Final report for GNE21-264
Project Information
This project aimed to assess the interactive effect of neonicotinoid pesticides and the pathogen Crithidia bombi on colony health of the common eastern bumble bee Bombus impatiens and evaluated drought as a climatic event that could exacerbate the exposure of bees to pesticides through pollen from seed-treated plants. We approached these questions through two separate experiments: a greenhouse study testing if drought increased neonicotinoid-pesticide concentrations in pollen of crops seed treated with systemic pesticides (objective 1), and a laboratory experiment assessing the consequences of neonicotinoid-pesticide exposure on pathogen transmission dynamics and colony behavior (objective 2).
In objective 1, we selected three crops that are commonly seed-treated with systemic pesticides (thiamethoxam, imidacloprid): sunflower, squash, and cotton; these crops are also agriculturally relevant for the northeastern United States region. We cultivated the crops in a greenhouse under controlled conditions, regulating factors such as temperature, light and substrate, while varying irrigation across three gradients– from fully watered to drought– measured as percent volumetric water content (VWC). Then, we collected pollen samples and quantified pesticide concentrations using Liquid Chromatography/Mass Spectrometry (LC/MS). In objective 2, we conducted experiments with microcolonies of bumblebees (Bombus impatiens) to test the effects of chronic exposure to the neonicotinoid pesticides thiamethoxam on pathogen transmission, bee survival, and colony behavior. We assembled microcolonies of 10 individuals, infecting three of them with the pathogen Crithidia bombi (designated as pathogen donors) and seven uninfected bees (recipients). Bees were provided ad libitum access to pesticide-treated diets –Control: 0, Low:0.5 and Medium:1 ppb thiamethoxam– through sucrose solutions. After 10 days, we dissected the bees and quantified the number of viable Crithidia bombi cells. We assessed effects on colony behavior by recording daily observations on four key actions: i) moving–walking around the colony, ii) stationary–remaining still with no interaction with other workers, iii) incubation–caring for the brood, and iv) feeding– consuming either pollen or nectar.
For Objective 1, we detected neonicotinoids in the pollen of all three crops studied; however, their final concentrations were not correlated with drought stress. Among the contaminants, the neonicotinoid thiamethoxam was the most prevalent, with particularly high concentrations in squash (32%) that exceeded the oral hazard thresholds for bee consumption.
In the experiment for objective 2, we found that bumblebee colonies exposed to the neonicotinoid thiamethoxam exhibited a significant reduction in pathogen infection as well as transmission rates, along with lower levels of sucrose consumption. The neonicotinoid treatment did not affect survival, but three observed behaviors were significantly affected by treatments: mobility and nursing responses decreased, while stationary behaviors increased.
Overall, our results suggest that systemic pesticides accumulate in pollen, but the likelihood for bees to encounter them does not increase during drought events. Our lab results indicate, surprisingly, that pesticide exposure reduced pathogen infection and transmission; this could be because pesticide-exposed bees had reduced consumption, and previous work has found that starvation reduces infection by this pathogen. Furthermore, when bees consume these neonicotinoid pesticides in their diet, important colony dynamics and behaviors are disrupted; for instance, bee mobility and incubation behaviors are significantly reduced, potentially compromising colony fitness. Given that pathogens constitute another significant cause of bee declines, understanding their potential synergistic effects with pesticide exposure, especially neonicotinoids, is an important step. Our results suggest that consumption of neonicotinoids decreases pathogen transmission and prevalence within the colony; however, we simultaneously observed a disruption of individual bee behavior and, consequently, colony dynamics. While the reduction of pathogens may positively impact bumblebee colonies, our research shows that this effect may be overshadowed by the behavioral changes leading to social disruptions within the colony that may have consequences for the overall long-term health and survival of the bumblebee colonies.
Our study provides important insights into the complex interplay between pesticides, pathogens, and environmental factors in shaping the health and resilience of bumblebee colonies. We conclude that farmers should avoid using seed-treated crops with systemic pesticides and instead explore more sustainable alternatives that prioritize pollinator health.
Project Objectives
Objective 1. We assessed the impact of drought stress on pesticide concentration in the pollen of three major US agricultural crops that are commonly seed-treated with systemic pesticides.
We selected three crops that are regularly seed-treated with pesticides: sunflower, cotton and squash. Plants of each crop were randomly assigned to one of three drought treatments (well-watered, moderate water, and drought). We collected pollen samples throughout the summer and fall 2021 and sent them to the Cornell Chemical Ecology Core Facility for pesticide testing. If drought increases pesticide concentrations in pollen, it would provide critical insight on how climate change may exacerbate the risk of pesticide exposure for bee pollinators in major crops. Conversely, if drought does not affect pesticide concentrations, this project would contribute valuable data quantifying ranges of pesticide residue levels across different crops, enabling more accurate assessments of pollinator exposure risk.
Hypothesis 1. Drought stress will increase pesticide concentrations in pollen from seed-treated crops.
Objective 2. We conducted an experimental study to test the effects of chronic exposure to the neonicotinoid thiamethoxam on the transmission dynamics of the pathogen Crithidia bombi in bumble bee colonies (Bombus impatiens).
This laboratory experiment was carried out during the summer of 2023, following approval for some methodological adjustments. We manually tracked interactions between bees within the colony, assessing pathogen prevalence and infection intensity. If pesticide exposure reduces interaction rates by disrupting social behavior, bee may become less likely to acquire pathogens from their nestmates. However, if exposure to the neonicotinoid-pesticide increases individual bee susceptibility to pathogens, transmission could still rise even with reduced or unaffected contact rates. Previous research has shown that pesticides–especially neonicotinoids– weaken bee immune systems, making them more vulnerable to pathogens. However, there is limited information available on how pesticides influence pathogen transmission patterns within colonies, making this a crucial area of investigation.
Hypothesis 2. Bees exposed to pesticides will interact less in the colony, potentially leading to lower transmission rates of Crithidia bombi. However, individuals who become infected will have a high infection intensity due to a weakened immune system in response to pesticides.
The purpose of this project is to elucidate the implications of drought, pesticides and pathogens for bees in agricultural landscapes. Pollinators are declining at concerning rates (Potts et al., 2010) threatened by multiple stressors, including pesticides and pathogen infections. Over the past 65 years, global pesticide use in agriculture has been expanding dramatically (Maroni et al., 2006), with neonicotinoid insecticides seeing particularly widespread adoption (Jeschke et al., 2011). In the US, neonicotinoid use has increased by 80% since their introduction in 1994 (Douglas & Tooker, 2015), with at least 300 million acres of US cropland currently treated with these compounds (DiBartolomeis et al., 2019; Douglas & Tooker, 2015). Approximately 90% of neonicotinoid applications involve systemic seed treatments for major field crops, such as corn, soybean, wheat, cotton, and sorghum (Douglas & Tooker, 2015). These systemic insecticides are taken up by the plant during germination, persisting for months throughout all plant tissues including pollen and nectar, posing a significant risk to pollinators.
