in either adult preference or offspring performance.
Together these initial findings suggest that there may be additional defenses of A. vittatum beetles, related to body size, which reduces the performance of their natural enemies.
Aim 3
How do plant defenses sequestered by A. vittatum relate to offspring parasitoid performance?
At this time, we are unable to determine the effect cucurbitacins have on offspring C. setosa parasitoids. We have some results from preliminary analyses that show the presence of cucurbitacins in both host beetle tissue and offspring parasitoid tissue but have not determined how performance may be impacted.
Objective 3 - Determine the effects of parasitism on A. vittatum performance
Aim 1, Aim 2, and Aim 3
How does parasitism affect A. vittatum survival?
How does parasitism affect A. vittatum fecundity?
How does parasitism affect A. vittatum consumption?
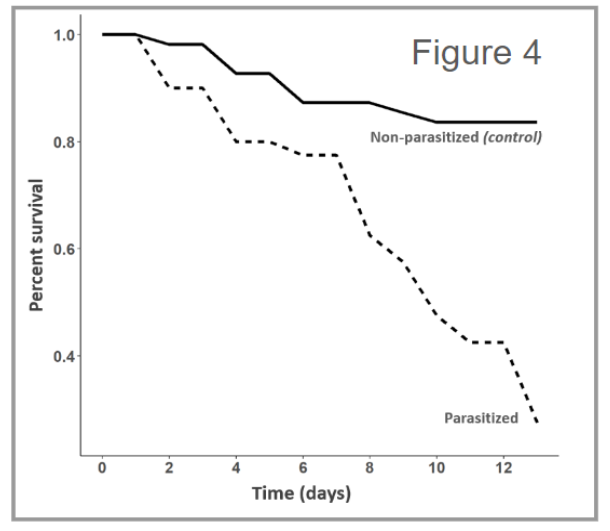
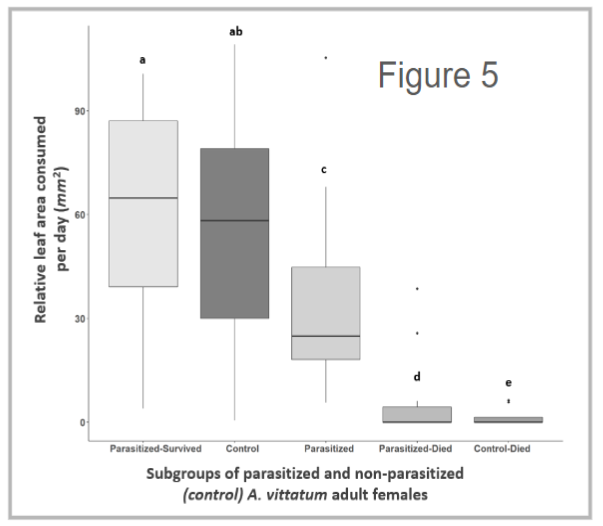
From a preliminary analysis, we found that parasitism was highly effective at reducing adult beetle survival (n = 98, p < 0.001) (Figure 4). Specifically, we found that about 75% (n = 32) died with about 66% of those that died yielding offspring parasitoids (n = 21). These individuals are referred to as the Parasitized sub-group in the following paragraph whereas the remaining females (n = 11) that survived the full length of the trial are referred to as the Survivors sub-group. Next, we observed that parasitized females were less likely to oviposit eggs than non-parasitized controls (n = 32, X2 = 4.36, df = 1, p < 0.05) and that Survivors were just as likely to oviposit eggs compared to non-parasitized controls (n = 11, X2 = 0.02 df = 1, p = 0.89). Lastly, we found that parasitized beetles consumed about 50% less leaf material than non-parasitized control beetles and that Survivors consumed just as much as non-parasitized control (n = 96, X2 = 46.762, df = 4, p < 0.001) (Figure 5).
Together these initial results suggest that parasitism by C. setosa is fairly effective at reducing both adult beetle survival and leaf consumption. These findings also suggest there are additional mechanisms for reducing beetle populations such as laying eggs less often. Furthermore, these results suggest that any reduction in leaf consumption in parasitized beetles may be mediated by mortality rather than by infection itself.
Aim 4
How does parasitism affect A. vittatum pheromone production?
