Progress report for GNE22-276
Project Information
Agriculture contributes about 18% of all greenhouse gases (GHG) global emissions and 10% of US emissions. Dairy farms are an important source of GHG emissions including enteric fermentation, manure management, and soil management. Methane (CH4) from enteric fermentation is the largest source of emissions with about 45% of the total GHG emissions of the full dairy farm system. Most of the efforts for the abatement of CH4 emissions focus on sources with high concentration of CH4. However, combustion for energy recovery is not feasible in enclosed agricultural systems due to the low concentration of CH4 in those emissions. Engineered biofiltration technology is a potential solution for the mitigation of emissions converting CH4 into carbon dioxide (CO2) and water. The objective of this work is to design a pilot-scale biofiltration system for the mitigation of CH4 emissions from dairy livestock facilities ventilation air. I will build a bank of six biofilters to study the effect of process conditions on biofilter performance. CH4 concentration and CO2 production will be measured in the inlet and outlet of the system to calculate the CH4 elimination capacity as performance criteria of the system. Experimental data will be gathered at different process conditions for the calibration and validation of a mechanistic mathematical model that is being simultaneously developed. Both the experimental analysis and the model validation will serve as tools to propose engineered microbial biofiltration technology suitable to be implemented in dairy farms, contributing to more sustainable agricultural practices.
The overarching goal of this proposal is to design and test a microbial biofiltration system for the biological oxidation of CH4-contaminated air from ventilation air of dairy livestock facilities. I propose an experimental approach to investigate the effect of process conditions on the biofiltration system performance. In addition, I propose to use the experimental data as an input to a mechanistic process model, that I am currently developing as part of my dissertation, to elucidate the relationships between process conditions with the mass transfer phenomena and the biodegradation kinetics of the system. I will pursue the following research objectives:
Objective 1. Demonstrate the feasibility of pilot-scale biofilters to biologically oxidize low concentration CH4.
Objective 2. Investigate the effect of different operation conditions on the CH4 biofilter performance.
Objective 3. Identify the correlations among the operation conditions and the mass transfer and biodegradation kinetics of the system.
Objective 1 includes the design and building of a pilot-scale biofiltration system. The biofilter performance will be assessed by quantifying the elimination capacity of CH4. In objective 2, I will evaluate the effect of CH4 inlet concentration and inlet air flow rate on the CH4 oxidation capacity of the system since those variables are known to have an influence on the CH4 oxidation20. To evaluate the effect of process conditions on the mass transfer and biodegradation kinetics of the process in Objective 3, I will use the gathered experimental data from Objective 2 as the input for a mechanistic mathematical model of the system that I am currently developing as part of my dissertation. In that way, mass transfer and biodegradation parameters will be identified as a function of the process conditions and correlations could be established.
The purpose of this project is to design a pilot-scale biofiltration system for the mitigation of methane emissions from dairy livestock facilities ventilation air. Dairy farms are sources of GHG such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), and are responsible for about 50% of CH4 of the emissions from anthropogenic sources1. Enteric fermentation, manure management, and soil management are the greatest contributors of CH4 emissions in dairy farms2. CH4 from enteric fermentation is the largest source of emissions with about 45% of the total GHG emissions of the full dairy farm system3. Due to the low concentration of CH4 in dairy farms ventilation air, combustion for energy recovery or existing methods to separate CH4 from air4 are either unfeasible because of the methane concentration thresholds or require high energy demands to treat such low methane concentration5, which make them impractical to implement in commercial dairy farms.
Dairy cattle are responsible for 25% and 53% of the total emissions from enteric fermentation and manure management emissions, respectively6. Since an optimum ventilation system is needed over time for animal well-being, a low concentration of methane is constantly being released into the atmosphere generating a significant carbon footprint in the environment7. Instead, biofilters can be used to biologically treat the CH4 emissions from the waste gas allowing the farmers to have a dairy system with less net GHG emissions. Biofiltration has been used mostly for the treatment of odor or ammonia-contaminated air but the applications for CH4 mitigation are very limited8. CH4-oxidizing bacteria in CH4 biofilters use low concentration methane as a carbon source and oxidize the CH4 into CO2 and water, decreasing the Global Warming Potential (GWP) of the emissions by 28-34-fold.9
A broad range of CH4 removal efficiencies (13%-99%) is reported in experimental setups of CH4 biofiltration10–13, due to the empirical nature of the biofilter design, the lack of understanding of the underlying phenomena, and the poor ability to control process conditions. This unpredictable nature of the system limits its applicability in the field8. Compaction, clogging, and channeling of the packing media are also drawbacks of the process operation at a larger scale14. In addition to the operational challenges of the system, the effects of process conditions on mass transfer phenomena and biodegradation kinetics, which are closely related to the system performance, need to be studied. Therefore, understanding the relationships between process conditions and their effect on biofilter performance is a necessary step to design stable and reliable engineered biofiltration systems. Such systems can contribute to a reduction of the net GHG emissions of dairy farms systems towards more sustainable agriculture.
