Final report for GNE22-282
Project Information
The recent reintroduction of industrial hemp (Cannabis sativa L.) as a crop for food, grain, oils, and fiber in Pennsylvania provided an opportunity for farmers to expand their operations to include this high-value commodity. While the benefits projected by the National Hemp Association, including job creation, rural economic growth, and annual financial gains, were impressive, much remains to be learned about this crop to ensure its success in Pennsylvania's agricultural landscape. In particular, insect pests pose a global concern in agriculture, resulting in significant annual crop losses. Given the novelty of this crop and the strict regulations on pesticide use, it has become essential to provide information about insect pests that could lead to direct crop losses through plant consumption and indirect losses via disease transmission, as well as alterations in important plant metabolites such as THC, which can impact yield and profitability. Furthermore, understanding the extent to which native biological control agents (predatory arthropods and parasitoids) regulate pest insects is crucial to disentangling.
To enhance fundamental understanding of the system and contribute to the development of sustainable control strategies, this project:
- Identified the most prevalent insect pests affecting hemp crops in the region, as well as their biological control agents and the occurrence of both types of organisms throughout the 2022 growing season.
- Evaluated the impacts of the most common insect pests on plant yield and defensive metabolites.
- Initiated studies to assess the efficacy of local biological control agents in managing pests.
In the first year of insect sampling, Cannabis aphids (Phorodon cannabis) were the most common pest found on Pennsylvania hemp farms, followed by thrips, leafhoppers, leaf beetles, and plant bugs. These pests exhibited differences in population, impact, and occurrence between CBD and fiber hemp crops, which helped prioritize pest management efforts based on hemp type. Natural enemy communities displayed significant diversity, with generalist predators preventing harmful infestations. While insect damage was observed across hemp farms, it generally did not impair plant growth potential. Greenhouse experiments revealed the high reproductive capacity of cannabis aphids in younger plants, along with varying degrees of tolerance among fiber hemp varieties when faced with severe infestations.
Research Conclusions: The study demonstrated the importance of minimal pesticide use and biological control to sustain populations of beneficial insects in hemp ecosystems. It also highlighted how insect diversity and dynamics varied across hemp types and farms, emphasizing the need for tailored pest management strategies. Challenges included managing large volumes of insect samples, adapting research methods for a novel crop, and mitigating insect and pathogen contamination in experimental plants. Despite these obstacles, this work provided critical insights into pest management and biological control for industrial hemp, laying a foundation for sustainable agricultural practices in Pennsylvania.
- Determine the composition of insect pests and biological control agents throughout the crop growing cycle in Pennsylvania.
Understanding the organisms that interact with crop plants is essential to our success in developing integrated pest management strategies for novel cropping systems. This study identified not only the common pest species on industrial hemp plants but also the biological control agents (predators and parasitoids) that utilize these plants to find prey resources across commonly grown CBD and fiber hemp cultivars. By doing this, we begin to establish a pest management framework that catalogs our pests throughout the growing period and evaluates the potential for biological control to reduce their damage. Importantly, this addresses a significant knowledge gap in the Northeastern region.
- Evaluate the impact of insect pests on plant defensive metabolites in three industrial hemp cultivars.
Insect pests can contribute to dramatic plant defoliation in cropping systems, resulting in crop loss. I evaluated the damage caused by common pests on industrial hemp and assessed how feeding by herbivorous pests might influence plant defenses, potentially leading to crop loss through increased plant metabolites that are federally regulated (THC, Tetrahydrocannabinol). The federal government strictly regulates the level of THC in industrial hemp to below 0.03%, and farmers are required to test their crop plants before harvest. If plants show THC levels greater than 0.03%, then the entire crop must be destroyed because it is classified as a Schedule 1 Drug. Understanding which pests trigger high levels of THC during specific phenological stages is crucial for farmers to prevent significant loss of income and livelihood.
- Evaluate the efficacy of biological control agents on pest management.
The value of beneficial biological control agents can contribute tremendously to pest management. Because pesticidal input is limited in industrial hemp, I started assessing the efficacy of common biological control agents in reducing populations of target pests identified in Objective 1. This information can be used to conserve or augment biological control agents in hemp farms to achieve pest control.
The purpose of this project is to determine the community of insect pests on industrial hemp, elucidate their impact on this high-value commodity, and develop an understanding of how pest management can be achieved in this new system.
In Pennsylvania, industrial hemp is a novel crop; the state government distributed the first commercial grow permits in 2019. Though the expansion of industrial hemp in Pennsylvania is relatively new, crop acreage has been increasing. During 2021, the planted area for fiber, seeds, grain, CBD, and oils reached over 350 acres (National Agricultural Statistics Service - NASS 2022) and is expected to rise in the coming years. The versatility of this crop and the high value of hemp products have increased the interest of farmers who seek alternative crops to increase their income and livelihood.
Like all plants, hemp hosts a variety of arthropod herbivores that have the potential to become important pests. Evidence of the diversity of insect pests in this crop is mounting with ~20 recognized herbivorous pests identified, including generalist herbivores such as the corn earworm, tarnished plant bug, hemp russet mite, and specialist herbivores such as the cannabis aphid (Schreiner and Cranshaw 2020; Ajayi and Samuel-Foo 2021). However, current pest lists come largely from the Southern and Western United States. Because Pennsylvania farmers have only been growing hemp for 4 years, there is much to learn about this system, including what pests represent a threat to hemp production and the income of farmers. Additionally, novel crops like hemp face extensive restrictions in terms of pesticidal input as they wait for safety and efficacy research results on various chemicals. It is clear that alternative management strategies, like biological control, are essential for the success of this crop, and the development of those strategies relies on an intimate understanding of the pests present.
This project advances our current knowledge and benefits sustainable agriculture by determining not only the herbivorous pests found in industrial hemp fields in Pennsylvania but also by evaluating how insect pests damage the plants in ways that impact yield directly – via feeding – or indirectly, by altering important and highly regulated plant metabolites like THC (Tetrahydrocannabinol), which if upregulated can lead the plant to “go hot,” a term used to describe a plant that exceeds the legal limit of THC (0.03%). If this happens, the farmer must then destroy the crop in its entirety which would be a devastating financial loss. Every year, an estimated 20% of hemp farms in the U.S. are non-compliant with THC levels, which represents a challenge that we must understand in Pennsylvania (Cowee 2019).
Altogether, the results of this study allow us to begin defining a sustainable integrated pest management plan for farmers growing this novel crop that is specific to our region by revealing important pests and their seasonality through crop stages, their influence on crop health, important plant metabolites (THC) and the potential for predatory insects and parasitoids to manage pest populations via biological control.
Works Cited:
- Ajayi OS, Samuel-Foo M (2021) Hemp Pest Spectrum and Potential Relationship between Helicoverpa zea Infestation and Hemp Production in the United States in the Face of Climate Change. Insects 12:940. https://doi.org/10.3390/insects12100940
- Cowee M (2019) Hemp THC levels estimated to exceed 0.3% limit in 20% of lots. In: Hemp Ind. Dly. https://hempindustrydaily.com/chart-20-of-hemp-lots-will-exceed-0-3-thc-limit-next -year-usda-estimates/. Accessed 15 Apr 2022
- National Agricultural Statistics Service - NASS (2022) National Hemp Report. Department of Agriculture USDA, United States.
- Schreiner M, Cranshaw W (2020) A Survey of the Arthropod Fauna Associated with Hemp (Cannabis sativa) Grown in Eastern Colorado. J Kans Entomol Soc 93:113–131. https://doi.org/10.2317/0022-8567-93.2.113
Research
1. Determine the composition of insect pests and biological control agents throughout the crop growing cycle in Pennsylvania
To evaluate the community of arthropod organisms in industrial hemp, weekly sampling of the herbivorous insects and potential biological control agents (predators and parasitoids) will be taken in 10 commercial plantings of industrial hemp across Pennsylvania. Sampling will take place throughout the growing season, from first sign of vegetation until harvest (~June – Oct). Sampling throughout the season will allow us to determine pest and biological control agent population fluctuations over time. The industrial hemp farms chosen will represent commercial farming practices, be geographically distinct from one another, and contain our three hemp varieties of interest (cv. Joey, Henola, and Bialobrzeskie). During each sampling, 30 plants per farm will be visually surveyed, counted, and all arthropods present on the plant will be recorded and collected for further identification. Collected individuals will be captured manually, with buccal aspirator or net, and transferred to the Lab of Arthropod Ecology and Trophic Interactions at Penn State for identification, pinning and kept as vouchers. All individuals collected will be identified to the functional group, family level and where is possible to genus or species level using taxonomic keys. In addition to visual sampling, yellow sticky traps and pitfall traps will be used to sample aerial and ground arthropods, respectively. Pitfalls and sticky traps will be changed out weekly. Plant height and phenology at each farm will be recorded, and plant tissue will be reserved from candidate individuals at the beginning (vegetative state), middle (flowering) and end of season (post-flowering) for plant metabolite analysis. Landscape and habitat context data (habitat type, acreage of farm, age of farm, other crops adjacent, evidence of insect damage on plants, weather conditions) will also be collected for each of the sites.
I will explore and visualize similarities or dissimilarities of the community composition between the three hemp cultivars, crop stages and locations using PCoA (Principle Coordinates Analysis) and will statistically assess the differences in community composition using PERMANOVA for both pests and biological control agents. This will allow us to determine if one cultivar harbors more or fewer pests or beneficial biological control agents than others, if plant phenology influences insect community composition, and if insect community composition is consistent over geographic space.