In total, we quantified the emissions of 29 groups of male adult A. vittatum (n = 174). We were able to detect vittatalactone in 21 out of 23 (91.3%) non-parasitized (control) groups but only in 4 out of 6 or (66.0%) of parasitized (experimental) groups. From a preliminary analysis, we found that groups of parasitized males were less likely to release detectable concentrations of vittatalactone (n = 29, X2 = 4.4722, df = 1, p < 0.05). Groups of non-parasitized males released a median of 59.14 mg/mL of vittatalactone whereas groups of parasitized males released a median of 31.46 mg/mL of vittatalactone. Using a one-tailed Wilcoxon Rank Sum Test we found no difference in vittatalactone emissions (n = 29, w = 86, p = 0.1866)
These initial findings may suggest that while parasitism may not impact the concentration of male beetle’s pheromone emission, it does seem to impact whether they call or not.
Additional Aim
How does the presence of parasitoids affect A. vittatum survival and herbivory?
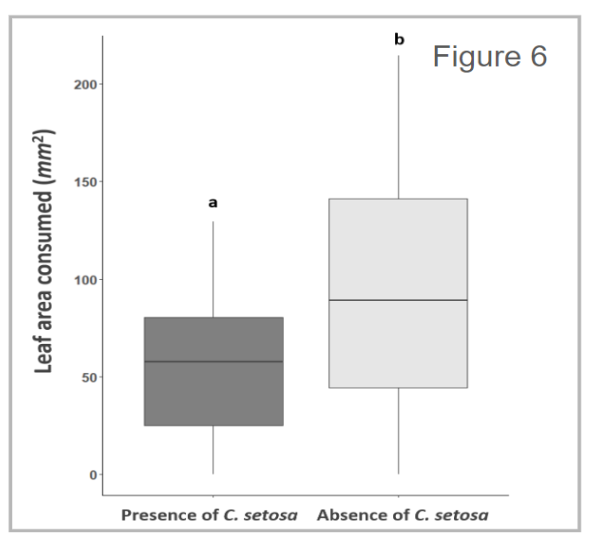
From a preliminary analysis, we found that overall, adult females in the presence of parasitoids consumed about 54% less leaf material than beetles in the absence of parasitoids (n = 58, w = 243, p = 0.011) (Figure 6). There was no effect however on mortality, with all females surviving the twenty-four-hour feeding assay.
These initial findings may suggest that the presence of parasitoids may be enough to reduce aboveground consumption.
Figures
Figure 1 – Scatter plots display the relationship between A. vittatum adult abundance (x-axis) and the parasitoid, C. setosa, adult abundance (y-axis) fit using a Poisson regression and split by A. vittatum adult sex. 1A displays the relationship between A. vittatum adult males and parasitoid abundance whereas 1B displays the relation between A. vittatum adult females and parasitoid abundance. Dots represent the total number of beetles recorded on individual plants.
Figure 2 – Box plots display the difference in total average cucurbitacins, including both total average metabolized cucurbitacins and total average un-metabolized cucurbitacins sequestered by parasitized and non-parasitized wild-caught A. vittatum adults.
Figure 3 – Scatter plots display the probability of wild-caught A. vittatum adults being infected with a C. setosa offspring parasitoid and then developing into a subsequent adult fly as a function of A. vittatum adult mass. 3A specifically displays the probability of wild-caught A. vittatum adults being infected with a C. setosa offspring parasitoid fly as a function of A. vittatum adult mass. 3B specifically displays the probability of emerging parasitoids successfully developing in an adult fly as a function of A. vitality of adult mass. Dots represent the individual parasitoids either emerging or eclosing from host beetles.
Figure 4 - The plot displays the average percent survival of A. vittatum adult females over time. On average parasitism reduced A. vittatum survival by 56% compared to non-parasitized control females.
Figure 5 - Boxplot displays the variation in relative leaf area consumed measured in subgroups of parasitized and non-parasitized (control) A. vittatum adult females. Relative leaf area removed was calculated by dividing the total leaf area removed by the number of days the individual survived. Relative leaf area removed across subgroups was then compared using a Kruskal-Wallis Rank Sum Test and a nonparametric pairwise comparison. Letters denote significance between subgroups.
Figure 6 - Boxplot displays the leaf area consumed measured between female adult A. vittatum beetles in the presence or absence of C. setosa parasitoids. A. vittatum beetles in the presence of C. setosa parasitoids consumed about 54% less leaf area than beetles in the absence of parasitoids.