Unfortunately, neonicotinoids are highly prevalent in ecosystems as they accumulate in soils and waterways where they are absorbed by non-target plants in natural landscapes (Bonmatin et al., 2015). Bees can be exposed to neonicotinoids and other systemic pesticides while foraging on pollen and nectar from crops (Tsvetkov et al., 2017; Wallner, 2009; Woodcock et al., 2017) as well as from wild flowers (Botías et al., 2015). Nevertheless, chronic pesticide exposure is more likely to occur in agricultural fields (Hladik et al., 2016; Krupke et al., 2012)
Mounting evidence shows that neonicotinoids impair learning and memory, increase susceptibility to pathogens, and reduce overall productivity and nest performance in bees (Feltham et al., 2014). For instance, sublethal exposure to neonicotinoids has been shown to increase the proliferation of Nosema ceranae (Doublet et al., 2015; Van der Sluijs et al., 2013; Vidau et al., 2011), black queen cell virus (BQCV) (Doublet et al., 2015), and deformed wing virus (DWV) (Prisco et al., 2013), all of which compromise bee resilience and survival. The simultaneous presence of pathogens and pesticides in agricultural fields creates a complex array of potential interactions, exposing pollinators to significant and poorly understood risks.
The effects of pesticides and pathogens on colony dynamics have been traditionally assessed separately but not in combination. A recent study tracking individual bumble bee workers within the colony demonstrated that exposure to field-realistic doses of neonicotinoids altered colony behavior, disrupting nursing and thermoregulatory efforts (Crall et al., 2018). Another study focused on pathogens showed that a higher proportion of infected individuals introduced to the colony increased infection intensity and prevalence in bumble bee colonies (Bombus impatiens) (Pinilla-Gallego et al., 2020). However, the mechanisms by which pesticide exposure influences pathogen transmission within colonies remain largely unexplored.
In addition to these challenges, extreme climatic events, such as drought, are becoming increasingly frequent and severe globally (Wilhite et al., 2014). These climatic shifts have the potential to significantly affect resource quality and pesticide exposure. Drought stress can constrain plant physiological development, resulting in lower photosynthetic rates, reduced growth and lower yields (Fathi & Tari, 2016; Liang et al., 2020). Drought may also negatively impact pollinators by reducing the nutritional quality of pollen and nectar (Wilson Rankin et al., 2020), and potentially increasing pesticide concentrations in pollen (Garavito, 2017). Thus, the rising frequency of drought events could have negative effects on bee pollinators, but these effects remain almost entirely unexplored.
Research
Objective 1
Overview
We planted 270 neonicotinoid seed-treated plants representing three major agricultural crops–cotton treated with imidacloprid, squash and sunflower with thiamethoxam–from spring through early Fall of 2021. The study was conducted in the Research and Education greenhouse facilities at the University of Massachusetts Amherst. We manipulated irrigation levels, recorded growth measurements, and collected pollen samples which were subsequently analyzed for pesticide residues.
Crop choice and plant propagation
The crops selected for this study represent a diverse range of agricultural uses: sunflower (seeds, oil, and cut flowers), squash (fruits), and cotton (fiber). These crops were chosen based on the following criteria:
- Reliance on pollinators for yield: Yields in our focal crops are improved by bee pollination; sunflower (Chambó et al., 2011) and squash (Hurd et al., 1971; Stoner, 2020) are entirely dependent on pollinators for productivity. While cotton is primarily self-pollinated, bee pollination has been shown to enhance production by 12% (Pires et al., 2014).
- Sufficient pollen production for chemical analysis: Sunflower and squash are exceptionally abundant pollen producers, while cotton is expected to generate enough pollen to enable pesticide residue analysis.
- Use of seed-treated material: These three crops are commonly seed-treated with pesticides, including more than half of cotton plantations (62%) (Hitaj et al., 2020), and nearly all commercial sunflower crops (Bredeson & Lundgren, 2015). Treated squash seeds are readily available (obs.), but there are fewer statistics about national use.
- Regional importance: Two of the three crops chosen for this study are commonly grown in New England (sunflower and squash), and the third is abundantly grown in the southern US. All are economically important nationally: sunflower (1,558 million acres), squash (46,700 acres), and cotton (12 million acres) (NASS, 2017).
The seedlings were transplanted into pots of varying sizes to accommodate the specific needs of each crop: 3 gallons (sunflower), 5 gallons (squash), or 2 gallons (cotton). All the plants were maintained under a 14-hour photoperiod with ambient temperatures set at 75oF during the day, and 68oF at night. After transplanting, all crops were supplemented with a controlled-release fertilizer (Osmocote, 14-14-14). The soil substrate used was ProMix BX, which is a blend enriched with mycorrhizae, consisting of 75% Canadian sphagnum peat moss, perlite, vermiculite, and a pH adjuster. Cotton plants, which became infested with aphids after 6 weeks, were treated with foliar applications of the insecticidal soap M-PEDE for two weeks. Following blooming, Neem oil to was used to continue managing the infestation.
Study design
We arranged the plants in a randomized complete block design on greenhouse benches. Full irrigation was provided up to the pre-blooming phase to ensure vigorous plant growth before initiating the drought treatments, which began 50, 40, and 90 days after sowing for sunflower, squash, and cotton, respectively. Drought treatments were maintained at specific volumetric water content (VWC) levels: above 30% for well-watered plants, 20–29% for moderate water stress, and below 20% for drought conditions.
Soil moisture content was monitored twice per week using a Time Domain Reflectometry (TDR) sensor. Crop growth metrics, including height, flower diameter, and flower number, were recorded regularly. Pollen samples were collected throughout the summer and for an additional two months into the fall of 2021.
We optimized pollen collection strategies per crop to maximize pollen yields, as follows:
- Sunflower: Floral heads were positioned face down over a piece of paper, and gently shaken to release pollen. Since sunflowers are compound flowers with florets blooming progressively, this process was repeated every two days over a 1–2 week period while the inflorescence remained receptive.
- Squash: Pollen was collected by gently scraping the anthers with tweezers. Sampling was conducted every morning for two months, as male squash flowers open exclusively in the morning and last for only a single day.
- Cotton: Given its low pollen production, we collected entire anthers over a four-month period to ensure adequate sample volume.
All pollen samples were collected in 1.5 ml centrifuge tubes, labeled, and immediately placed on dry ice in a cooler during greenhouse collection. Upon returning to the lab, the samples were stored at -20°C to preserve quality.
Pollen sample analysis
After concluding the greenhouse work in October 2021, I sorted the pollen stored in the freezer, pooling samples within each crop and treatment to create composite samples weighing 0.5 -1 g. This process was highly labor-intensive, as I sorted and combined thousands of individual pollen samples from single flowers. We sent the samples for analysis in December 2021 to the Cornell Chemical Ecology Core Facility services to quantify pesticide concentrations. The Core Facility team, with extensive experience in pollen processing, conducted the analysis using Liquid Chromatography/Mass Spectrometry (LC/MS). Given that the minimum amount of pollen required per sample for successful analysis was 0.5 g, pooling plants within treatments was necessary in some cases to meet this threshold. This was particularly critical for cotton (total 18; 4-8 samples/treatment), which produces relatively low amounts of pollen, whereas sunflower (total 40;11-15 samples/treatment) and squash (total 60;13-26 samples/treatment) yielded ample quantities, simplifying the preparation process.
To evaluate the potential risk of toxicity to bees, we calculated the Hazard Quotient (HQ), which compares the pesticide concentration in pollen to toxicity reference values (LD50—the lethal dose that kills 50% of a test population). HQ values were then compared to the EPA's threshold of concern (>0.4) to determine the level of toxicity for bees.