In addition, this observational survey will result in a table that consists of all the pests and beneficial biological control agents in Pennsylvania industrial hemp, the seasonality of herbivore pests throughout the crop cycle and the description of pest damage, which I will turn into an extension bulletin for growers in our state.
2. Evaluate the impact of insect pests on plant defensive metabolites and yield in three industrial hemp cultivars
Understanding how insect pests alter plant defenses in industrial hemp is crucial to ensure minimal crop losses and expanding our knowledge of insect-plant interactions in this new farming system. Direct consumption of leaf material and floral buds can directly affect yield by diminishing plant resources and eliminating marketable portions of the plant. Insect herbivory is also known to affect a myriad of plant traits, including plant metabolites. While these changes typically have no true influence in crop production, modification of plant metabolites in industrial hemp could be devastating for farmers. This is because industrial hemp is federally regulated and one particular metabolite, Tetrahydrocannabinol (THC), must remain under the threshold of 0.03%. If during the required testing a farmer’s crop is found above the 0.03% threshold, they must destroy the crop in its entirety – leading to a complete loss of revenue. Recent work suggests that herbivory by the corn earworm, Helicoverpa zea, can increase levels of THC above legal limits in some varieties of hemp grown for CBD (Jackson et al. 2021), highlighting the importance of understanding these dynamics across commonly grown hemp cultivars (Jackson et al. 2021). This early work suggests that hemp cannabinoids may act as defensive compounds and it is suspected that other herbivorous pest can similarly alter the levels of these important compounds. Further research will be needed to understand the potential impact of the different insect pest and natural enemies present in Pennsylvania hemp varieties.
Here I hypothesize that pest damage will induce plant defenses in industrial hemp that will change the composition and concentration of plant metabolite compounds, and that the impact of herbivory on plant defense may differ based on the plants phenological stage (vegetative, flowering, post-flowering).
To evaluate these hypotheses, I will measure chlorophyll content as proxy for plant health and yield and cannabinoids to understand the influence of herbivory on plant metabolites such as THC. Experiments will be done on the three aforementioned hemp cultivars which will be exposed to pest insects (infested treatment) or pest-free (non-infested treatment) during the various phenological stages (vegetative, flowering and post-flowering). I will focus on two major pests of industrial hemp that are projected to be problematic in this system: the cannabis aphid (Phorodon cannabis) and the corn earworm (Helicoverpa zea) (Lagos-Kutz et al. 2018; Ajayi and Samuel-Foo 2021). A colony of P. cannabis was established in the Arthropod Ecology and Trophic Interactions Lab in 2021 and a colony of H. zea will be field collected or purchased commercially and established in the laboratory.
The three commercial varieties of hemp will be grown in a walk-in growth chamber (24C, 18:4 L:D photoperiod) for three weeks before running the experiment. The infested treatment will receive 5 pest individuals, either P.cannabis aphids or H. zea larvae, inoculated at either the vegetative stage, flowering stage or the post-flowering stage, whereas the non-infested controls will remain pest-free. Vegetative infestation will occur three weeks after planting, growth (n=20), flowering infestation will occur once flower buds appear (n=20), and post-flowering infestation will occur once flowers senesce, and seed set begins (n=20). At each phenological stage, I will also have 20 non-infested control plants (N=120 experimental plants / pest species / cultivar).
Photosynthesis measurements will be taken with a fluorometer three days post-infestation on the uppermost extended leaves to assess chlorophyll content of the plant when experiencing herbivory by insect pests. On the same day, the composition and concentration of cannabinoid compounds, including Cannabidiol (CBD), Cannabiniol (CBN) and THC, will be evaluated using High Performance Liquid Chromatography (HPLC) in the Center for Chemical Ecology Core Facility at Penn State. Sample leaves will be taken from each plant, 3-days post infestation, from the uppermost developed leaves. For the extraction of Cannabinoids, leaf tissue will be flash frozen with liquid nitrogen and stored at -80 °C until further processed. Processing will begin with leaf tissue ground using a Geno/Grinder 2000 (SPEX Sample Prep, Metuchen, NJ) with 6mm stainless steel balls under cryogenic conditions. A 100 mg aliquot of this ground tissue will then be transferred to a 2 mL microcentrifuge tube containing 0.6 g of 2 mm zirconia beads and will receive 1.5 mL of extraction solvent consisting of ethanol with 2 mg/mL 4-biphenyl carboxylic acid (BPCA) as an internal standard. Samples will then be homogenized for 6 minutes using a FastPrep homogenizer (MP Biomedicals) and then centrifuged for 5 min at 10,000 rpm. An aliquot of the supernatant will then be removed and filtered through a 0.2 µm syringe filter for analysis by HPLC.
HPLC Analysis of Cannabinoids: Cannabinoids present in the leaf extracts will be separated using 5 µL injections onto a Shimadzu Prominence HPLC system fitted with a reverse-phase Restek Raptor ARC-18 column (150 mm x 4.6 mm x 2.7 µm) maintained at 40 °C with a flow rate of 1.5 mL-min. Mobile phase A will consist of HPLC-grade water with 5 mM ammonium formate and 0.1% formic acid; mobile phase B will be HPLC-grade acetonitrile with 0.1% formic acid. An isocratic hold for 7 min at 77% B will be followed by a linear gradient to 95% B over 2 min, a 1 min hold at 95% B, and then a 4 min re-equilibration at 77% B. Full spectra will be recorded from 190-400 nm by UV-DAD, with quantification at 228 nm using relative responses to the internal standard and external calibration to neutral and carboxylate cannabinoid standards of cannabidvarin (CBDV and CBDVA), cannabidiol (CBD and CBDA), cannabigerol (CBG and CBGA), tetrahydrocannabivarin (THCV and THCVA), cannabichromene (CBC and CBCA), as well as cannabinol (CBN), ∆9-tetrahydrocannabinol (∆9-THC), ∆8-tetrahydrocannabinol (∆8-THC), tetrahydrocannabinolic acid A (THCA-A), Cerilliant (Round Rock, TX)
The concentration of chlorophyll and the main cannabinoids (THC, CBN, CBD) will be assessed as a function of treatment, pest species, and cultivar using generalized linear models. The results will illustrate the potential impact of two pest species on plant defensive chemistry at different plant stages. Importantly, this study will provide insight into whether herbivory by these herbivorous pests could render the crop unmarketable by increasing THC levels.
3. Evaluate the efficacy of biological control agents on pest management
Functional response tests will be performed on colony-reared P. cannabis and H. zea and generalist predators to explore the efficiency of these predators as density-dependent mortality factors. For each pest, an appropriate predator was chosen based on ubiquity across the Pennsylvania landscape and the propensity to consume the prey. The lady beetle, Harmonia axyridis, a generalist predator well known for its capacity to feed on several species of aphids will be used in trials with P. cannabis. Podisus maculiventris, a generalist stink bug predator, will be used in experiments with H. zea as it is commonly found feeding on lepidopteran larvae. Colonies of both of H. axyridis and P. maculiventris have been established in the Arthropod Ecology and Trophic Interactions lab since 2019, adding wild-caught individuals annually, and will be used in the experiments.
To evaluate the predation potential of each of these predators, four life stages of H. axyridis, first instar, second instar, third instar, adult female and male will be evaluated by exposing them to different number of prey aphids (10, 20, 40, 80, 100). Three larval stages (2nd, 3rd, 4th) as well as the adult stage of P. maculiventris will exposed to H. zea larvae at various densities (1, 3, 5, 7, 10). Predators will be isolated in 16 oz deli cups and starved for 24 h prior to experiments. Experiments will begin at the same time every day, beginning once a predator is introduced into the experimental arena. The experimental arena will consist of fresh hemp leaves, placed on moistened filter paper in petri dishes and infested with different number of P. cannabis or H. zea. Predators will be removed from arenas after 24 h with no prey replacement occurring during the experiment.
The experiment will be replicated 10 times in a randomized complete block design with ten blocks representing time and five treatments. A one-way analysis of variance (ANOVA) will be used to compare differences in the mean attack rate (prey remaining after 24 hours) at each prey density. A t-test will then be used to compare average predation rates between sexes and instars of each predator. Consumer-resource interactions (i.e. the functional response) will be evaluated in R Core Team (2020) where selecting, fitting and comparing functional response models will be done using the package “FRAIR”.
Works Cited:
- Ajayi OS, Samuel-Foo M (2021) Hemp Pest Spectrum and Potential Relationship between Helicoverpa zea Infestation and Hemp Production in the United States in the Face of Climate Change. Insects 12:940. https://doi.org/10.3390/insects12100940
- Jackson B, Gilbert L, Tolosa T, et al (2021) The Impact of Insect Herbivory in the Level of Cannabinoids in CBD Hemp Varieties. In Review
- Lagos-Kutz D, Potter B, DiFonzo C, et al (2018) Two Aphid Species, Phorodon cannabis and Rhopalosiphum rufiabdominale, Identified as Potential Pests on Industrial Hemp, Cannabis sativa L., in the US Midwest. Crop Forage Turfgrass Manag 4:180032. https://doi.org/10.2134/cftm2018.04.0032
-
Insect pests and biological control agents in Pennsylvania hemp crops throughout the growing season.
Description of Hemp Fields
To ensure representative sampling across various ecological conditions during the insect survey in the 2022 growing season, we selected seven hemp fields across six counties in Central and Southeastern Pennsylvania. Four farms grew hemp for fiber/grain; the other three were planted with CBD hemp. According to the farmer's practices, the hemp fields had single or mixed varieties within the same plot. The number of samplings at each farm varied based on harvest time, with a more extended sampling period for CDB fields (See Table 1). Every week from June until October, we visited the fields to observe insect damage signs on the plants and record, count, and collect the insects directly interacting with these crops. We also installed yellow sticky traps to give a better sense of the insects flying between crop plants over more extended periods of time.