Research
I propose to perform a series of experiments to evaluate the effect of key process conditions (i.e., CH4 concentration of the inlet-air and inlet flow rate) on the CH4 biofilter performance. This information will also allow me to identify the effect of the process conditions on the mass transfer and biodegradation kinetics of the system to later implement the correlations in a mechanistic process model of the system that I am currently developing as part of my dissertation.
Objective 1. Demonstrate the feasibility of pilot-scale biofilters to biologically oxidize low-concentration methane.
I will build a pilot-scale biofiltration setup consisting of a bank of six pilot-scale biofilter columns connected to a gas system where air and CH4 will be provided. Each of the biofiltration columns will be made in-house following an optimized column modular design that we developed on a previous biofiltration-related project, as follows: the biofilter columns will be made of polycarbonate tube with an inner diameter of 0.15 m and a height of 1 m. A three-modular design of the columns is considered. The advantages of having a modular design include the minimization of compaction difficulties10, easy packing and unpacking process of the columns11, and more flexibility and quick response against possible clogging or channeling14. The standard biofilter packing media for the columns will consist of a 50:50 mix of compost obtained from the Penn State Organic Materials Processing Center (OMPEC) facility mixed and woodchips provided by the Penn State Office of the Physical Plant Grounds Maintenance21.
Two different sets, each one with three biofilters, are considered. Set 1 will be used for the baseline experiments and set 2 will be devoted to the experiments related to objective 2, where different CH4 inlet concentrations and inlet flow rates will be tested. Each set will have a pair of mass flow controllers to control the CH4:air inlet concentration ratio. In addition, each of the biofilters will have a rotameter at the inlet that allows for setting different inlet flow rates. Tubing, connections, and adapters will be used to connect each of the sets to the CH4 and air supplier and a humidifier will be used to control moisture content in the inlet gas.
The biofilters require a considerable stabilization period to reach steady-state conditions14. To account for that stabilization period, I propose a consecutive building process for the six biofilters. That is, the first batch of the building period will be dedicated to constructing and setting up the first set of three biofilters. Once built, the stabilization period begins at specified conditions for about two months. Inlet and outlet air samples will be taken twice per week to monitor CH4 and CO2 concentration during the stabilization period and guarantee that the system has reached a steady state. While the first set is in the two-month stabilization period, I will start the building process and tunning of the other set of three biofilters, which also will need a stabilization period of two months to reach a steady state. In this way, death times are avoided since the two sets of biofilters will be in different stages through the project timeline allowing us to test different conditions in each of them.
Objective 2. Investigate the effect of different operation conditions on methane biofilter performance. Operation conditions such as CH4 inlet concentration and inlet flow rate have an important effect on the performance of methane biofilters10,11,22,23. Three levels, i.e., high, baseline, and low for each of the variables, i.e., CH4 inlet concentration and flow rate will be tested to determine the biofilter performance. The biofilter performance will be determined by measuring CH4 concentration in the inlet and outlet air streams of the system. We will also monitor the concentrations of CO2. The concentration of gases will be measured by gas chromatography. CH4 biofilters performance will be analyzed by the elimination capacity. To achieve this objective, I am proposing two tasks:
Task 1: Baseline experiment
The first set of three biofilters will be built in-house. I will operate the first set of biofilters under baseline conditions of CH4 inlet concentration, which correspond to CH4 concentration from an average dairy farm ventilation air. A preliminary two-month stabilization period is considered, during which a constant flow of methane-contaminated air will flow through the biofilters. This baseline set will operate under the same conditions throughout the whole project timeline. This will allow me to evaluate the effect of time, and the corresponding possible packing material deterioration such as the presence of compaction, clogging, and channeling effects on the CH4 oxidation capacity. I will take samples from the inlet and outlet air streams of the biofilters 2-3 times per week, and I will quantify CH4 and CO2 concentrations.