Statistical Analysis
Statistical analyses were performed using R version 2022.07.2 (R Core Team, 2024). We analyzed the effects of drought treatments on crop growth metrics (height, flower diameter, and number of flowers) and pollen pesticide concentrations using generalized linear mixed models implemented in the glmmTMB package. Growth measures were analyzed with a gaussian distributions, while the number of pesticides detected was modeled using a Poisson distribution. Drought treatments were included as fixed predictors, with greenhouse bench position included as a random predictor to account for spatial variation.
Objective 2
Overview
We tested the effect of neonicotinoid-pesticide treatments on the transmission of the pathogen Crithidia bombi in bumble colonies of Bombus impatiens and performed an additional experiment with individual bees to eliminate the impact of social interactions on transmission. For the colony experiment, we placed the bees in microcolonies of ten individuals, inoculating three of them (donors), and then providing the pesticide treatments through sucrose solutions. For the individual trials, we placed bees in individual containers with their assigned sucrose diet treatment. In all the experiments, we measured sucrose consumption, observed behavioral changes, and after 10 days we dissected the bees to assess pathogen transmission and prevalence.
Update on study design
We made an upgrade to the study design for objective 2. In the original proposal, we proposed using cutting-edge equipment with cameras and specialized software to track disease transmission within the bumble bee colony when exposed to sublethal doses of pesticides. However, we had to face obstacles over the Spring and Summer of 2022 that imposed time constraints, delaying the set-up of the automated tracking system. To overcome these challenges, we simplified the methods using a more time-efficient technique typically employed in the laboratory for similar studies. Instead of using the automatic system, we manually tracked disease transmission by observing social interactions and behaviors and correlating them with final infection state. This approach allowed us to effectively gather the necessary data within the available timeframe; this change was approved by NE SARE in April 2023.
Study design
As we observed a substantial mortality rate from the highest pesticide dose in the individual trials, we chose to use only the two lower-dose treatments in the microcolony experiments. We established microcolonies of 10 bumblebee workers using commercial colonies (Bombus impatiens, Koppert®). Microcolonies can estimate reproduction and mimic colony conditions, while allowing for more replicates than full colonies (Tasei & Aupinel, 2008). We arranged three microcolonies from each of three parent colonies, assigning each microcolony randomly within parent colony to one of three pesticide treatments (6 replicates per treatment). After completing the first round, we repeated this process twice, for a total of three trials and 18 replicates per treatment. The pesticide doses for the sucrose solution treatments were informed by the literature and the greenhouse experiment (Obj 1); we used as reference pesticide concentrations detected in squash pollen as this was the crop with the highest concentrations detected out of the three crops (Control = 0, Low = 0.5 ppb, Medium = 1 ppb ppb). We continuously provided the colonies with a 30% sucrose solution containing thiamethoxam (PESTANAL®, Sigma-Aldrich) to simulate chronic exposure.
We prepared stock solutions of the pesticide by dissolving 5.0 mg of thiamethoxam in 50 mL of deionized water and then used these to achieve the specific treatment concentrations. Next, we inoculated three individuals per microcolony with the gut pathogen Crithidia bombi (hereafter Crithidia), following standard laboratory protocols (Adler et al., 2020; Jonathan J. Giacomini et al., 2018), to simulate conditions where newly-exposed foragers return to the colony. We then observed interactions between bees and behavioral changes in the microcolonies for ten days, after which we dissected all bees to count Crithida cells, assessing the effects of the pesticide on the transmission of these pathogens. We measured sucrose consumption in each experiment (per colony and per bee) by weighing the sucrose upon provision and approximately 48 h later, repeating this process for 10 days.
Individual contacts between donor and worker bees were observed and documented three times per colony per day, with each observation lasting five seconds, over a ten-day period. The microcolonies were kept in a dark room and only red lights were used for the observations, as the visual system of bumble bees is less sensitive to this color (Paulk et al., 2008). To assess whether pesticides affect pathogen infection independent of social interactions that influence transmission, I conducted separate individual trials. In these trials, infected bumble bee workers were fed each concentration of thiamethoxam using the same diet treatments. After two weeks, I dissected the bees and assessed infection intensity. This experiment tested the hypothesis that pesticides would increase individual infection intensity due to a weakened immune system, and the results would be used to inform the interpretation of how disease spreads in the microcolony experiment.
Statistical analysis
We used generalized linear mixed models employing the glmmTMB package with binomial distribution to assess presence/absence of infection and Poisson distribution to evaluate Crithidia cell counts as infection intensity; we used a cox proportional hazard to model survival from the coxme package. Fixed predictors included pesticide treatments, while inoculation date and colony id served as random predictors.
We also performed behavior analysis using manual two-step hurdle models to manage zero-inflated and overdispersed count data. First, we assessed the incidence of each behavior (moving, stationary, incubating, feeding) using a binomial distribution (active/ inactive). Next, we modeled only the active bees within each behavior using a Poisson distribution to assess their response to pesticide treatments. This study sheds light on how chronic exposure to systemic pesticides affects behavior within the colony, individual susceptibility to infection, and transmission dynamics, providing insights into an important but understudied consequence of pesticide exposure for the sustainability of agricultural landscapes.
Results
Objective 1
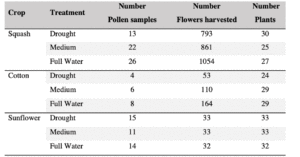
We successfully harvested 119 pollen samples distributed across 82 squash, 98 sunflower and 82 cotton plants, to assess the impact of drought stress on pesticide concentration in the pollen of these three major US agricultural corps, commonly seed-treated with systemic pesticides. Floral production varied among the selected crops, and lower-water treatments amplified these differences by decreasing the number of flowers and pollen production across the three crops (Table 1; Figure 1).
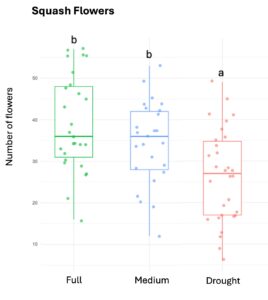
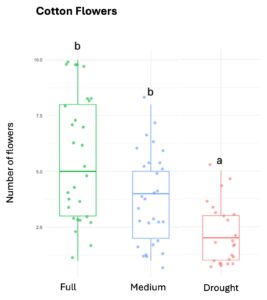
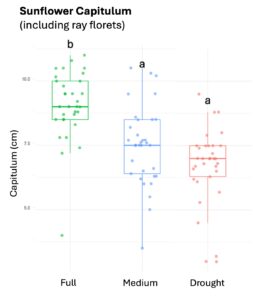
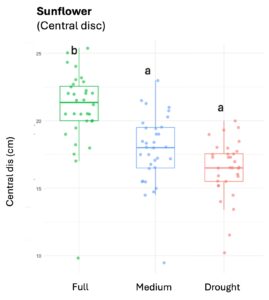
Squash plants exhibited 24% reduction of floral production under drought conditions and 18% under medium water treatments (X2 = 19.214, d.f = 2, p < 0.001). Cotton plants experienced a more substantial reduction in floral production with decreases of 67% under drought conditions and 32% under medium treatments water treatments (X2 = 37.44, d.f = 2, p < 0.001). For sunflowers, we used floral head diameter measuring entire capitulum (including ray florets) and central disc (disc florets only) as proxies for floral productivity. We found a significant negative correlation between low-water treatments and both floral traits (Figure 1); the capitulum diameter decreased by 20%, and 10% under drought and medium water conditions, respectively (X2 = 50.57, d.f = 2, p < 0.001), and the corolla-center diameter decreased by 25% and 16% under the same conditions (X2 = 51.8, d.f = 2, p< 0.001).