Table 1. Hemp farms surveyed for insect communities in Central and Southeastern Pennsylvania during the 2022 growing season.
|
Site |
Location |
County |
Hemp type |
Variety |
Number of samplings |
|
1. Shaffer Farm |
Central PA |
Clearfield |
CBD |
Stormy Daniels |
11 |
|
2. Lazy Moon |
Central PA |
Blair |
CBD CBG |
Mix Hawaiian Haze and White |
14 |
|
3. Russell E. Larson Agricultural Research Center (Rock Springs/ CBD) |
Central PA |
Center |
CBD |
Mix of Varieties |
10 |
|
4. Russell E. Larson Agricultural Research Center (Rock Springs fiber) |
Central PA |
Center |
Fiber/grain |
Sour Kush, OG |
9 |
|
5. Coexist |
Southeast PA |
Berks |
Fiber |
Bialobrzeskie |
7 |
|
6. Cedar Meadow Farm |
Southeast PA |
York |
Fiber/grain |
Henola, Yuma |
10 |
|
7. Southeast Agricultural Research and Extension Center (SEAREC) |
Southeast PA |
Lancaster |
Fiber |
Bialobrzeskie |
9 |
The hemp fields presented diverse crop management practices across cultivars. Most were established in agricultural lands, often surrounded by corn crops, with a few located near urban zones. Fiber and grain fields were planted with high densities either using no-till or conventional tilling methods. In contrast, CBD plots were planted with lower densities in smaller areas using black plastic mulch beds, fabric mulch, or natural soil covers for weed control. Harvest timing and production cycle also differed. CBD generally had a more extended growing period than fiber hemp (Figure 1).
In addition to our project funded by the Northeast SARE program, we secured additional funding from the Pennsylvania Department of Agriculture to enhance the scope of our research across the state. This allowed us to conduct a second year of insect sampling throughout the 2023 growing season in the central and southeastern counties. To explore variations in the insect community composition among cultivars, locations, and hemp types, we included twenty individual variety plots established within two experimental and three commercial farms.
We selected the same six fiber/grain varieties for surveying at each experimental farm, resulting in 12 sampling sites. In the commercial farms, six plots were established with different CBD cultivars, while two of the plots cultivated fiber varieties (See Table 2). We conducted vacuum sampling in each site, yellow sticky and pitfall traps. Due to the increased number of samples, the identification process is still ongoing; however, the results of this second sampling year will be shared through Penn State Extension in field days, conferences, and a scientific publication.
Table 2. Hemp farms surveyed for insect communities in Central and Southeastern Pennsylvania during the 2023 growing season.
|
Site |
Location |
County |
Hemp type |
Variety |
Number of samplings |
|
1. Rhodes Farm |
Central PA |
Clinton |
CBD |
Merlot |
7 |
|
Box |
7 |
||||
|
Trinity |
7 |
||||
|
2. Russell E. Larson Agricultural Research Center (Rock Springs) |
Central PA |
Center |
Fiber |
Bialobrzeskie |
5 |
|
Carmenecta |
5 |
||||
|
Fedora 17 |
5 |
||||
|
Futura 83 |
5 |
||||
|
Henola |
5 |
||||
|
Orion 33 |
5 |
||||
|
3. Southeast Agricultural Research and Extension Center (SEAREC) |
Southeast PA |
Lancaster |
Fiber |
Bialobrzeskie |
5 |
|
Carmenecta |
5 |
||||
|
Fedora 17 |
5 |
||||
|
Futura 83 |
5 |
||||
|
Henola |
5 |
||||
|
Orion 33 |
5 |
||||
|
4. Cedar Meadow Farm |
Southeast PA |
York |
Fiber |
Yuma S |
5 |
|
CBD |
Green Cherry |
7 |
|||
|
Hawaiian Haze |
7 |
||||
|
Fifth Element |
7 |
||||
|
5. Coexist |
Southeast PA |
Berks |
Fiber |
Bialobrzeskie |
5 |
Insect Survey 2022: Visual Sampling and Yellow Sticky Traps
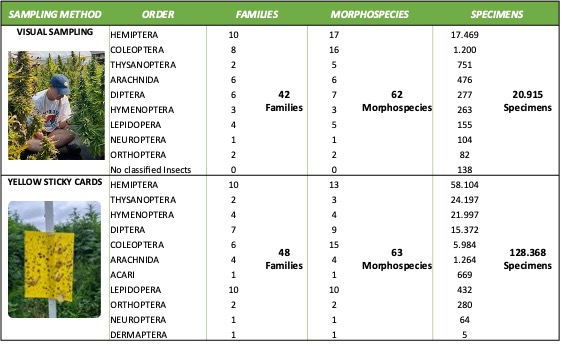
During the 2022 growing season, we used two sampling methods to increase our knowledge of the insect communities in hemp crops in the Northeast region. Visual sampling allowed us to detect insects directly interacting with the hemp plants and observe their populations, behaviors, damage, and impacts on the crop. We recorded and identified 20,915 arthropods, including insects and arachnids (Table 3). Several weaknesses were noted using visual sampling. This method may not capture all insect species, particularly small parasitoid wasps and leafhoppers that can be easily disturbed and may evade observation while approaching and manipulating the plants. In addition, This method can be time-consuming, making it less practical and more costly to sample across a larger number of sample sites (hemp farms.
The yellow sticky traps allowed us to monitor a broad range of flying insects for longer periods during sampling and to complement the visual sampling, addressing some of these potential biases. These traps captured significantly more specimens than visual sampling (128,368 specimens). Both methods captured a similar number of families and morphospecies. Hemiptera and Thysanoptera were highly detected in both types of sampling; however, visual sampling was more effective in detecting spiders, while sticky cards were more successful in capturing Hymenopterans and Dipterans (Table 3).
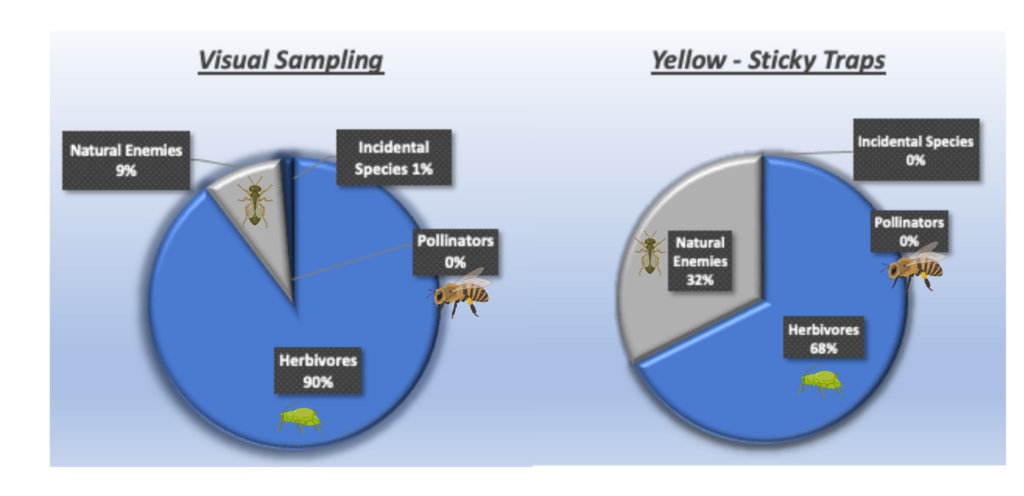
When categorized by functional groups, herbivorous insects were the most predominant group observed in yellow sticky traps and visual sampling, representing between 67.6% and 89.8% of the collected specimens, respectively. Natural enemies were the second most abundant insect group, better represented on the sticky cards than in visual sampling. Incidental species—not directly related to the crop—accounted for 1%, and pollinators contributed less than 1% (Figure 2).
Table 3. Summary of specimens captured in hemp crops using visual sampling and yellow-sticky cards during the 2022 insect survey.
Differences in insect community composition in hemp crops
Exploring the differences between the insect communities found in Pennsylvania hemp crops can help us understand how herbivores and natural enemies vary across different hemp types, varieties, locations, and other factors that might influence their populations within the crops. To assess these differences, we first visualized how distant these insect communities are between samples using an NMDS plot (Non-Metric Dimensional Scaling). Then, we assessed whether these differences were statistically significant using a permutational multivariate analysis of variance (PERMANOVA).
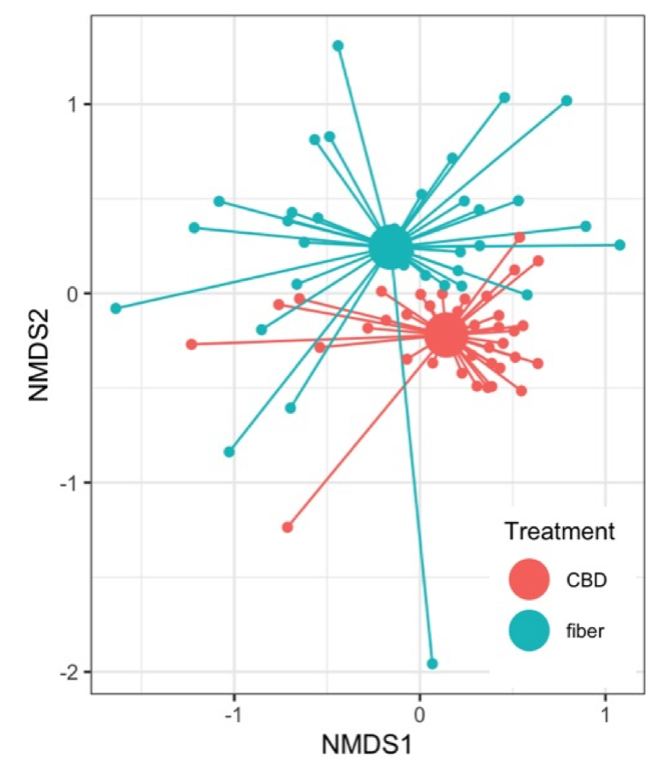
Figure 3 shows the community composition in CBD and fiber crops. Each point in the graph represents the insect communities sampled in CBD (teal points) and fiber crops (red points). The proximity of the points indicates how similar the communities are. The samples for CBD and fiber form separate and distant clusters, indicating distinct differences in the insect communities between the two types of hemp. The PERMANOVA analysis confirmed that the composition of insect species significantly varied between CBD and fiber crops (F = 8.5261, p = 0.001). This can be explained by the differences in plant structure, cultivation practices, and chemical composition between these cultivars. Additionally, the fiber samples appear more dispersed from the central point than those in CBD, indicating greater variability in the insect communities within the fiber crops.