Task 2: Evaluate the effect of CH4 inlet concentration and inlet flow rate on the methane oxidation capacity
For the second set of biofilters, high-level CH4 inlet concentration and low-level CH4 inlet concentration, as compared to the baseline condition, will be tested. My hypothesis is that the changes in the elimination capacity are significant between these two levels10. Four sets of experiments will be performed. Aleatory triplicates are considered to average out the effects of unknown and uncontrollable nuisance factors in the design. In addition, it is known that process conditions affect and produce changes in the microbial community distribution of the biofilter in the long run14. Therefore, to avoid influences between one experiment and the following ones, I will run experiments 5 and 6 under low CH4 concentration conditions first, since it is more likely that high CH4 concentration conditions can influence further experiments. I will operate the set of biofilters under low conditions of CH4 inlet concentration until a steady state is reached. During this stabilization period, a constant flow of CH4-contaminated air will flow through the biofilters, and samples will be taken 2-3 times per week to monitor the changes in CH4 concentration. Once the system reaches a steady state, experimental variations on the flow rate are introduced for runs 5 and 6.
Table 1. Experiments under low CH4 inlet concentration
and two levels of air inlet flow rate
|
|
CH4 inlet concentration |
Low |
Low |
Low |
|
Air inlet flow rate |
Run 5 |
Low |
High |
High |
|
Run 6 |
High |
Low |
Low |
For runs 5 and 6, a preliminary two-month stabilization period is considered. Thereafter, each of the replicates per process conditions will be held for one month. That is, a run is considered to run per three months total. Samples will be taken 2-3 times per week from the inlet and outlet air streams and CH4 and CO2 will be quantified.
Once runs 5 and 6 are finished, the CH4 inlet concentration conditions will switch to high level and two month stabilization period will occur. Once the system reaches a steady state, experimental variations on the flow rate are introduced for runs 7 and 8, each of the replicates per process conditions will hold for one month. That is, a run is considered to run per three months total. Samples will be taken 2-3 times per week from the inlet and outlet air streams and CH4 and CO2 will be quantified.
Table 2. Experiments under high CH4 inlet concentration
and two levels of air inlet flow rate
|
|
CH4 inlet concentration |
High |
High |
High |
|
Air inlet flow rate |
Run 7 |
High |
Low |
High |
|
Run 8 |
Low |
High |
Low |
No progress has been done so far in this objective since we first need to finish the complete experimental setup for the bank of biofiltration columns. I expect to start this objective in about 2-3 months, once the experimental setup is finished.
Objective 3. Identify the correlations among the operation conditions and the mass transfer and biodegradation kinetics of the system. The experimental design proposed in Objective 2 will allow me to gather a significant set of data including CH4 concentration, flow rates, CO2 concentration, and CH4 elimination capacity for different process conditions. In addition, as a part of my dissertation, I am currently working on developing a mechanistic model describing the phenomena taking place in the biofilter considering the mass transfer, transport phenomena, and reaction occurring at the biofilm level. To that aim, microorganisms are considered to grow on the surface of a solid medium where the microbial degradation of CH4 to CO2 and water follows a Michaels-Menten-based kinetics24,25. Mass balances for the CH4 and CO2 will be formulated in both the gas phase and the gas/liquid interface. The mathematical model will consist of a set of ordinary differential equations that will be solved using Python.
For Objective 3, I propose to use the obtained experimental data as input for the model for the identification of the mass transfer parameters and biodegradation kinetics parameters using optimization techniques. Having data on different process conditions will allow me to identify the effect of the process conditions on the value of the process parameters. My hypothesis is that the inlet flow rate has a greater influence on the process parameters since high flow rates can accelerate compaction problems in the packing material and low flow rates can limit mass transfer in the system. I expect that the outcome of this objective will be the obtention of a correlation for each of the process parameters as a function of the CH4 inlet concentration and inlet air flow rate.
Progress on this objective has been done only using data from the literature. I expect to start using data from my experiments once objective 2 is in about 30% of execution.
The following are the outputs and deliverables from the project:
Objective 1. Demonstrate the feasibility of pilot-scale biofilters to biologically oxidize low-concentration CH4.
- Three-modular design column
This design process was a collaborative effort with Shakthi N Suresh, a recent graduate with a master’s degree in engineering design. The images used in this section have been sourced from his thesis, which centers on the specific design of the three modular biofilter columns.