Squash was the most productive pollen-bearing crop of the three, contributing 51% of the 119 pollen samples collected, followed by 33% derived from sunflower, and cotton with the remaining 15%. Collecting pollen from cotton was particularly challenging, as the typical low production dropped dramatically under drought conditions (67%). Despite four months of consistent anther collection, we still ended up with a small sample size (Table 1). Therefore, we conducted preliminary analyses on cotton plants to estimate general patterns but not to draw any definitive conclusions.
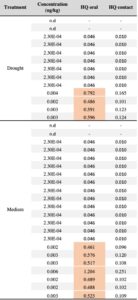
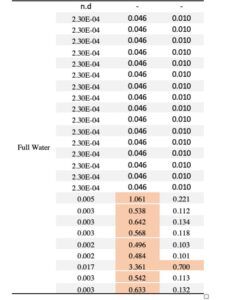
We detected at least one pesticide contaminant in 96% of squash (N= 9 pesticides), 72% of cotton (N=10 pesticides) and 50% of sunflower samples (N=8 pesticides). In total, 19 different compounds were identified across pollen samples, including nine fungicides, five herbicides, three insecticides and one synergist (Table 2). None of the drought treatments predicted either the presence or concentration of pesticides in pollen in any of the crops; the chi-squared value did not exceed 1.36, and the lowest p value was 0.71 suggesting that drought stress does not increase pesticide concentrations in pollen. Among the 19 compounds detected across crops, the neonicotinoid insecticide thiamethoxam was the most prevalent contaminant across all crops, although more frequently detected in squash pollen (92%), followed by cotton (16%), and sunflower (2.5%). Interestingly, cotton was found to contain traces of thiamethoxam but no imidacloprid, even though the provider stated that the treatment involved a pesticide mix with imidacloprid as the primary active ingredient. Notably, 20 squash samples had thiamethoxam at concentrations exceeding the oral hazard threshold (HQ) for bee consumption, while one sample surpassed the contact hazard threshold (Table 3).
Objective 2
We tested the effects of chronic exposure to the neonicotinoid thiamethoxam on the transmission dynamics of the pathogen Crithidia bombi in bumble bee colonies (Bombus impatiens). Our results consistently show that the pesticide treatments reduced the pathogen infection, with the highest dose having the most effect. The spread of pathogen also was significantly reduced under medium and low treatments with no significant difference observed between the two. In addition, we also conducted individual trials feeding infected bumble bee workers each concentration of thiamethoxam using the same diet treatments and dissecting two weeks later to assess infection intensity. Our results were consistent with the microcolony trials; we found the lowest infection rates at high pesticide concentrations. This experiment allowed us to isolate the pesticide effect from social interactions to inform our colony trials.
Laboratory trials
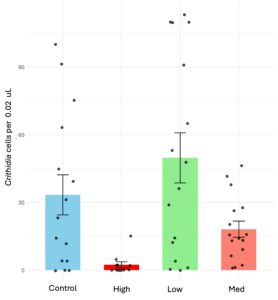
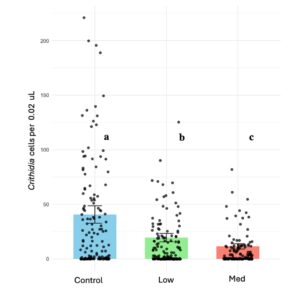
Chronic exposure to thiamethoxam significantly reduced Crithidia cell counts in bumblebees (X2 = 30.66, d.f = 3, p < 0.001) that were individually exposed to the pesticide. Bees subjected to high dosages (1.5 ppb) showed a dramatic reduction, with Crithidia counts nearly 20 times lower than those in the control group, while medium dosages (1 ppb) led to a 1.72-fold reduction compared to the control (Figure 2A). However, mortality rates for bees exposed to high dosages remained notably elevated at 44%, compared to 5% to 16% in the other groups (X2 = 8.56, d.f = 3, p = 0.035).
After completing the individual trials, we proceed with the colony trials; this experiment aimed to assess the interactive effect of pathogen-pesticide dynamics in the colony, including social interactions. Consistent with the individual trial findings, microcolonies fed thiamethoxam exhibited around 2.5 to 5 times lower Crithidia counts under low and medium treatments, respectively (X2 = 38.941, d.f = 2, p < 0.001); both treatments reduced significantly Crithidia counts, with the medium treatment showing the most substantial reduction (Figure 2B). Additionally, infection spread was lower under both pesticide doses (X2 = 14.69, d.f = 2, p < 0.001), with no significant difference between the two. Survival rates remained similar for all treatments, with no significant effects with exposure to thiamethoxam across colonies (X2 = 0.935, d.f. = 2, P = 0.626); also, the number of individual contacts between bee donors (inoculated with Crithidia) and bee recipients increased with the number of recipients alive (X2 = 17.03, d.f. = 1, P < 0.001), while it did not respond to pesticide treatments.
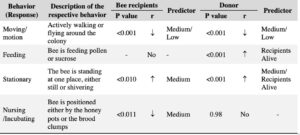
Despite the reduced presence of the pathogen Crithidia, we observed disruptions in several behaviors critical for colony growth and development including brood incubation, social interactions, feeding and overall motion (Table 4). For instance, bee mobility was reduced in both donors (X² = 23.64, d.f = 2, p < 0.001) and recipients (X² = 30.361, d.f = 2, p < 0.001), by both pesticide treatments, with no significant differences between the two. We also observed a significant increase in stationary behavior—the response variable—in both bee recipients (X² = 9.08, d.f = 2, p < 0.010) and donors (X² = 16.22, d.f = 2, p < 0.001) specifically under medium pesticide concentrations. For bee donors, the best-fit models included the number of living recipients as an additive predictor, with moving and stationary behaviors as the response variables.
Additionally, incubation activity was significantly reduced in recipients under the medium pesticide treatment (X² = 8.86, d.f = 2, p = 0.011), while it remained unaffected in bee donors (X² = 0.037, d.f = 2, p = 0.98).
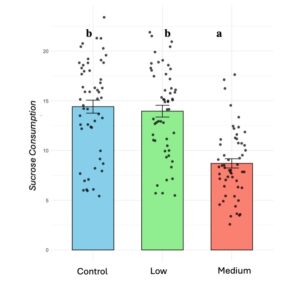
Sucrose consumption significantly decreased in colonies exposed to medium pesticide treatments (X² 67.92, d.f =2, p < 0.001) (Figure 3). Additionally, we observed a significant withdrawal in nursing activities from bee recipients under medium pesticide treatments (X² = 8.869, d.f = 2, P = 0.011), but no effect on bee donors.
Discussion
In objective one, we found that drought stress triggered physiological responses that reduced the quantity and size of floral resources across three crops, but it did not increase the concentration of systemic pesticides as we hypothesized. Cotton experienced the most severe reduction in floral production, with 67% decrease in flower counts under drought conditions. Squash and sunflower were more moderately but significantly affected, with squash producing 24% fewer floral units and sunflower capitula being 20 to 25% smaller in size (Figure 1). Consistent with other studies (Phillips et al., 2018), our results indicate substantial reductions in floral resources with crop-specific variations; these differences likely reflect the crop’s life histories, reproductive strategies, and cultivar-specific traits (Descamps et al., 2021; Phillips et al., 2018). Prior research suggests that squash and sunflower exhibit some degree of resilience and adaptability to drought stress (Bannayan et al., 2011; Brandenberger et al., 2019; Hussain et al., 2018; Kireva, 2020), whereas cotton shows lower tolerance, with floral resources being among the first to be traded off (Loka & Oosterhuis, 2012; Zonta et al., 2017). Thus, our findings underscore the significant impact of drought on the amount and size of floral resources, with variations reflecting the unique characteristics of each crop.