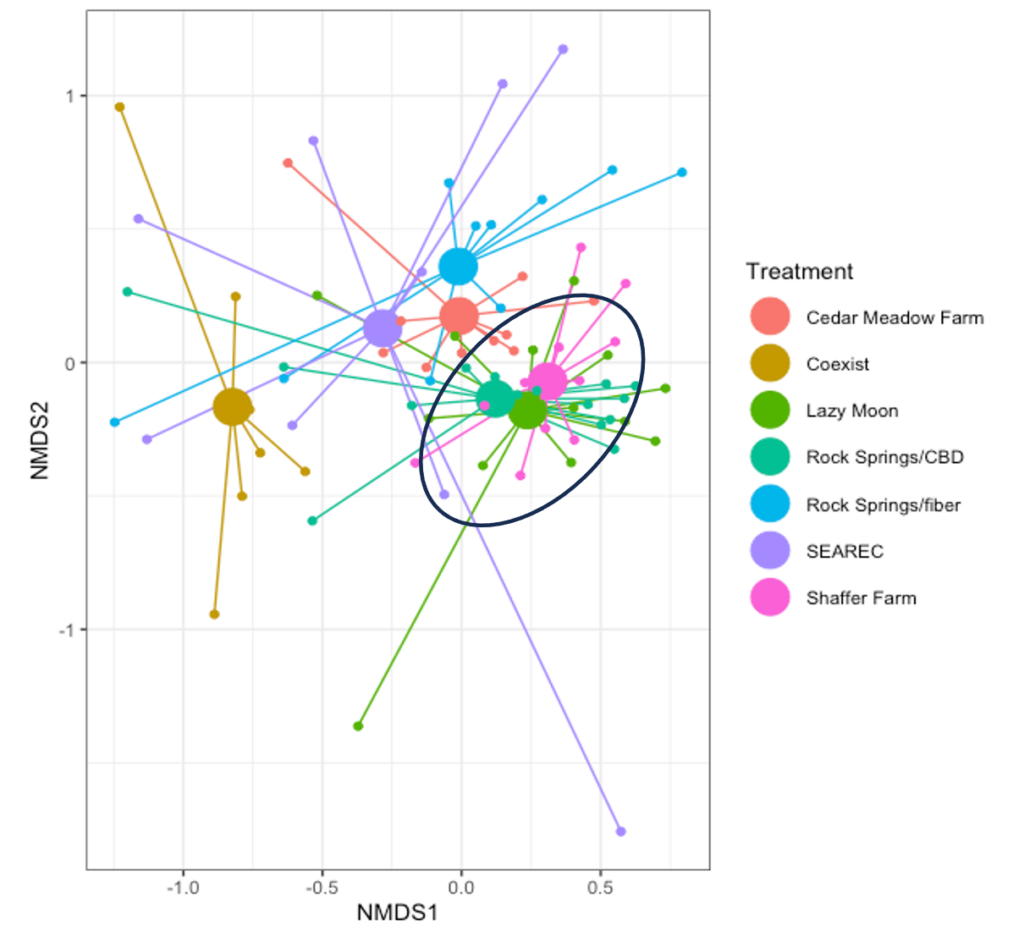
From this, we visualized the insect communities across hemp farms. While each farm seems to have distinct communities, these overlap in CBD farms. Interestingly, the arthropod communities across our fiber plots did not overlap. The PERMANOVA analysis confirmed that insect communities are significantly different between hemp farms (F = 5.6232, p = 0.001), indicating that aside from the type of hemp, there are factors specific to the sample sites that are shaping the composition of the insect community, especially in the fiber farms (Figure 4).
Key differences in our fiber farms include the landscape type—urban versus agricultural—the surrounding vegetation, prior crops, and practices such as tillage or non-tillage, mixed varieties, and varying plant densities. The upcoming analysis will delve deeper into these communities, focusing on their distribution across sampling sites, types of hemp, occurrence over time, and diversity.
Prevalent Insect Pests Interacting with Hemp Crops in Pennsylvania
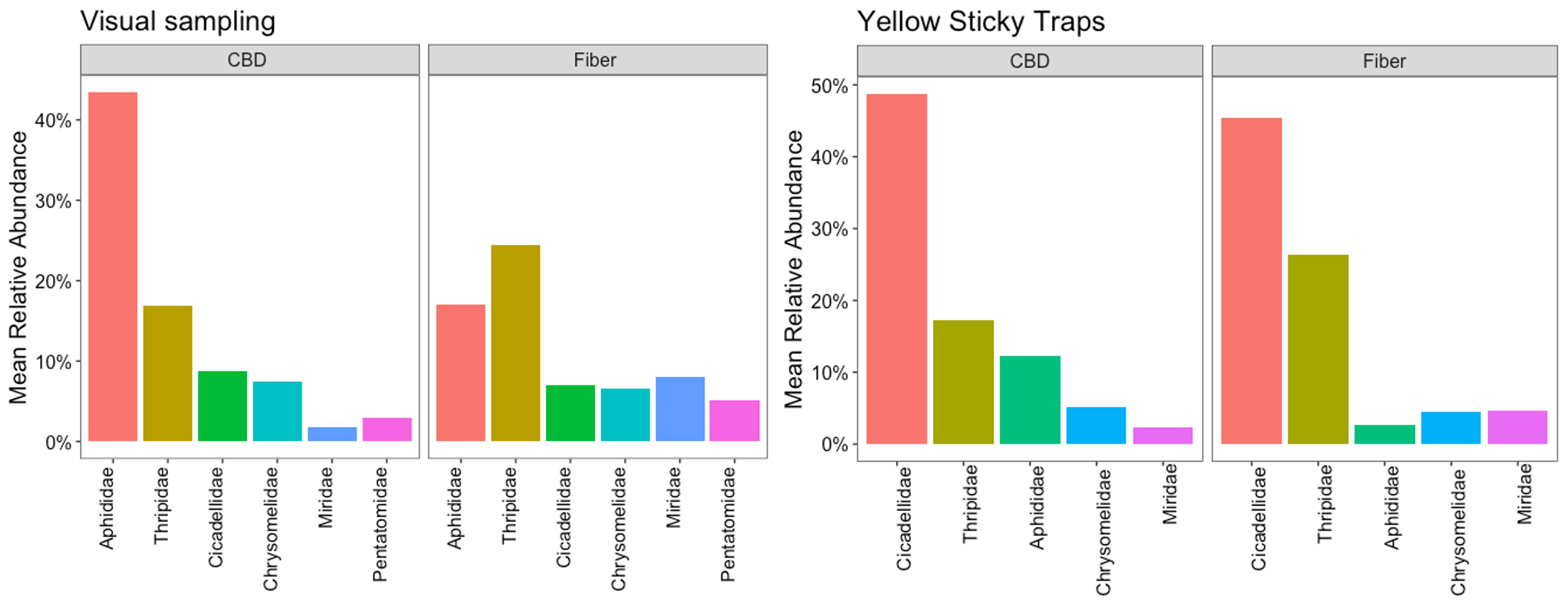
The most prevalent insect pests interacting with hemp crops in Pennsylvania were determined based on the herbivore species with the highest relative abundance. Relative abundance measures the proportion of each herbivore species to the total number of individuals from all species collected during the insect samplings. To better understand the ecological roles of herbivore species with similar niches and functions, we grouped the specimens by families, calculated the relative abundance of herbivore families for each sample (plant or sticky card), and estimated the mean relative abundance in each sampling event, farm, hemp type, and crop phenological stage to observe patterns.