In this project, our overarching goal was to better understand the biology of Celatoria setosa and its potential as a biological control agent. We asked three key questions; one, what factors influence parasitoid abundance and preferences, two, how do preferences relate to offspring performance, and three, how do beetles respond to parasitism? From our three key questions, we have two key takeaways or important research conclusions to date.
First, we have found that these natural enemies are highly effective at reducing beetle survival, leaf consumption, and male and female fitness (Objective 3). Specifically, we found a four-fold reduction in survival, a 50% reduction in leaf consumption, and reductions in both egg-laying and pheromone emission likelihood. Together these findings provide evidence that this species is beneficial despite the possible nuances of parasitoid biology. Second, we found that beetle abundance specifically male beetle abundance was most strongly correlated with C. setosa abundance (Objective 1). This important finding suggests that to have a source population of natural enemies, there may need to be a source population of A. vittatum beetles as well.
Our results are still in their preliminary stages but they could impact future farming practices in the following ways. First, it may be more practical to rear C. setosa parasitoids and release them than facilitate their presence with a source beetle population or even border crops (Platt et al. 1999). Augmented control is common with some natural enemies and has just recently been shown successful with another common species of cucurbit herbivore pest (Boyle et al. 2023). Second, some of our more experimental findings suggest that parasitism may disrupt pheromone communication in A. vittatum beetles by reducing the likelihood of releasing pheromone at all. Moreover, striped cucumber beetle pheromone, vittatalactone, has been found to attract additional insect herbivore pests of cucurbits (Brzozowski et al. 2021; Weber et al. 2022). It is possible that further research into the manipulation of pheromone behavior through C. setosa parasitoids could impact the behavior of multiple pests simultaneously.
References
Barrett, M. R., Filgueiras, C. C., & Willett, D. S. (2021). Using Cucumis sativus, Acalymma vittatum, Celatoria setosa, and generalist pollinators as a case study for plant–insect interactions. Arthropod-Plant Interactions, 15(5), 637–644. https://doi.org/10.1007/s11829-021-09852-2
Boyle, S. M., Salom, S., Schultz, P., Lopez, L., Weber, D. C., & Kuhar, T. P. (2023). Augmentative biological control for squash bug (Hemiptera: Coreidae) using the egg parasitoid, Hadronotus pennsylvanicus (Hymenoptera: Scelionidae). Environmental Entomology, 52(5), 779-786.
Brodeur, J., & McNeil, J. N. (1989). Seasonal microhabitat selection by an endoparasitoid through adaptive modification of host behavior. Science, 244(4901), 226-228.
Brodeur, J., & Vet, L. E. (1994). Usurpation of host behaviour by a parasitic wasp. Animal Behaviour, 48(1), 187-192.
Brzozowski, L. J., Weber, D. C., Wallingford, A. K., Mazourek, M., & Agrawal, A. A. (2021). Trade-offs and synergies in management of two co-occurring specialist squash pests. Journal of Pest Science. https://doi.org/10.1007/s10340-021-01379-y
Coco, A. M., Lewis, M. T., Fleischer, S. J., & Tooker, J. F. (2020). Parasitoids, Nematodes, and Protists in Populations of Striped Cucumber Beetle (Coleoptera: Chrysomelidae). Environmental Entomology, nvaa116. https://doi.org/10.1093/ee/nvaa116
Coudron, T. A., Kelly, T. J., & Puttler, B. (1990). Developmental responses of Trichoplusia ni (Lepidoptera: Noctuidae) to parasitism by the ectoparasite Euplectrus plathypenae (Hymenoptera: Eulophidae). Archives of Insect Biochemistry and Physiology, 13(1‐2), 83-94.
Cuny, M. A., Bourne, M. E., Dicke, M., & Poelman, E. H. (2021). The enemy of my enemy is not always my friend: Negative effects of carnivorous arthropods on plants. Functional Ecology, 35(11), 2365-2375.
Diver, S., & Hinman, T. (2008). Cucumber beetles: organic and biorational integrated pest management. National Center for Appropriate Technology, 20.