We found no correlation between pesticide presence in pollen and drought stress in any of the evaluated crops. However, we identified eight to ten different pesticide contaminants per crop, with squash pollen containing the highest levels of contamination among the three crops studied (Table 2). While the detection of neonicotinoids in pollen was expected, given their common use in as seed-coating treatments as systemic insecticides, the presence of numerous other pesticides– predominantly fungicides and herbicides– was unexpected. These compounds are not typically systematically absorbed by the plants, raising questions about their source and pathways into squash pollen.
The presence of the insecticide thiamethoxam in squash pollen was particularly concerning, as it was detected in 56 out of 61 samples across treatments with 20 samples exhibiting high concentrations (Table 3). Our results indicate that thiamethoxam concentrations in squash pollen reached levels that pose a substantial risk of oral toxicity to bees. One third of the squash samples exhibited acute HQ values greater than 0.4, surpassing the EPA’s threshold of concern (Table 3) and suggesting a potential risk of chronic toxicity to bees. These findings are particularly concerning given that the use of neonicotinoid-coated seeds in squash crops has been widely adopted as an IPM strategy often considered safer for pollinators compared to spray applications. Several studies have documented the transfer of thiamethoxam from seed treatments to pollen and nectar in cotton and sunflower crops (Jiang et al., 2018; Laurent & Rathahao, 2003; Lundin et al., 2015; Ward et al., 2023; Zaharia et al., 2023), while only one has assessed this phenomenon in squash crops and its detrimental effects on bees (Obregon et al., 2022). Consistent with this recent study, our experiment provides evidence that the use of thiamethoxam-treated seeds can result in high pesticide concentrations in squash pollen with potential toxic risks for bees, regardless of drought conditions.
Overall, the combined effects of drought and insecticide seed coatings reduced both the quantity and quality of floral resources. These changes can have cascading impacts on the diet and survival of wild and managed bees, which rely heavily on pollen and nectar for sustenance. While we found no correlation between drought and pesticide concentrations in pollen, it is important to note that our study was limited to three crops grown under controlled greenhouse conditions. Further research is needed to understand the impacts of drought on neonicotinoid seed-treated crops under field-realistic conditions.
In objective two, we found that thiamethoxam-laced diets influenced pathogen counts and transmission rates in bumblebee microcolonies, but not in the way we had predicted. We hypothesized that pesticide exposure would increase infection intensities, likely due to a weakened immune system. However, contrary to our expectations, we observed a significant reduction in Crithidia counts, a result consistently demonstrated across laboratory experiments with individual bees and microcolonies (Figure 2A and B). Additionally, colonies exposed to thiamethoxam showed lower Crithidia transmission rates. Our findings contrast with a recent study by Siviter et al., (2022), which assessed the combined effect of Crithidia and a single exposure event to thiamethoxam at different dosages but found no effect on pathogen intensity. However, as our study investigated chronic exposure to thiamethoxam, the two studies are not directly comparable.
This unexpected finding suggests two possible explanations: either thiamethoxam may have direct anti-parasitic effects on Crithidia, which would be surprising given previous studies reporting negative outcomes from thiamethoxam and Crithidia co-exposure (Baron et al., 2017; Fauser et al., 2017; Fauser‐Misslin et al., 2014); or reduced food consumption (or other changes in host physiology) could lead to decreased pathogen counts due to starvation, as demonstrated in prior studies (Conroy et al., 2016; Logan et al., 2005). Considering that thiamethoxam is probably the most used neonicotinoid-insecticide worldwide (Botías et al., 2015) and Crithidia one of the most prevalent pathogens in bumblebee populations (Gillespie, 2010; Shykoff & Schmid-Hempel, 1991), understanding the interaction between this pesticide and pathogen is critical. Further research is necessary to elucidate the mechanisms by which thiamethoxam influences Crithidia dynamics including whether its effects are driven by direct toxicity to the parasite, indirect impacts on host physiology, or other unexplored factors.
Furthermore, despite the reduced presence of the pathogen Crithidia, we observed significant disruptions in behaviors critical for colony growth and development (Table 4). The loss of mobility and disengagement from nursing behaviors were strongly significant in both thiamethoxam treatments which aligns with similar results reported for honeybees (Wu-Smart & Spivak, 2016) and bumblebees (Crall et al., 2018); after two days of chronic exposure, bees progressively started to experience loss of mobility by adopting stationary positions, often accompanied by trembling and involuntary spasms followed by paralysis. These results are consistent with previous studies that reported abnormal behaviors in bumblebees following chronic exposure to thiamethoxam and imidacloprid, including trembling, falling and other sudden involuntary movements (Crall et al., 2018; Ludicke & Nieh, 2020; Tosi et al., 2017). This suggests that a key reason for decline in essential colony tasks–such as nursing, cleaning, and foraging– is that bees enter a state of paralysis, losing control of their bodies. This loss of functionally poses a significant threat to overall colony health and sustainability. Additionally, we observed a significant decrease of sucrose consumption in response to medium pesticide treatments.
Our findings highlight a complex and unexpected interaction between chronic thiamethoxam exposure and Crithidia bombi dynamics in bumblebee microcolonies. Contrary to our hypothesis, thiamethoxam significantly reduced Crithidia counts and transmission rates. However, it also induced severe disruptions in behaviors critical for colony health and sustainability. Bees exposed to thiamethoxam exhibited loss of mobility, tremors, and paralysis, consistent with neurotoxic effects reported in previous studies. These impairments significantly reduced participation in essential tasks such as nursing and foraging, which are vital for colony growth and development. Given the widespread use of thiamethoxam and the prevalence of C. bombi in bumblebee populations, further research is needed to elucidate the underlying mechanisms driving these interactions. This includes assessing whether the effects are due to direct toxicity to the pathogen, indirect impacts on host physiology, or other unexplored factors. Understanding these dynamics is critical for balancing pest management practices with the conservation of pollinator health and agroecosystem sustainability.
References
Adler, L. S., Fowler, A. E., Malfi, R. L., Anderson, P. R., Coppinger, L. M., Deneen, P. M., Lopez, S., Irwin, R. E., Farrell, I. W., & Stevenson, P. C. (2020). Assessing Chemical Mechanisms Underlying the Effects of Sunflower Pollen on a Gut Pathogen in Bumble Bees. Journal of Chemical Ecology, 46(8), 649–658. https://doi.org/10.1007/s10886-020-01168-4
Bannayan, M., Rezaei, E., & Alizadeh, A. (2011). Climatic Suitability of Growing Summer Squash (Cucurbita pepo L.) as a Medicinal Plant in Iran. Notulae Scientia Biologicae, 3. https://doi.org/10.15835/nsb.3.2.5846
Baron, G. L., Jansen, V. A. A., Brown, M. J. F., & Raine, N. E. (2017). Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nature Ecology & Evolution, 1(9), 1308–1316. https://doi.org/10.1038/s41559-017-0260-1
Bonmatin, J.-M., Giorio, C., Girolami, V., Goulson, D., Kreutzweiser, D. P., Krupke, C., Liess, M., Long, E., Marzaro, M., Mitchell, E. A. D., Noome, D. A., Simon-Delso, N., & Tapparo, A. (2015). Environmental fate and exposure; neonicotinoids and fipronil. Environmental Science and Pollution Research International, 22(1), 35–67. https://doi.org/10.1007/s11356-014-3332-7
Botías, C., David, A., Horwood, J., Abdul-Sada, A., Nicholls, E., Hill, E., & Goulson, D. (2015). Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environmental Science & Technology, 49(21), 12731–12740. https://doi.org/10.1021/acs.est.5b03459
Brandenberger, L., Shrefler, J., Damicone, J., & Rebek, E. (2019). Squash and Pumpkin Production.