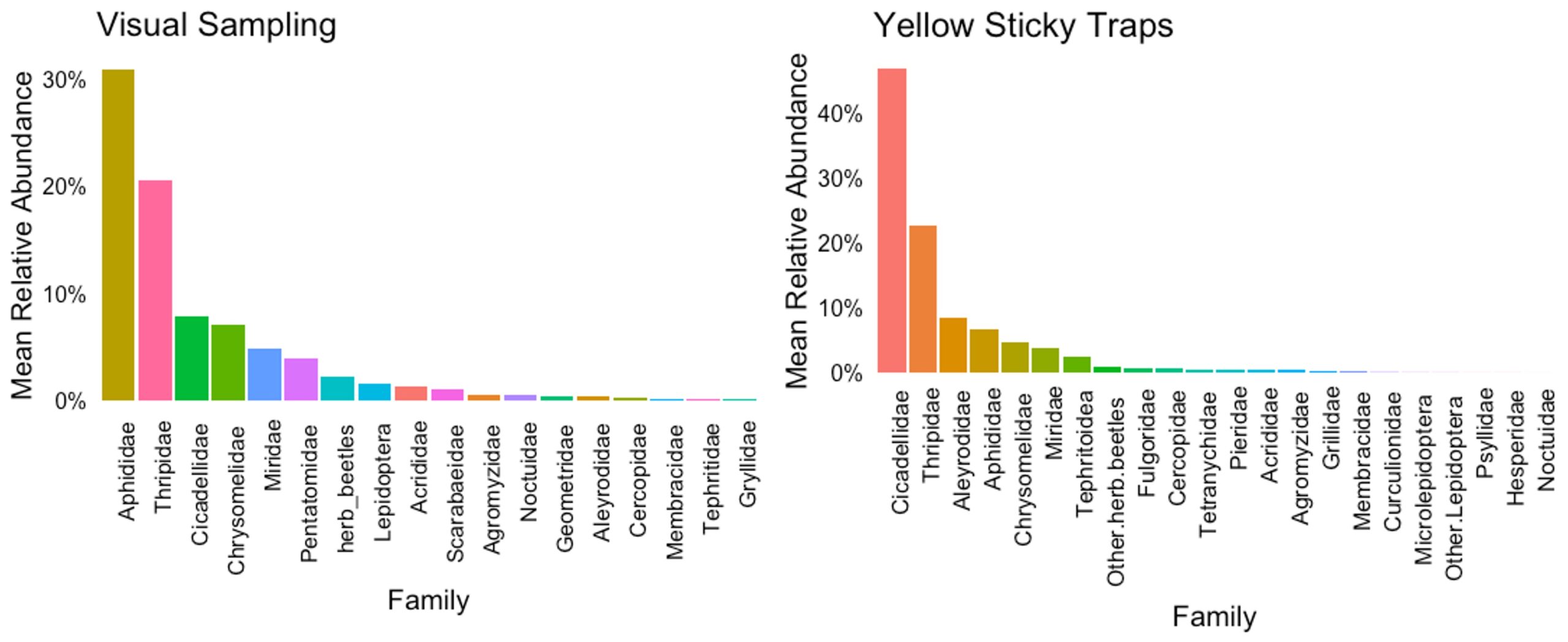
Based on the visual samplings, aphids (Aphididae) and thrips (Thripidae) are the insect families with the highest mean relative abundance; together, their abundance represents 50% of the herbivores feeding on hemp. Leaf beetles (Chrysomelidae), leafhoppers (Cicadellidae), stink bugs (Pentatomidae), and plant bugs (Miridae) were also abundant but less prevalent than aphids and thrips (Figure 5). In the sticky traps, leafhoppers (Cicadellidae) were the most abundant herbivore group, representing 40%. These insects are more likely to be detected in sticky traps than in visual samplings due to their distinct jumping behavior to evade predators and move between plants. Thrips were the second most prevalent pests, with a relative abundance of 25%, consistent with the visual observations, followed by whiteflies (Aleyrodidae), leaf beetles (Chrysomelidae), and plant bugs (Miridae). The aphid population was still significant but less abundant in this sampling method. The aphid population in sticky traps was represented mainly by alate aphids (Figure 5).
Herbivore Families in Hemp Crops
The composition of these prevalent insect pests varied across hemp farms and sampling methods. In the visual samplings, aphids (Aphididae), thrips (Thripidae), leaf beetles (Chrysomelidae), and leafhoppers (Cicadellidae) were widely distributed across all hemp farms, while stink bugs and plant bugs were found in 86% of the sampling sites. Their relative abundance showed different patterns. In Lazy Moon, Rock Springs/CBD, Shaffer, and Cedar Meadow farms, the insect pest communities were dominated by aphids, followed by thrips and leaf beetles. Meanwhile, in Coexist, Rock Springs/fiber, and SEAREC, the populations of insect pests were more evenly distributed, with thrips being slightly more abundant. On the other hand, the yellow sticky traps confirmed that leafhopper and thrips populations play an important role in the composition of insect pests across hemp farms (Figure 6).
Prevalent Insect Pest in Hemp Farms
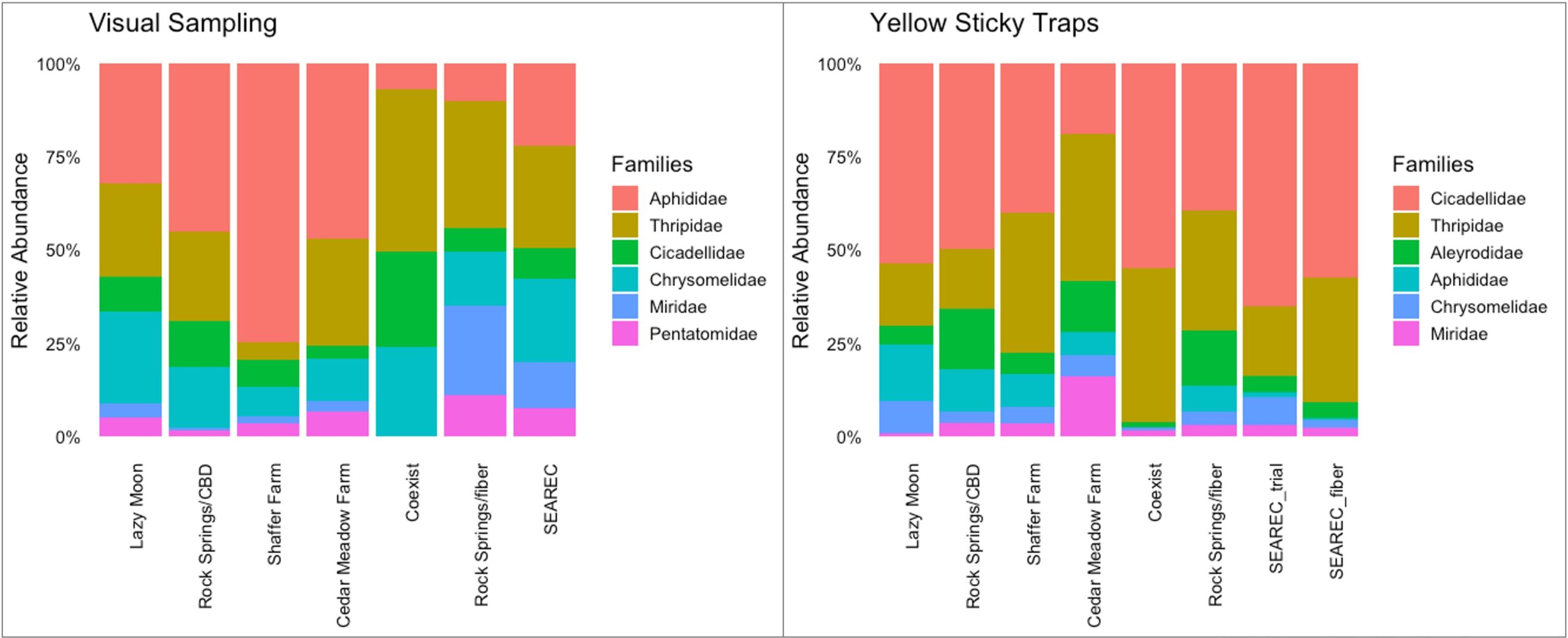
The group of prevalent insect pests was the same in CBD and fiber crops; however, their relative abundance had different degrees of relevance for each type of hemp during the 2022 growing season. In the visual samplings, aphid populations were considerably more important for CBD hemp than they were for fiber, where aphids held second place. Thrips were more relevant for fiber crops than they were for CBD hemp. Leafhoppers and leaf beetles had the same level of representation for both types of hemp, while plant bugs and stink bugs were slightly more relevant for fiber crops. Interestingly, the sticky traps showed consistency regarding the importance of these herbivores for CBD and fiber (Figure 7). This indicates that the interactions of these herbivores with the crop will have a different impact on CBD and fiber, which has implications for pest management and helps to refine pest monitoring priorities for both crop systems.
Prevalent Insect Pest in CBD and Fiber Hemp
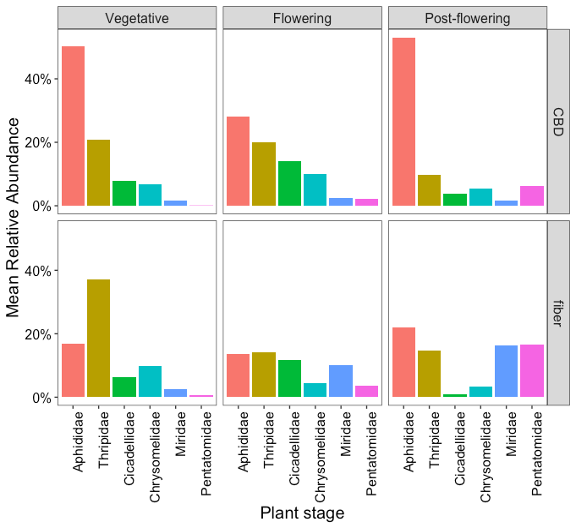
Dynamics of Common Pests Throughout the Crop Cycle in CBD and Fiber Hemp.
Recognizing the differences in pest insect communities in hemp helped us identify the dominant herbivores in each type of hemp. Not every prevalent pest performs or interacts with CBD and fiber plants in the same way, resulting in different degrees of pest dominance and varying impacts of herbivory. To understand the extent of the risks that these prevalent pests pose to each type of hemp and to plan accordingly for the measures to mitigate their impact, the next step was to determine when in the lifecycle of the crops these prevalent insects occur, dominate, or increase their populations. Figure 8 shows the mean relative abundance of each prevalent pest for CBD and fiber crops at each phenological stage. This information will be used to inform pest management practices, targeting those herbivores that have the potential to be more problematic.