The biofilter column comprises three modules, each module measures 33 cm in length and 15 cm in diameter. These modules are constructed using polycarbonate tubes. These modules are stacked and secured using 3D-printed Polylactic acid (PLA) flanges at both ends, ensuring an airtight seal with gaskets, screws, and wingnuts. The entry point for the air-CH4 gas mixture is at the bottom, which then travels through the packing media enclosed within the modules. The 3D-printed base unit has been designed to include an optional drainage feature, while 3D-printed legs offer versatility for standalone placement or mounting on a base to create repeatable units. Figure 1 shows the CAD design for the modular biofilter and the pictures of the prototype in the laboratory.


A mixing chamber and a humidifier were also built using polycarbonate tubes and 3D-printed parts. The mixing chamber allows us to mix appropriately the air and CH4 streams, while the humidifier ensures that the incoming gas mixture is adequately saturated, preventing dryness in the packing material and ensuring optimal moisture levels for microbial growth within the compost. For the final prototype, we mounted it on a wooden structure alongside the humidifier and mixing chamber, presenting an initial design concept for the structure intended to house the nine biofiltration columns (see Figure 2).
Although this initial design was successfully constructed, we encountered several drawbacks during the construction process, particularly in achieving the necessary airtight system critical for our application. This design analysis exposed the limitations of 3D-printed components in maintaining the requisite airtight integrity for our specific applications. These challenges raised concerns about scaling up to accommodate nine biofilter columns. As a result, we opted to transition to the single-module design column, aiming for a more streamlined and compact design while building upon the insights and design lessons gained from our experience with the three-modular design column.
- One-modular design column
This design process was a collaborative effort with Tate Greiger, an undergraduate researcher in the Department of Engineering Science.
The biofilter column body was made from a polycarbonate tube with an internal diameter of 15 cm and a height of 1.2 meters. To cap both ends of the column, we employed Acetal Resin (Delrin®) for machining the lids. To ensure airtight sealing between the polycarbonate tube and the lids, O-ring piston seals were utilized. All the additional parts that do not require airtight characteristics were 3D printed. The entry point for the air-CH4 gas mixture is at the bottom, which then travels through the packing media enclosed within the column. A 5cm chamber was integrated at the base to ensure the uniform mixing of the inlet air-CH4 stream before it enters the packing material. Within this chamber, a drainage system was implemented to manage potential leachate during biofilter operation. All the fittings used to connect the column with the tubing are of the push-to-connect fittings suitable for airtight conditions.
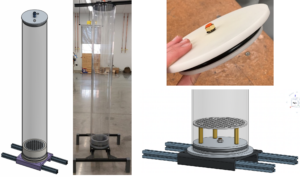
The biofilter column is attached to an aluminum extrusion structure, providing both stability and the convenience of mobility, all within a compact design that is well-suited for easy and efficient scalability. Figure 3 illustrates the various components that make up the biofilter column. To confirm the absence of any leaks and validate the suitability of the design for scaling up to nine columns, a leak test was conducted using the bubble test method.
After validating the design's airtight integrity, we proceeded to build the remaining eight biofilters. To house this bank of biofiltration columns, we designed three aluminum extrusion structures, with each structure capable of accommodating up to three biofiltration columns. This configuration enables us to efficiently execute the various conditions outlined in our experimental design.
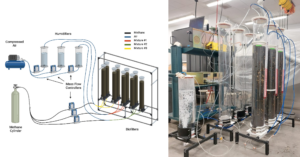
Fittings and tubing have been installed in the bank of biofiltration system and the bank of biofilters system has been proved to be airtight. Rotameters are used to monitor inlet flow in each of the columns. Preliminary tests have also been executed to assess the packing material process and evaluate the overall flow conditions once the columns are packed.
This specific objective stands at 100% completion.
Objective 2. Investigate the effect of different operation conditions on the CH4 biofilter performance.
With the completion of the experimental setup, my current focus is on running the proposed experimental design. To accomplish this, we implemented in-line concentration measurements using FTIR technology from California Analytical Instruments. The FTIR uses Fourier Transfer Infrared technology for measuring concentrations of multiple gases within a sample. This equipment enables monitoring of greenhouse gas (GHG) concentrations from low ppb to high % ranges making it well-suited for our specific application. The FTIR instrument can measure a range of gases, including CH4, CO2, and N2O, all of which are highly relevant to our application. Furthermore, we intend to monitor additional gases such as nitric oxide, sulfur hexafluoride, carbon monoxide, and others, as the equipment provides the capacity to measure them within the sample. Gathering data on these gases holds the potential to offer valuable insights and generate new hypotheses about potential metabolic pathways involved in methane biodegradation.
To employ the FTIR, we have established a connection between the outlet stream of each biofilter and a multi-port sampler (Figure 5). Through this multi-port sampling system, we can program a measurement cycle for each biofilter, enabling us to capture the gas concentrations in the outlet stream of each.