Ellers-Kirk, C., & Fleischer, S. J. (2006). Development and Life Table of Acalymma vittatum (Coleoptera: Chrysomelidae), a Vector of Erwinia tracheiphila in Cucurbits. Environmental Entomology, 35(4), 875–880. https://doi.org/10.1603/0046-225X-35.4.875
Ferguson, J. E., & Metcalf, R. L. (1985). Cucurbitacins: Plant-derived defense compounds for diabroticites (Coleoptera: Chrysomelidae). Journal of Chemical Ecology, 11(3), 311–318. https://doi.org/10.1007/BF01411417
Fischer DC (1981) Tachinid parasitoids of Acalymma vittata, Diabrotica undecimpunctata, and Diabrotica virgifera. Masters Thesis, Univ Illinois at Urbana-Champaign, USA
Fischer DC (1983) Celatoria diabroticae Shimer and Celatoria setosa Coquillett: Tachinid parasitoids of the diabroticite Coleoptera. PhD Thesis, Univ Illinois at Urbana-Champaign, USA
Gould, G. E. (1942). The Biology and Control of the Striped Cucumber Beetle (Doctoral dissertation, Purdue University).
Haber, A. I., Wallingford, A. K., Grettenberger, I. M., Ramirez Bonilla, J. P., Vinchesi-Vahl, A. C., & Weber, D. C. (2021). Striped cucumber beetle and western striped cucumber beetle (Coleoptera: Chrysomelidae). Journal of Integrated Pest Management, 12(1), 1.
Hladun, K. R., & Adler, L. S. (2009). Influence of leaf herbivory, root herbivory, and pollination on plant performance in Cucurbita moschata. Ecological Entomology, 34(1), 144-152.
Harvey, J. A. (2005). Factors affecting the evolution of development strategies in parasitoid wasps: The importance of functional constraints and incorporating complexity. Entomologia Experimentalis et Applicata, 117(1), 1–13. https://doi.org/10.1111/j.1570-7458.2005.00348.x
Hoballah, M. E. F., & Turlings, T. C. (2001). Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evolutionary ecology research, 3(5), 583-593.
Houser, J. S., & Balduf, W. V. (1925). The striped cucumber beetle: Diabrotica vittata Fabr.
Ingerslew, K. S., & Finke, D. L. (2017). Mechanisms underlying the nonconsumptive effects of parasitoid wasps on aphids. Environmental Entomology, 46(1), 75-83.
Karban, R., & English-Loeb, G. (1997). Tachinid parasitoids affect host plant choice by caterpillars to increase caterpillar survival. Ecology, 78(2), 603-611.
Kaiser, M. C., & Heimpel, G. E. (2016). Parasitoid‐induced transgenerational fecundity compensation in an aphid. Entomologia Experimentalis et Applicata, 159(2), 197-206.
Metcalf, R. L., & Lampman, R. L. (1989). The chemical ecology of diabroticites and cucurbitaceae. Experientia, 45(3), 240–247. https://doi.org/10.1007/BF01951810
Miller, S. M. (2005). Biological control tactics for suppression of adult striped cucumber Acalymma vittatum, with natural enemy parasitoid, Celatoria setosa, and insect parasitic nematode, Heterorhabditis bacteriophora (Doctoral dissertation, The Ohio State University).
Morris, B. D., Smyth, R. R., Foster, S. P., Hoffmann, M. P., Roelofs, W. L., Franke, S., & Francke, W. (2005). Vittatalactone, a β-Lactone from the Striped Cucumber Beetle, Acalymma v ittatum. Journal of Natural Products, 68(1), 26–30. https://doi.org/10.1021/np049751v
Reed, D. K., Reed, G. L., & Creighton, C. S. (1986). Introduction of Entomogenous Nematodes into Trickle Irrigation Systems to Control Striped Cucumber Beetle (Coleoptera: Chrysomelidae)1. Journal of Economic Entomology, 79(5), 1330–1333. https://doi.org/10.1093/jee/79.5.1330
Rojas, E. S., Batzer, J. C., Beattie, G. A., Fleischer, S. J., Shapiro, L. R., Williams, M. A., Bessin, R., Bruton, B. D., Boucher, T. J., Jesse, L. C. H., & Gleason, M. L. (2015). Bacterial Wilt of Cucurbits: Resurrecting a Classic Pathosystem. Plant Disease, 99(5), 564–574. https://doi.org/10.1094/PDIS-10-14-1068-FE
Rypstra, A. L., & Buddle, C. M. (2013). Spider silk reduces insect herbivory. Biology letters, 9(1), 20120948.