- Aphids (Hemiptera: Aphididae)

During the visual sampling conducted in 2022, the cannabis aphid (P. cannabis) (Figure 9) emerged as the most prevalent pest affecting hemp crops in Pennsylvania. This exotic pest specializes in plant species within the hemp family (Cannabaceae) and was first documented as a pest of hemp in the United States in 2016. Initial reports indicated its presence in hemp grain cultivars in Colorado and indoor cultivation facilities in Virginia (Cranshaw et al., 2018). Since then, the cannabis aphid has disseminated across several states where hemp is cultivated.
In Pennsylvania, P. cannabis was detected in both CBD and fiber hemp crops and was consistently present at our sampling sites across central and southeastern counties (Figure 6). The aphid populations were significant, with their presence first observed during the vegetative phase of the crops. However, in CBD hemp, the highest populations occurred throughout the post-flowering stage, persisting until harvest (Figure 8). Aphid infestations were notably more abundant in CBD hemp compared to fiber hemp (Figure 7). Typically, the production cycle for CBD is longer than that for fiber, resulting in an extended period of vulnerability for CBD plants, where aphid infestations can be problematic. This highlights the importance of understanding their impact on crop yield and plant metabolites, such as THC. These sap-sucking insects can cause direct damage to plants by extracting nutrients and indirect damage, such as the production of honeydew, which promotes the growth of sooty mold and the transmission of plant viruses, including cucumber mosaic virus (CMV) and alfalfa mosaic virus. (AMV).
- Herbivore Thrips (Thysanoptera: Thripidae)

Three generalist herbivore thrips species were frequently observed in hemp crops: onion thrips (Thrips tabaci), western flower thrips (Frankliniella occidentalis), and soybean thrips (Neohydratothrips variabilis). These rasping-sucking insects consume the cellular contents of leaves, resulting in punctures and scrapes that can diminish the foliage's appearance and increase the plants' susceptibility to pathogens (Gill et al. 2015) (Figure. 10). The presence of thrips in hemp is of particular concern due to their ability to feed on a wide range of host plants, survive through winter in soil and plant debris, and attack hemp crops since the establishment. Despite a significant abundance of thrips in most of the farms where we conducted the insect samplings, we did not observe detrimental damages that could affect the quality of the crops; however, in our experience growing hemp indoors for experiments, we observed significant thrip damage, especially in smaller plants, resulting in reduced plant vigor and quality.
Thrips populations were notably prevalent during the vegetative stage of the crops, especially for fiber, and declined as they progressed toward the post-flowering stage (Figure 12). In relation to the type of hemp, thrips populations were more abundant in fiber hemp than in CBD (Figure 7).
- Leaf Beetles (Coleoptera: Chrysomelidae): Flea Beetles and Spotted Cucumber Beetles.

Various chrysomelid species were often observed in hemp crops, particularly several flea beetle genera, such as Altica sp., Systena sp., and Crepidodera sp., and spotted cucumber beetles (Diabrotica undecimpunctata) (Figure 11). These beetle populations were similarly prevalent in CBD and fiber hemp (Figure 7), reaching peak levels during the flowering periods (Figure 8).
Flea beetles are known to cause significant damage by feeding on leaves, creating characteristic small holes that could reduce the plants' photosynthetic capacity and stunt growth if infestations are severe. In addition to foliage damage, spotted cucumber beetles are known vectors of plant pathogens, such as bacterial wilt caused by Erwinia tracheiphila in plants from the family Cucurbitaceae and other essential crop families (Bragard et al. 2020). The presence of beetles could represent a potential threat; however, until now, this bacterial disease has not been reported in hemp. Furthermore, the effect of this group of chewing herbivores on plant metabolites such as THC and CBD has not yet been investigated.
- Leafhoppers (Hemiptera: Cicadellidae)

Leafhoppers were represented by diverse species that are common pests in other crops in the region, such as the potato leafhopper (Empoasca fabae), corn leafhopper (Dalbulus maidis), and the aster leafhopper (Macrosteles quadrilineatus), among others. Although no significant damage was observed in the crops, it’s important to note that many of these species have the potential to harm hemp by introducing pathogens or causing direct feeding damage, especially when conditions favor population growth (Figure 12). In Colorado and Arizona, there have been reports linking leafhoppers to the transmission of plant viruses in CBD and fiber hemp crops (Giladi et al. 2020; Hu et al. 2021; Stats et al. 2023).
- Stink Bugs (Hemiptera: Pentatomidae) and Plant Bugs (Hemiptera: Miridae)

Stink bugs and plant bugs, especially tarnished plant bugs were frequently found among the piercing-sucking insects in hemp crops (Figure 13). These pests were particularly prevalent in fiber hemp farms (Figure 7), with their populations becoming more pronounced during the flowering and post-flowering stages (Figure 8). This indicates that these growth stages are critical for monitoring and managing infestations. This seasonal increase aligns with their feeding preferences and the nature of the damage they inflict.
Stink and plant bugs have been reported feeding on flowers, seeds, and delicate plant tissues. This feeding can lead to challenges like seed deformation, bud abortions, and distorted flower structures. These issues might lower germination rates and alter the seeds' physical appearance for hemp growers focusing on seeds and grain. Similarly, such insects can diminish the crop's commercial value in flower production (Britt et al. 2019). While we’re learning more about the roles of stink bugs and plant bugs in hemp cultivation, there’s still much to discover about their long-term impact on yield and the levels at which they start to cause economic harm.
- Importance of Less Abundant Herbivores

Other species, like Japanese beetles (Coleoptera: Scarabaeidae), were less abundant, but when present, they were observed causing significant defoliation and skeletonization in fiber/grain cultivars (Figure 14), which could lead to reduced photosynthetic capacity and crop yield. They were also observed in mating aggregations on male flowers, suggesting that hemp could become a hospitable host for these damaging beetles. Japanese beetles can feed on various plants and are commonly present across the Eastern United States, making them a potential emerging pest for hemp cultivation.

The corn earworm (Lepidoptera: Noctuidae) is recognized as a significant pest of hemp crops, particularly for CBD and grain varieties, where larvae feed on flowers, buds, and developing seeds (Figure 15). While its abundance was low during our pest monitoring in 2022, and no significant impact was observed in the sampling sites, its presence became more prominent at the end of the 2023 growing cycle. It is critical to conduct studies to understand how the planting season, as well as the proximity of hemp fields to corn crops, could play a role in the impact of corn earworm infestations, especially in CBD varieties. Planting CBD crops late in the season, between July and October—beyond the corn harvesting period—may increase the risk of corn earworms migrating into hemp fields due to this spatial and temporal overlap.
Incidence and Severity of Insect Pest Damage on Hemp Crops
Insect damage in the hemp crops was categorized based on the feeding behavior of the different pests interacting with the plants. Chewing damage is caused by herbivores with strong mandibles, including caterpillars, inchworms, leaf beetles, and grasshoppers, primarily affecting leaves and flower buds. Scraping damage results from piercing-sucking insects, such as thrips, aphids, whiteflies, leafhoppers, plant bugs, and stink bugs, which extract fluids from the plant foliage, stems, and seeds. Boring damage is caused mainly by lepidopteran stem borers that tunnel into the stems, affecting the plant structure. Mining damage is often inflicted by dipteran and lepidopteran leaf miners who create tunnels inside the leaf tissue while completing their larval stage—lastly, bud rot is attributed to the corn earworm feeding and excrement within flower buds (Figure 16).