Bredeson, M. M., & Lundgren, J. G. (2015). Thiamethoxam Seed Treatments Have No Impact on Pest Numbers or Yield in Cultivated Sunflowers. Journal of Economic Entomology, 108(6), 2665–2671. https://doi.org/10.1093/jee/tov249
Chambó, E. D., Garcia, R. C., Oliveira, N. T. E. de, & Duarte-Júnior, J. B. (2011). Honey bee visitation to sunflower: Effects on pollination and plant genotype. Scientia Agricola, 68(6), 647–651. https://doi.org/10.1590/S0103-90162011000600007
Conroy, T. J., Palmer-Young, E. C., Irwin, R. E., & Adler, L. S. (2016). Food Limitation Affects Parasite Load and Survival of Bombus impatiens (Hymenoptera: Apidae) Infected With Crithidia (Trypanosomatida: Trypanosomatidae). Environmental Entomology, 45(5), 1212–1219. https://doi.org/10.1093/ee/nvw099
Crall, J. D., Switzer, C. M., Oppenheimer, R. L., Ford Versypt, A. N., Dey, B., Brown, A., Eyster, M., Guérin, C., Pierce, N. E., Combes, S. A., & de Bivort, B. L. (2018). Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science, 362(6415), 683–686. https://doi.org/10.1126/science.aat1598
Descamps, C., Quinet, M., & Jacquemart, A.-L. (2021). The effects of drought on plant–pollinator interactions: What to expect? Environmental and Experimental Botany, 182, 104297. https://doi.org/10.1016/j.envexpbot.2020.104297
DiBartolomeis, M., Kegley, S., Mineau, P., Radford, R., & Klein, K. (2019). An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLOS ONE, 14(8), e0220029. https://doi.org/10.1371/journal.pone.0220029
Doublet, V., Labarussias, M., Miranda, J. R. de, Moritz, R. F. A., & Paxton, R. J. (2015). Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environmental Microbiology, 17(4), 969–983. https://doi.org/10.1111/1462-2920.12426
Douglas, M. R., & Tooker, J. F. (2015). Large-Scale Deployment of Seed Treatments Has Driven Rapid Increase in Use of Neonicotinoid Insecticides and Preemptive Pest Management in U.S. Field Crops. Environmental Science & Technology, 49(8), 5088–5097. https://doi.org/10.1021/es506141g
Fathi, A., & Tari, D. B. (2016). Effect of Drought Stress and its Mechanism in Plants. International Journal of Life Sciences, 10(1), Article 1. https://doi.org/10.3126/ijls.v10i1.14509
Fauser, A., Sandrock, C., Neumann, P., & Sadd, B. M. (2017). Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecological Entomology, 42(3), 306–314. https://doi.org/10.1111/een.12385
Fauser‐Misslin, A., Sadd, B. M., Neumann, P., & Sandrock, C. (2014). Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. Journal of Applied Ecology, 51(2), 450–459. https://doi.org/10.1111/1365-2664.12188
Feltham, H., Park, K., & Goulson, D. (2014). Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology, 23(3), 317–323. https://doi.org/10.1007/s10646-014-1189-7
Garavito, A. (2017). Pollen Nutrition, Pesticides, and Pathogens: Interactive Effects on Honey Bee Health [Thesis]. https://doi.org/10.13016/M20P29
Gillespie, S. (2010). Factors affecting parasite prevalence among wild bumblebees. Ecological Entomology, 35(6), 737–747. https://doi.org/10.1111/j.1365-2311.2010.01234.x
Hitaj, C., Smith, D. J., Code, A., Wechsler, S., Esker, P. D., & Douglas, M. R. (2020). Sowing Uncertainty: What We Do and Don’t Know about the Planting of Pesticide-Treated Seed. BioScience, 70(5), 390–403. https://doi.org/10.1093/biosci/biaa019
Hladik, M. L., Vandever, M., & Smalling, K. L. (2016). Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Science of The Total Environment, 542, 469–477. https://doi.org/10.1016/j.scitotenv.2015.10.077
Hurd, P. D., Linsley, E. G., & Whitaker, T. W. (1971). Squash and Gourd Bees (peponapis, Xenoglossa) and the Origin of the Cultivated Cucurbita. Evolution, 25(1), 218–234. https://doi.org/10.2307/2406514
Hussain, M., Farooq, S., Hasan, W., Ul-Allah, S., Tanveer, M., Farooq, M., & Nawaz, A. (2018). Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agricultural Water Management, 201, 152–166. https://doi.org/10.1016/j.agwat.2018.01.028
Jeschke, P., Nauen, R., Schindler, M., & Elbert, A. (2011). Overview of the Status and Global Strategy for Neonicotinoids. Journal of Agricultural and Food Chemistry, 59(7), 2897–2908. https://doi.org/10.1021/jf101303g
Jiang, J., Ma, D., Zou, N., Yu, X., Zhang, Z., Liu, F., & Mu, W. (2018). Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere, 201, 159–167. https://doi.org/10.1016/j.chemosphere.2018.02.168
Jonathan J. Giacomini, J. L., David R. Tarpy, E. C. P.-Y., & Rebecca E. Irwin, L. S. A. (2018). Medicinal value of sunflower pollen against bee pathogens. https://doi.org/10.1038/s41598-018-32681-y
Kireva, R. (2020). Sunflower irrigation in conditions of water deficit. 3.
Krupke, C. H., Hunt, G. J., Eitzer, B. D., Andino, G., & Given, K. (2012). Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLOS ONE, 7(1), e29268. https://doi.org/10.1371/journal.pone.0029268
Laurent, F. M., & Rathahao, E. (2003). Distribution of [14C]Imidacloprid in Sunflowers (Helianthus annuus L.) following Seed Treatment. Journal of Agricultural and Food Chemistry, 51(27), 8005–8010. https://doi.org/10.1021/jf034310n
Liang, G., Liu, J., Zhang, J., & Guo, J. (2020). Effects of Drought Stress on Photosynthetic and Physiological Parameters of Tomato. Journal of the American Society for Horticultural Science, 145(1), 12–17. https://doi.org/10.21273/JASHS04725-19
Logan, A., Ruiz-González, M. X., & Brown, M. J. F. (2005). The impact of host starvation on parasite development and population dynamics in an intestinal trypanosome parasite of bumble bees. Parasitology, 130(6), 637–642. https://doi.org/10.1017/S0031182005007304
Loka, D., & Oosterhuis, D. (2012). Water stress and reproductive development in cotton. https://www.semanticscholar.org/paper/WATER-STRESS-AND-REPRODUCTIVE-DEVELOPMENT-IN-COTTON-Loka-Oosterhuis/02462b02c7850ed6ce046f1ab2ec7f173540f1fd
Ludicke, J. C., & Nieh, J. C. (2020). Thiamethoxam impairs honey bee visual learning, alters decision times, and increases abnormal behaviors. Ecotoxicology and Environmental Safety, 193, 110367. https://doi.org/10.1016/j.ecoenv.2020.110367
Lundin, O., Rundlöf, M., Smith, H. G., Fries, I., & Bommarco, R. (2015). Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps. PLOS ONE, 10(8), e0136928. https://doi.org/10.1371/journal.pone.0136928
Maroni, M., Fanetti, A. C., & Metruccio, F. (2006). Risk assessment and management of occupational exposure to pesticides in agriculture. La Medicina Del Lavoro, 97(2), 430–437.