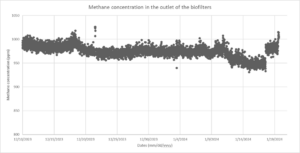
Currently, we are running 1000 ppm methane at a flow of 2lpm as stabilization conditions for the biofilters. The stabilization period has run for about 1 month, Figure 6 shows the methane concentration during this time, which as expected ranges from 950-1010 ppm.
This task is currently at a 20% completion stage. As the next steps, I expect to start the runs for the experimental design in the next couple of months, where the system will run for around 6-7 months continuously. Gas concentrations of the outlet streams of all the biofilters will be taken.
Objective 3. Identify the correlations among the operation conditions and the mass transfer and biodegradation kinetics of the system.
Progress on this objective has been made only using data from the literature. I expect to start using data from my experiments once objective 2 is in about 50% of execution.
From what I have done with the literature data, I developed a mathematical model. The equations yield a system of partial differential equations. I employed the Finite Element Method in Python to approximate and solve this system of differential equations. Initially, I examined the impact on the airflow by considering low, medium, and high flow levels while keeping the CH4 inlet concentration constant at 9500 ppm (Figure 7). According to the results, lower flow rates result in a more effective removal capacity for the system. This effectiveness, however, is limited at higher flow rates. Furthermore, the production of CO2 follows a similar pattern. This trend also raises concerns about potential compaction issues the system may encounter in the long term, highlighting the importance of avoiding very high air flow rates to prevent such compaction problems.
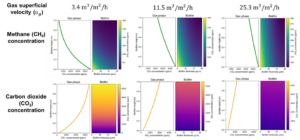
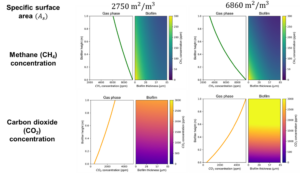
An additional analysis focuses on the influence of packing material type and specific surface area on the system's performance (Figure 8). Figure 8a depicts a standard laboratory-scale biofilter, featuring a blend of 50% organic material and 50% inorganic material, while Figure 8b showcases a biofilter containing compost as the sole organic material, offering nearly 2.5 times greater specific surface area compared to the mixed material scenario. According to the results, an increase in available surface area, facilitating both mass transfer and biodegradation, leads to enhanced methane conversion. Nevertheless, in practical applications, employing solely compost as the packing material is not advisable, as it tends to be prone to compaction and clogging over time. Thus, a balanced ratio of organic to inorganic material in the packing of the filter is a more sustainable approach.
This task is currently at 20% completion. The next steps include validating the model with real data from the experiments and the progress of objective 2.
Note: All the progress has been made in teamwork where a master's student and an undergraduate student have been helping us to assess all the activities related to the project objectives.
Education & Outreach Activities and Participation Summary
Participation Summary:
I have shared the results of this proposed work with specialized audiences during scientific conferences and symposia at the local, regional, and national levels, in the form of poster and oral presentations. The shared results include:
- González, C. Methane Biofiltration Systems and Their Engineering Challenges. Microbial Technologies to Mitigate Methane Emissions Cross-track Symposium: Climate Change and Microbes (CCM) ASM MICROBE 2023. Houston-Texas. June 15- 19 (Invited Speaker)
- González, C., Peacock, V., Geiger, T., Vasco-Correa, J. 2023. Air biofiltration technology for the mitigation of diluted methane emissions. Penn State Climate Solutions Symposium. Penn State Institutes of Energy and Environment IEE. May 22-23. Poster.
- González, C., Peacock, V., Geiger, T., Vasco-Correa, J. 2023. Assessment of a bank of biofiltration system for dilute-methane emissions abatement. ASABE Annual International Meeting. Omaha-Nebraska. July 08-12. Poster
I also expect to produce a peer-reviewed article to be submitted for publication in a scientific journal. The journal options include Journal of Environmental Management, Environmental Science and Pollution Research, and Journal of Environmental Chemical Engineering.
The proposed research will allow exploring the further design of the technology and pathways for future implementation in the field. Thus, outreach to dairy farmers and policymakers will happen in the future when the technology is more mature and ready for deployment. The outreach strategy includes sharing with farmers, policymakers, and stakeholders the biofiltration technology as an affordable opportunity to implement sustainable practices on farms with a potential economic benefit. Penn State extension resources and relationships related to dairy farms, and animals and livestock are available for that purpose.