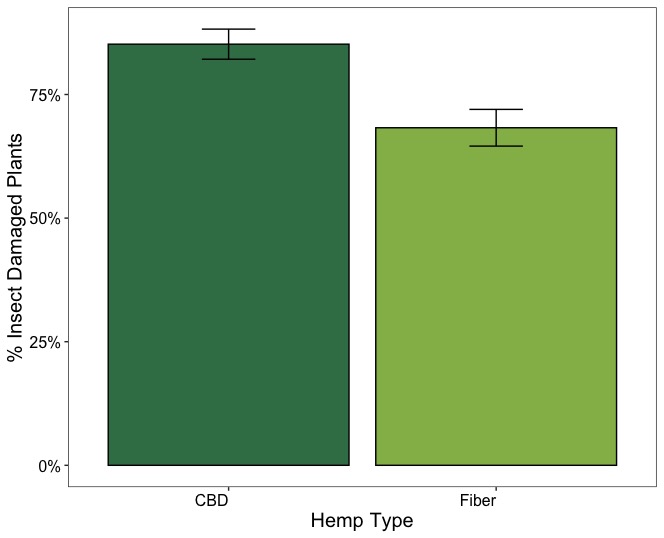
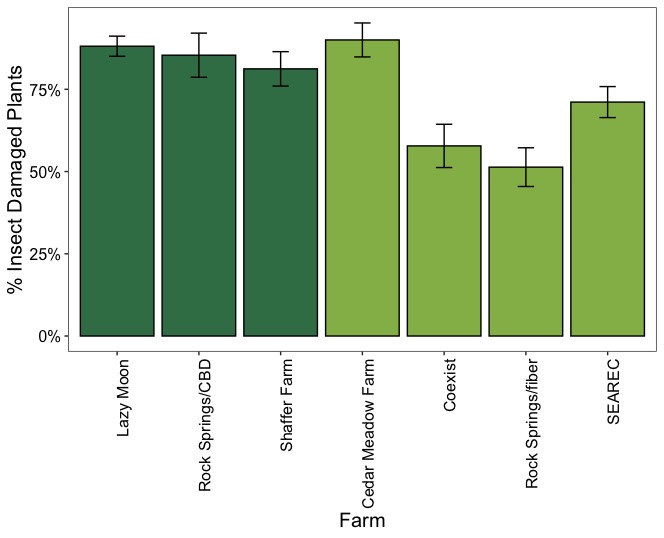
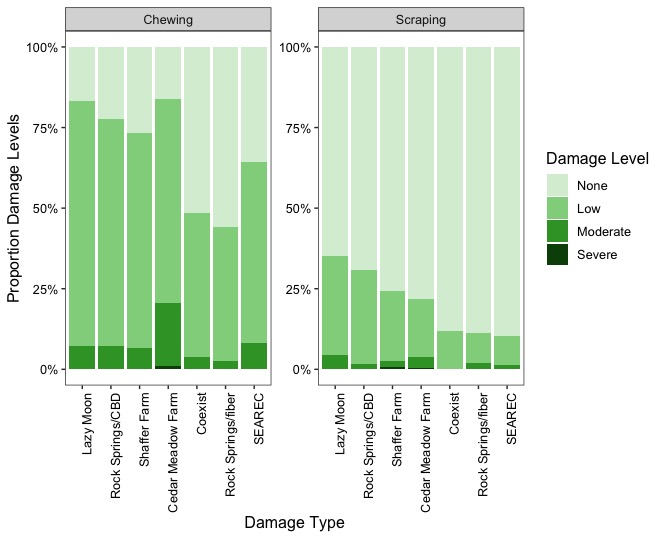
Figure 17 illustrates the overall proportion of plants with insect damage across CBD and fiber hemp types. In CBD hemp, the incidence of insect damage was higher, with approximately 85% of plants affected, whereas in fiber hemp, the proportion was lower, at around 68%. Variations in the proportion of insect-damaged plants were also observed across hemp farms. Regardless of the severity of insect damage and its impact on the crop, in CBD farms (Lazy Moon, Rocksprings, and Shaffer Farm), insect feeding signs were consistently high, with over 81 % of herbivory incidence on the plants. In contrast, the proportion of plants with insect feeding signs was lower in fiber hemp farms, except at Cedar Meadow farm, which had a similar damage incidence to the CBD farms (Figure 18).
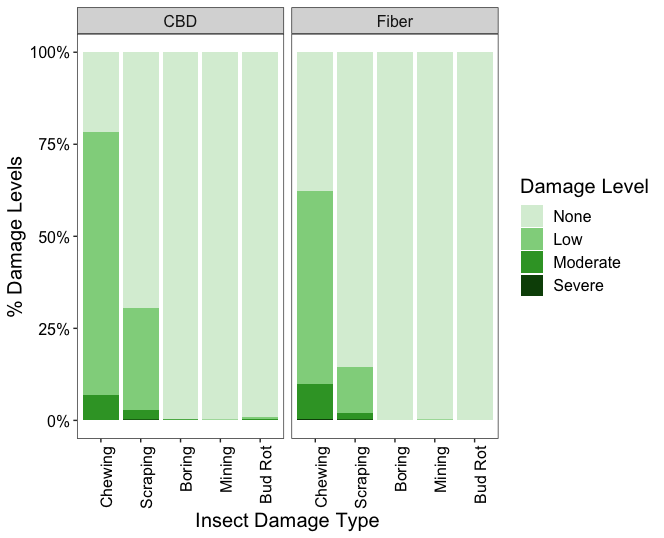
Figure 19 shows the proportion of plants at each damage level (0= none, 1= low, 2= moderate, and 3= severe) for each type of damage (chewing, scraping, boring, mining, and bud rot) in CBD and fiber hemp crops. The damage caused by chewing insects was the most prevalent among both hemp types, followed by piercing-sucking insects. Approximately 78% of CBD plants showed chewing damage, with most of this damage rated at low levels. Similarly, about 64% of the fiber plants exhibited chewing damage, mainly concentrated at low levels. Scraping damage was less frequent, occurring in 30% of CBD plants and 15% of fiber plants, predominantly at low levels. Boring, mining, and bud rot damages were insignificant across CBD and fiber hemp crops. Examining the type of damage and severity across hemp farms, chewing and scraping damages were widespread, with a higher incidence on CBD farms. However, these damages occurred mainly at low severity in all sampling sites (Figure 20)
These results show that while CBD hemp experiences a higher incidence of insect damage than fiber, the severity remains predominantly low across all observed damage types. Chewing and piercing-sucking insects are the primary herbivores affecting hemp crops in Pennsylvania. Despite their widespread presence, the low severity of damage suggests that current pest pressure does not significantly impact crop yield. Therefore, intensive pest management interventions may not be necessary under current conditions. Continued monitoring is recommended to detect changes in pest damage incidence and severity.
Prevalent Natural Enemies in Pennsylvania Hemp Farms
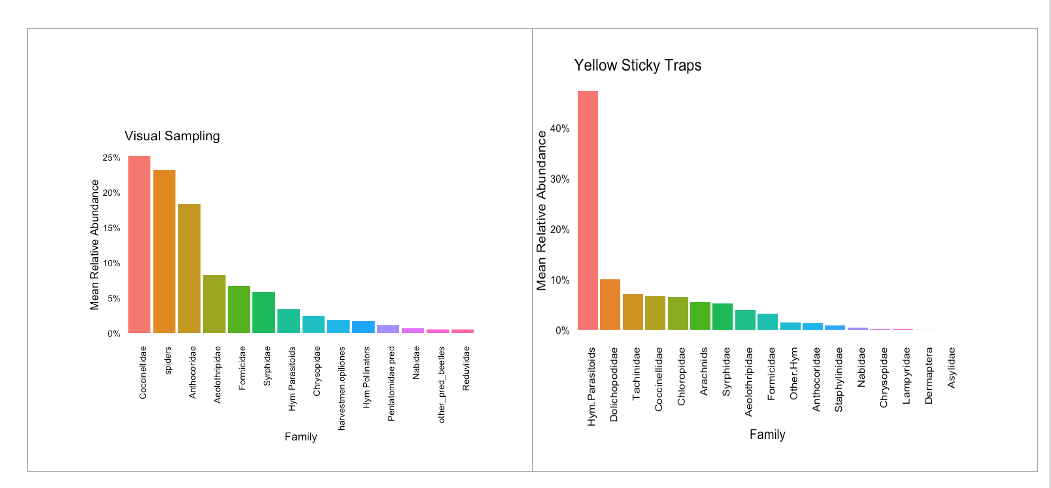
The visual sampling indicated that lady beetles (Coccinellidae), spiders (Arachnids), and minute pirate bugs (Anthocoridae) were the predominant groups of natural enemies foraging on hemp crops. Predatory thrips (Aelothripidae), ants (Formicidae), hoverflies (Syrphidae), and lacewings (Chrysopidae) were represented in moderate abundance. Additionally, several families of parasitic wasps (Hymenoptera) were observed; however, it’s possible that they are not fully represented in the visual sampling since some species are tiny and exhibit rapid flight, making their detention difficult (Figure 21).
The yellow sticky traps proved highly effective at capturing parasitoids belonging to a diverse array of hymenopteran families. This group accounted for 40% of the natural enemies collected using this method. Alongside lady beetles, arachnids, and hoverflies, frequently seen in visual sampling, the sticky traps also captured notable families such as Dolichopodidae, Tachinidae, and predatory frit flies (Chloropidae). However, these were less abundant than the parasitoid group (Figure 21).
Prevalent Natural Enemies in Hemp Crops
- Lady beetles (Coleoptera: Coccinellidae)

Lady beetles made up 25% of the predator observations, showcasing a rich diversity of native species, including the pink lady beetle (Coleomegilla maculata), the minute lady beetle (Diomus terminatus), the 14-spotted lady beetle (Propylea quatuordecimpunctata), and the seven-spotted lady beetle (Coccinella septempunctata), which were commonly observed. Additionally, the introduced multicolored Asian lady beetle (Harmonia axyridis) was also abundant in our samples (Figure 22); however, its populations did not dominate over native species such as the pink and minute lady beetles.
These coccinellid species serve as generalist predators, preying on several soft-bodied insect pests, such as aphids, scale insects, mealybug mites, tiny larvae, and insect eggs found on hemp. Some of them also consume pollen and nectar. Their presence in the crops suppresses populations of prevalent pests, such as cannabis aphids, highlighting the importance of preserving them in the hemp ecosystem to avoid detrimental pest effects.
- Spiders (Araneae)
Collectively, the spider families Thomisidae (crab spiders), Salticidae (jumping spiders), Araneidae (orb-weaver spiders), and Cheiracanthiidae (sac spiders) contribute an additional 24% to the total natural enemy population, emphasizing their vital ecological role in hemp crop systems. These spiders regulate pest populations and contribute to ecosystem stability through their diverse hunting strategies and habitats. As generalist predators, they prey on a wide range of arthropods, including pests and occasionally beneficial insects; their impact is particularly significant against abundant pests.
- Minute Pirate bugs (Hemiptera: Athocoridae)

Minute pirate bugs (Orius sp.) (Figure 23) rank as the third most prevalent general predator detected in hemp crops, accounting for approximately 18% of beneficial control agents. These voracious insects are typically released in biological control programs in field and greenhouse environments to mitigate significant agricultural pests, including aphids, whiteflies, spider mites, and thrips. In hemp crops, we frequently observed them preying on thrips. They can also occasionally consume pollen in instances of prey scarcity.