Singer, M. S., Mace, K. C., & Bernays, E. A. (2009). Self-Medication as Adaptive Plasticity: Increased Ingestion of Plant Toxins by Parasitized Caterpillars. PLOS ONE, 4(3), e4796. https://doi.org/10.1371/journal.pone.0004796
Smyth, R. R., & Hoffmann, M. P. (2003). A Male-Produced Aggregation Pheromone Facilitating Acalymma vittatum [F.] (Coleoptera: Chrysomelidae) Early-Season Host Plant Colonization. Journal of Insect Behavior; New York, 16(3), 347–359. http://dx.doi.org/10.1023/A:1024824025210
Smyth, R. R., Tallamy, D. W., Renwick, J. A. A., & Hoffmann, M. P. (2002). Effects of age, sex, and dietary history on response to cucurbitacin in Acalymma vittatum. Entomologia Experimentalis et Applicata, 104(1), 69–78. https://doi.org/10.1046/j.1570-7458.2002.00992.x
Smyth, R. R., & Hoffmann, M. P. (2010). Seasonal incidence of two co-occurring adult parasitoids of Acalymma vittatum in New York State: Centistes (Syrrhizus) diabroticae and Celatoria setosa. BioControl, 55(2), 219–228. https://doi.org/10.1007/s10526-009-9232-y
Stamp, N. E. (1981). Behavior of Parasitized Aposematic Caterpillars: Advantageous to the Parasitoid or the Host? The American Naturalist, 118(5), 715–725.
Toepfer, S., Haye, T., Erlandson, M., Goettel, M., Lundgren, J. G., Kleespies, R. G., Weber, D. C., Walsh, G. C., Peters, A., Ehlers, R.-U., Strasser, H., Moore, D., Keller, S., Vidal, S., & Kuhlmann, U. (2009). A review of the natural enemies of beetles in the subtribe Diabroticina (Coleoptera: Chrysomelidae): implications for sustainable pest management. Biocontrol Science and Technology, 19(1), 1–65. https://doi.org/10.1080/09583150802524727
Weber, D. C., Haber, A. I., Pasteur, K., Boyle, S. M., Kuhar, T. P., & Cornelius, M. L. (2022). Both the Squash Bug Anasa tristis and Horned Squash Bug Anasa armigera (Hemiptera: Coreidae) are Attracted to Vittatalactone, the Aggregation Pheromone of Striped Cucumber Beetle. Environmental Entomology, nvac079. https://doi.org/10.1093/ee/nvac079
Xiong, X., Michaud, J. P., Li, Z., Wu, P., Chu, Y., Zhang, Q., & Liu, X. (2015). Chronic, predator-induced stress alters development and reproductive performance of the cotton bollworm, Helicoverpa armigera. BioControl, 60, 827-837.
Zuk, M., Rotenberry, J. T., & Tinghitella, R. M. (2006). Silent night: Adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biology Letters, 2(4), 521–524. https://doi.org/10.1098/rsbl.2006.0539
Education & Outreach Activities and Participation Summary
Participation Summary:
Over the past few years, we have participated in multiple education and outreach events and plan to participate in upcoming events as well. For instance, each fall, Cornell University's Department of Entomology puts on an insect-themed fair, known as Insectapalooza, which draws in thousands of New York State residents and out-of-state bug enthusiasts, primarily families with young children, to learn about insects and our department's research. From 2021-2023, we have showcased insects collected from our collaborator's farms and demonstrated techniques we used to study them. In addition, we also have presented the results of our research, funded through the Northeast SARE Program at the annual Entomological Society of America Research Conference, ENTSOC in 2021, 2022, and 2023. We were also specially invited to the 2024 spring Eastern Branch ENTSOC meeting, this upcoming March. These conferences are open to the public and share findings at both local symposiums, such as the Eastern Branch, and international communities. Lastly, we planning to submit two to three manuscripts for publication in peer-reviewed journals which would not have been possible without the generous sponsorship of the Northeast SARE Program. We are hoping to have all of them open-access and available by the late spring of 2024. One of the exciting and unexpected outcomes of our education and outreach efforts was the inclusion of undergraduate student research in our funded projects. The students were able to participate in summer research, go on to present their experiences at their research conferences, and are included as authors in our upcoming publications.
Ongoing work beyond the Northeast SARE project scope will include finalizing our datasets, data management, and preparation of our upcoming presentations and publications.
Presentations
Entomological Society of America Meeting
[1] Acalymma vittatum facilitates neighboring insect herbivores Anasa tristis. Barrett, M.R., Brennan N.C., Weber, D.C., & Thaler, J.S. 10-minute Paper. National Harbor, MD, USA. (2023).