NASS. (2017). USDA. https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_2_US_State_Level/
Obregon, D., Pederson, G., Taylor, A., & Poveda, K. (2022). The pest control and pollinator protection dilemma: The case of thiamethoxam prophylactic applications in squash crops. PLOS ONE, 17(5), e0267984. https://doi.org/10.1371/journal.pone.0267984
Paulk, A. C., Phillips-Portillo, J., Dacks, A. M., Fellous, J.-M., & Gronenberg, W. (2008). The Processing of Color, Motion, and Stimulus Timing Are Anatomically Segregated in the Bumblebee Brain. Journal of Neuroscience, 28(25), 6319–6332. https://doi.org/10.1523/JNEUROSCI.1196-08.2008
Phillips, B. B., Shaw, R. F., Holland, M. J., Fry, E. L., Bardgett, R. D., Bullock, J. M., & Osborne, J. L. (2018). Drought reduces floral resources for pollinators. Global Change Biology, 24(7), 3226–3235. https://doi.org/10.1111/gcb.14130
Pinilla-Gallego, M. S., Williams, E. E., Davis, A., Fitzgerald, J. L., McArt, S. H., & Irwin, R. E. (2020). Within-Colony Transmission of Microsporidian and Trypanosomatid Parasites in Honey Bee and Bumble Bee Colonies. Environmental Entomology, 49(6), 1393–1401. https://doi.org/10.1093/ee/nvaa112
Pires, V. C., Silveira, F. A., Sujii, E. R., Torezani, K. R. S., Rodrigues, W. A., Albuquerque, F. A., Rodrigues, S. M. M., Salomão, A. N., & Pires, C. S. S. (2014). Importance of bee pollination for cotton production in conventional and organic farms in Brazil. Journal of Pollination Ecology, 13(0), Article 0. https://doi.org/10.26786/1920-7603(2014)20
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., & Kunin, W. E. (2010). Global pollinator declines: Trends, impacts and drivers. Trends in Ecology & Evolution, 25(6), 345–353. https://doi.org/10.1016/j.tree.2010.01.007
Prisco, G. D., Cavaliere, V., Annoscia, D., Varricchio, P., Caprio, E., Nazzi, F., Gargiulo, G., & Pennacchio, F. (2013). Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences, 110(46), 18466–18471. https://doi.org/10.1073/pnas.1314923110
Shykoff, J. A., & Schmid-Hempel, P. (1991). Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie, 22(2), 117–125. https://doi.org/10.1051/apido:19910204
Stoner, K. A. (2020). Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields. Agronomy, 10(8), Article 8. https://doi.org/10.3390/agronomy10081141
Tasei, J.-N., & Aupinel, P. (2008). Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie, 39(4), 397–409. https://doi.org/10.1051/apido:2008017
Tosi, S., Burgio, G., & Nieh, J. C. (2017). A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Scientific Reports, 7(1), 1201. https://doi.org/10.1038/s41598-017-01361-8
Tsvetkov, N., Samson-Robert, O., Sood, K., Patel, H. S., Malena, D. A., Gajiwala, P. H., Maciukiewicz, P., Fournier, V., & Zayed, A. (2017). Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science, 356(6345), 1395–1397. https://doi.org/10.1126/science.aam7470
Van der Sluijs, J. P., Simon-Delso, N., Goulson, D., Maxim, L., Bonmatin, J.-M., & Belzunces, L. P. (2013). Neonicotinoids, bee disorders and the sustainability of pollinator services. Current Opinion in Environmental Sustainability, 5(3), 293–305. https://doi.org/10.1016/j.cosust.2013.05.007
Vidau, C., Diogon, M., Aufauvre, J., Fontbonne, R., Viguès, B., Brunet, J.-L., Texier, C., Biron, D. G., Blot, N., Alaoui, H. E., Belzunces, L. P., & Delbac, F. (2011). Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema ceranae. PLOS ONE, 6(6), e21550. https://doi.org/10.1371/journal.pone.0021550
Wallner, K. (2009). Sprayed and seed dressed pesticides in pollen, nectar and honey of oilseed rape. Julius-Kühn-Archiv,No.423, 152–153.
Ward, L. T., Hladik, M. L., Guzman, A., Bautista, A., & Mills, N. J. (2023). Neonicotinoid Sunflower Seed Treatment, While Not Detected in Pollen and Nectar, Still Impacts Wild Bees and Crop Yield. Agrochemicals, 2(2), Article 2. https://doi.org/10.3390/agrochemicals2020018
Wilhite, D. A., Sivakumar, M. V. K., & Pulwarty, R. (2014). Managing drought risk in a changing climate: The role of national drought policy. Weather and Climate Extremes, 3, 4–13. https://doi.org/10.1016/j.wace.2014.01.002
Wilson Rankin, E. E., Barney, S. K., & Lozano, G. E. (2020). Reduced Water Negatively Impacts Social Bee Survival and Productivity Via Shifts in Floral Nutrition. Journal of Insect Science, 20(15). https://doi.org/10.1093/jisesa/ieaa114
Woodcock, B. A., Bullock, J. M., Shore, R. F., Heard, M. S., Pereira, M. G., Redhead, J., Ridding, L., Dean, H., Sleep, D., Henrys, P., Peyton, J., Hulmes, S., Hulmes, L., Sárospataki, M., Saure, C., Edwards, M., Genersch, E., Knäbe, S., & Pywell, R. F. (2017). Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science, 356(6345), 1393–1395. https://doi.org/10.1126/science.aaa1190
Wu-Smart, J., & Spivak, M. (2016). Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Scientific Reports, 6(1), 32108. https://doi.org/10.1038/srep32108
Zaharia, R., Trotuș, E., Trașcă, G., Georgescu, E., Șapcaliu, A., Fătu, V., Petrișor, C., & Mincea, C. (2023). Impact of Seed Treatment with Imidacloprid, Clothianidin and Thiamethoxam on Soil, Plants, Bees and Hive Products. Agriculture, 13(4), Article 4. https://doi.org/10.3390/agriculture13040830
Zonta, J. H., Brandão, Z. N., Rodrigues, J. I. D. S., & Sofiatti, V. (2017). Cotton response to water deficits at different growth stages. Revista Caatinga, 30(4), 980–990. https://doi.org/10.1590/1983-21252017v30n419rc
Tables and Figures
Table 1. Production of flowers and pollen categorized per crop and treatments; pollen samples weighted between 0.5 to 1 g.