[2] Eavesdropping on host-specific signals: Investigating how Acalymma vittatum colonization influences parasitoid behavior and performance. Barrett, M.R., Phillips, K., & Thaler, J.S. 10-minute Paper. Vancouver, BC, CA. (2022).
[3] Alternative outcomes in a trophic cascade: Acalymma vittatum, Cucumis sativus, and Celatoria setosa. Barrett, M.R., Filgueiras, C.C. & Willett, D.S. 10-minute Paper. Denver, Colorado, USA (2021).
[4] An updated method for rearing Celatora setosa: A parasitoid tachinid fly of Acalymma vittatum. Barrett, M.R., Filgueiras, C.C. & Willett, D.S. Poster. Denver, Colorado, USA (2021).
Cornell University - Department of Entomology - Graduate Student Symposium
[1] Barrett, M.R., Phillips, K., & Thaler, J.S. Investigating how Acalymma vittatum colonization influences parasitoid behavior and performance. 10-minute Paper. Cornell University, Ithaca, NY, USA. (2023).
Project Outcomes
For most major vegetable crop systems, natural enemies of recurring insect herbivore pests are typically well-known and studied. For example, it is now common knowledge that many species of lady beetles prey on aphid pests, and some species are even commercially available in which growers can purchase these organisms and release them for biological control. However, in some cases, natural enemies of common pests are mostly unknown. For example, Acalymma vittatum – striped cucumber beetle is a common pest of cultivated cucurbit crops, but the primary natural enemy, Celatoria setosa, a parasitoid tachinid fly, is not well-known, and two of the most insightful manuscripts describing them are unpublished. Our work researching Celatoria setosa and specifically investigating what factors influence their abundance in the field and how striped cucumber beetles, directly and indirectly, respond to them will serve as some of the first empirical studies to date, helping growers make decisions shortly.
Our work also has direct economic, environmental, and social benefits to our growers. First, our work highlights economic benefits by detailing exactly how these parasitoid natural enemies reduce pest beetle populations and their damage. Second, our work also reveals the benefits of integrating natural biological processes into pest control tactics which may help encourage growers to use fewer chemical insecticides given that parasitoids can reduce beetle populations by a four-fold decrease and damage by a 50% decrease. Lastly, through the completion of this project, I was able to collaborate closely with growers in the community and have conversations about the problems facing them today as well as the solution applied research can deliver. In summary, the experience provided by SARE Projects undoubtedly had a lasting impact on me as well as the growers who assisted me.
While sustainable agriculture is critical to future food security, it is also vital to preserving culture and the well-being of the greater agricultural community. One of the important pillars of sustainable agriculture is the integration of biological processes, such as the use of natural enemies to help control pest populations and damage in vegetable crop systems, rather than relying solely on pesticides. Moreover, while this project specifically generated new knowledge about a natural enemy of a major agricultural pest, I also gained valuable insight into the problems facing different stakeholder groups, which helped me reorganize my project goals to better serve the greater agricultural community. For example, the initial aims of the project focused on understanding the basic biology of Celatoria setosa, a parasitoid natural enemy of Acalymma vittatum – striped cucumber beetle, such as what factors influence their attraction. However, the growers who assisted me in this project were primarily concerned with practical information, such as when, how, and why are these natural enemies effective. Therefore, to integrate these needs, I learned new techniques and incorporated new experiments, such as investigating how parasitism may affect the ability of striped cucumber beetles to release their mating pheromone, which required new chemical skills such as HPLC or GC/MS and headspace volatile collection technique. Through the completion of this project, my approach to research in sustainable agriculture changed in that I now understand what types of results are needed by a variety of agricultural community groups and how to better serve them.
In addition, the completion of this project has shifted my future career and research aspirations. Before this experience, I primarily wanted to pursue research in fundamental areas of biology and ecology, whereas, after this experience, I would like to continue applied research. I am more enthusiastic about applied research given that this style is more community-orientated. Moreover, the completion of this project has also helped generate new questions and spurred new research, some of which has been recently funded. For example, in addition to the attraction of parasitoid natural enemies, striped cucumber beetles also attract other insect herbivore pests. This observation led to the application and award of a USDA-NIFA fellowship for continued research into the interactions of multiple agricultural pests.