Table 2. Summary of pesticide residues in pollen samples categorized by crop and pesticide type. Pesticide types: I=insecticide, F=fungicide, H=herbicide, S= synergist. For each detected residue, the table indicates the number of samples below (< LOQ) and above (> LOQ) the limit of quantification –the number in parenthesis indicates the number of samples that tested >LOQ for each compound–. For residues detected >LOQ, the mean concentration (ug/kg) and percentage of positive detections is listed. Contact and oral honey bee LD50 toxicity data were obtained from the Tomlin Pesticide Manual44, the ECOTOX database of the U.S. Environment Protection Agency (http://cfpub.epa.gov/ecotox/) and the AgriTox Database of the French government (http://www.agritox.anses.fr/index.php). Pollen Hazard Quotients (HQ) were determined for oral and contact exposure reflecting how bees encounter pesticides when foraging, by dividing average of residues (ug/kg pollen) for each compound by the respective honey bee oral and contact LD50 value (ug/bee). The HQ values highlighted in red exceed the EPA threshold of concern of 0.4 and indicate high toxicity of the compound for honey bees.
Table 3. Squash pollen samples testing positive for the neonicotinoid thiamethoxam (20 samples with concentrations >LOQ) categorized by treatments. The table presents the contact and oral hazard quotients (HQ= concentration / LD50) for each sample. HQ values exceeding the EPA’s level of concern (> 0.4) indicate high thiamethoxam toxicity to honey bees and are highlighted in red.
Table 4. Chronic exposure to the neonicotinoid insecticide thiamethoxam disrupts various behaviors in both bee donors (inoculated with the pathogen Crithidia bombi) and recipients. The correlation (r) represented with an arrow indicates the relationship between each observed behavior (response variables) and the pesticide treatments (predictors). For donors, the models also included the number of living recipients as an additional predictor.
Figure 1. The three crops varied in their floral productivity, measured in number of flowers for squash and cotton (top plots), and diameter of floral head for sunflower (bottom plots). Different letters indicate statistical significance from post-hoc analysis.
Figure 2. Effect of chronic exposure to thiamethoxam-laced diets on Crithidia infection in microcolonies of B. impatiens. (A) Crithidia bombi cell counts in individual trials. (B) Crithidia bombi cell counts in microcolonies; means estimated by a generalized linear model; error bars represent standard error back-transformed by emmeans; left axis (blue bars) represent proportion of individuals with zero infection; right axis (red bars), proportion of individuals that became infected. Different letters indicate statistical significance from post-hoc analysis.
Figure 3. Sucrose consumption decreases in bumblebee microcolonies (B. impatiens) when they are chronically exposed to medium doses of thiamethoxam. Different letters indicate statistical significance from post-hoc analysis.
Our research demonstrates that the combined effects of drought and neonicotinoid seed coatings reduce both the quantity and quality of floral resources. Drought reduces resource quantity in terms of floral size and number, while neonicotinoid seed coatings reduce quality due to the presence of pesticide contaminants in pollen. While we found no direct correlation between drought and pesticide concentrations in pollen, our work shows that crop responses vary, with some squash plants accumulating higher pesticide loads in pollen compared to sunflower and cotton. This variability poses differing levels of toxicity risk to both wild and managed pollinators.
Additionally, our findings underscore how chronic exposure to neonicotinoids disrupts essential colony behaviors necessary for colony growth and development, such as nursing and foraging. Surprisingly, pesticide exposure also reduced Crithidia counts and transmission rates. The mechanism for this is unknown but could be due to the reduced sucrose consumption we observed, which has previously been shown to reduce Crithidia infection.
While further research is needed to better understand the underlying mechanisms driving these pesticide-climate-pathogen interactions, our study raises awareness of the unintended detrimental effects of neonicotinoids to pollinators. Based on our findings, we strongly discourage the use of seed-treated crops with systemic pesticides, as their use exposed pollinators to pesticide residues through pollen, with negative effects on several aspects of bee behavior in colonies, potentially compromising the sustainability and resilience of our agricultural systems.
Education & outreach activities and participation summary
This grant has also allowed graduate student Carolina Munoz to connect with growers and beekeepers, providing valuable professional training for her career goal of working in extension education.
Participation summary:
We have reached different audiences over the past year to disseminate results, including academic scientists, beekeepers, and growers through the following events:
Webinars and talks:
- In-person oral presentation at the Annual Meeting Entomological Society of America – November, 2023. Washington, D.C.
- In-person oral presentation at the Essex beekeepers Association. November, 2023. Boston, MA. Stakeholders reached: 60.
- Webinar at Boston Area Beekeepers Association (BABA). January, 2024. Stakeholders reached: 24.
- Webinar at Vermont Beekeepers Association. January, 2024. Stakeholders reached: 50.
- In-person presentation at Tricounty Beekeeper Workshop, Wooster, OH. March 2, 2024. Stakeholders reached: 30.
- In-person presentation at Massachusetts Beekeepers Association Spring 2024 meeting. March 16, 2024. Stakeholders reached: 200.
Workshops/field days
- In-person workshop at Research Extension Assistant (REA) at the UMass Extension Vegetable Program. Center for Agriculture, Food and the Environment (CAFE). February 2024. Stakeholders reached: 25.
Journal Articles:
We are preparing two manuscripts for submission in early Spring 2025.
Project Outcomes
Our research contributes to the construction of more sustainable agricultural practices by providing evidence that neonicotinoid-treated seeds unintentionally expose bee pollinators to these harmful contaminants through pollen, thereby compromising their overall health. Through the laboratory experiments, we found evidence that bumble colonies exposed to neonicotinoids had reduced pathogen infection but also exhibited significant behavioral changes, including a reduction of vital colony tasks such as nursing and feeding, as well as a notable reduction in mobility. These impairments suggest that neonicotinoids disrupt critical colony functions necessary for their survival and reproduction.
Compounding these effects, climatic events such as drought can exacerbate the situation by reducing the availability of floral resources. In such scenarios, the presence of neonicotinoids in the limited pollen and nectar available creates an additive burden, compromising bees’ access to food both in terms of quality and quantity.
Collectively, these results underscore that the use of neonicotinoid-treated seeds is not a sustainable practice, as it resulted in concentrations of pesticides in pollen that negatively affected several aspects of pollinator behavior. To promote a more sustainable agricultural system, we recommend avoiding the use of neonicotinoid-treated seeds. This alternative practice minimizes harmful impacts on pollinators to support food security and environmental health.
Conducting this project provided invaluable insights into the complex interplay between pesticides, pathogens, and environmental factors in shaping the health and resilience of bees. The opportunity to conduct separate experiments–one focused on plants in the greenhouse setting and the other on bees in the laboratory—provided a unique and more holistic perspective. This dual approach significantly enriched the depth and breadth of our findings, allowing for a more comprehensive understanding of these interconnected dynamics.
Additionally, managing and training a team throughout the process further developed my leadership and organizational skills, making this experience both scientifically enriching and professionally transformative.
Through the talks and workshops with growers, beekeepers, farmers, and homeowners, I also came to appreciate the power of building community to share knowledge and raise awareness. Establishing connections with individuals passionate about supporting pollinators and committed to implementing more sustainable practices has been incredibly rewarding.
My next career step is to serve as the Director of Public Programming and Outreach at the Norcross Wildlife Foundation. In this position, I plan to continue my work in extension education and habitat management, while also fostering partnerships to enhance wildlife conservation efforts. I will prioritize raising awareness within the community and inspiring meaningful action to support sustainable practices and protect natural ecosystems.