- Ants (Formicidae), hoverflies (Syrphidae), and lacewings (Chrysopidae)
These predator families are essential components of the natural enemy community in hemp crops, playing a role as natural pest controllers and contributing to crop health and productivity through their ecological functions. Ants are generalist predators and scavengers, feeding on pests such as caterpillars, aphids, and insect eggs while disrupting pest colonies through their foraging behavior. Hoverflies, in their larval stage, are predators of aphids, scales, and thrips, while the adult flies also serve as pollinators. Lacewings are effective predators of small pests such as aphids, mites, and whiteflies.
- Parasitoid wasps (Hymenoptera: Ichneumonoidea, Chalcidoidea)
Parasitoid wasps exhibited considerable diversity in morphology and dimensions. The larger specimens were classified within the superfamily Ichneumonoidea, whereas most of the smaller examples were categorized under the superfamily Chalcidoidea. These parasitoid wasps are effective natural enemies used in biological control programs against significant agricultural pests such as caterpillars, aphids, whiteflies, mealybugs, scale insects, and beetles. We observed a high abundance of aphelinid specimens in sticky traps, a specialized group of endoparasites of aphids in the family of Aphelinidae.
The visual inspections of insects in Central Pennsylvania allowed us to discover an exotic wasp, Aphelinus maculatus Yanosh 1979 (Hymenoptera: Aphelinidae), parasitizing adults of the cannabis aphid P. cannabis in CBD hemp (Figure 24). There are very few records of A. maculatus worldwide; it was first described in Russia in 1979 (Yasnosh 1981) and most recently in China in 2016, attacking green peach aphids Myzus persicae (Chen and Li 2016). This is the first time that A. maculatus has been naturally found in Pennsylvania, attacking the cannabis aphid in cultivated CBD hemp. This increases the list of natural enemies to conduct biological control in hemp crops.

- Predatory flies (Diptera: Dollycopodidae, Tachinidae, Chloropidae)
Dolichopodidae, Tachinidae, and Chloropidae represent significant natural enemies in hemp ecosystems, often found on yellow sticky traps. Dolichopodidae, known as long-legged flies, help reduce populations of aphids, mites, and thrips, particularly during high-density outbreaks. Tachinidae, or tachinid flies, act as parasitoids that are vital in biologically controlling common pests such as caterpillars, beetles, and true bugs. Among the chloropids, Thaumatomyia sp. emerged as the predominant species, recognized as a specialized predator of root aphids during its larval stage.
The presence of these beneficial arthropods holds promising implications for biological control in this new crop system. They have the potential to mitigate the impact of detrimental herbivores. Moreover, their representation serves as valuable indicators of ecosystem health, fostering the adoption of sustainable farming practices.
Diversity index of insect pests and natural enemies in Hemp Crops
Understanding the diversity of the insects that interact with hemp crops serves as a measure of the stability and resilience of these agroecosystems to prevent pest outbreaks and autoregulate pest populations. The diversity index considers the number of species in a specific ecosystem (species richness) and how evenly the populations are distributed among those species (species evenness). Crops with high diversity support a broad array of natural predators that help maintain herbivore populations at levels that are not harmful to the crop, decreasing reliance on chemical pesticides. We can use this index in hemp crops to gain insights into crop management practices that can better support natural enemy communities, inform potential pest outbreak risks, evaluate the effectiveness of pest control interventions, and help identify when these interventions are needed.
The Shannon-Weiner Diversity Index was calculated for each functional group (herbivores and natural enemies) in CBD and fiber hemp. Despite the herbivores having a higher relative abundance among the functional groups, they were less diverse than natural enemies in both CBD and fiber crops, indicating that few families within the insect pests are dominating the herbivore communities. On the contrary, natural enemies were less abundant, but their communities were more diverse and more evenly distributed. Comparing the diversity of herbivore communities between hemp types, CBD had a lower index (1.813) than fiber (4.168), indicating a more uneven distribution of insect pest populations in CBD, dominated by cannabis aphids. Conversely, in the predator group, a greater diversity of species was observed in CBD hemp (10.07) compared to fiber hemp (6.247) (See Table 5).
Table 5. Total Shannon- Weiner Diversity Index for insect functional groups observed in CBD and fiber hemp during the 2022 visual survey in Pennsylvania.
|
Functional Group |
CBD |
Fiber |
|
Herbivores |
1.813 |
4.168 |
|
Predators |
10.07 |
6.247 |
To better understand how diversity changes as different species colonize and develop in plants at different times throughout the crop cycle, we calculated the Shannon-Weiner Diversity Index of herbivores and natural enemies for each sampling event.
In CDB crops, a variety of natural enemies have established rapidly since the early vegetative stage and consistently exhibited a more diverse community composition than the herbivore group throughout the entire growing season. This demonstrates the extraordinary capacity of CDB hemp crops to attract and sustain populations of diverse natural enemies, helping to mitigate pest pressure. Conversely, herbivore populations established more gradually during the vegetative stage, peaked in diversity during the flowering period, and then declined towards the end of the cycle, as cannabis aphids became the dominant insect species. A higher diversity of herbivores can be harmful when many damaging species affect the crop, or beneficial when balanced communities prevent any species from becoming overly dominant, allowing the crop to tolerate damage from various herbivores without hampering productivity (Figure 25a).
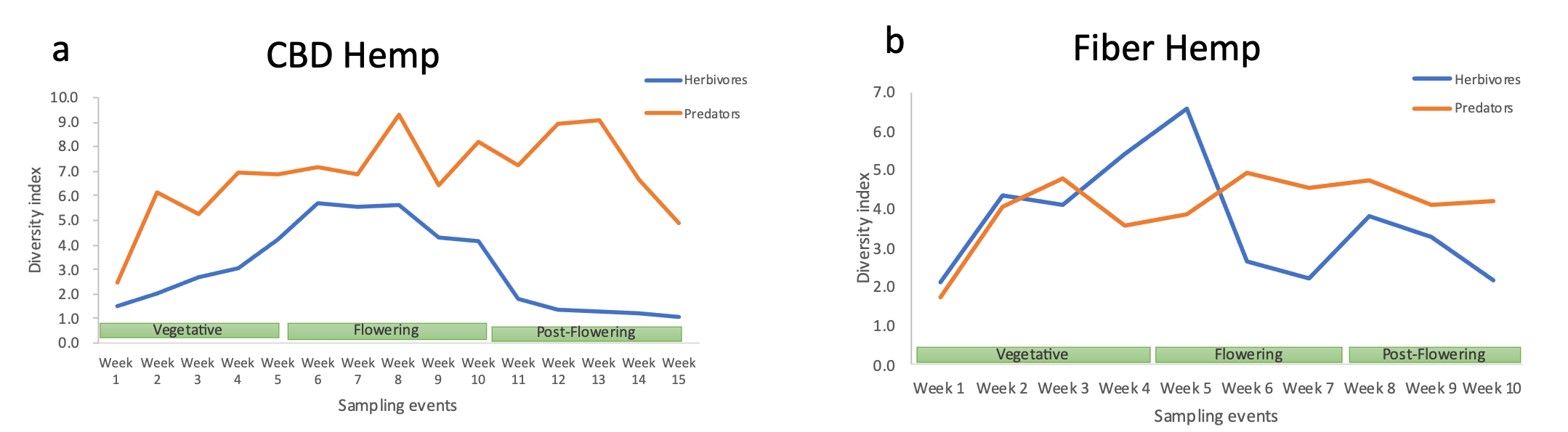
In fiber hemp crops, the diversity of pests and natural enemies increased at similar rates since the early vegetative stage; however, as the plants moved towards flowering, the natural enemy population decreased, while the diversity of herbivore insects rapidly surged. This indicates that lower populations of beneficial insects can lead to a higher number of pests and potentially more crop damage, requiring interventions with other control methods. During post-flowering, the diversity of natural enemies remained stable and higher than that of insect pests (Figure 25b).
- The impact of insect pests on plant defensive metabolites and yield in industrial hemp cultivars
The initial phase of this research has provided valuable insights into the key pest species affecting hemp crops in the region. Notably, the cannabis aphid (P. cannabis) has emerged as the most prevalent and widely distributed pest in CBD and fiber hemp crops throughout Pennsylvania. Significant populations of these aphids tend to establish early in the vegetative phase, with numbers peaking as the plants approach the flowering stage, especially for CBD hemp. However, the true impact of cannabis aphids on hemp production, particularly regarding plant yield, crop health, and the potential effect of aphid populations on important plant compounds such as THC and CBD — an area of critical concern for farmers given the crop legal regulations— remains unclear. One of the challenges in assessing the extent of aphid damage is the difficulty in quantifying or observing visible signs of harm, as these pests can infest plants at any phenological stage without obvious external symptoms. This lack of visible damage makes it difficult to gauge their actual effects on crop performance and determine thresholds to conduct control interventions with different methods.
The second goal of our broad hemp research at the Lab of Arthropod Ecology and Trophic Interactions at Penn State University is to assess the long-term impacts of cannabis aphid infestations on locally used hemp cultivars, particularly regarding yield loss and modifications in cannabinoid and volatile terpenes, as well as the plant's response to aphid feeding. With this proposal sponsored by the Sustainable Research and Education program (SARE), we began examining these effects on three different fiber varieties: Henola, Joey, and Bialobrzeskie, through greenhouse experiments conducted independently for each cultivar. To broaden our research scope, we also included one auto-flowering CBD variety for indoor cultivation and one regular photoperiod CBD variety (Abacus) in the greenhouse studies. In addition, to investigate aphid herbivory under more realistic conditions of a cropping system, we established a fiber plot with Bialobrzeskie and a CBD plot with the Abacus variety at the Russell E. Larson Agricultural Research Center during the 2024 growing season.
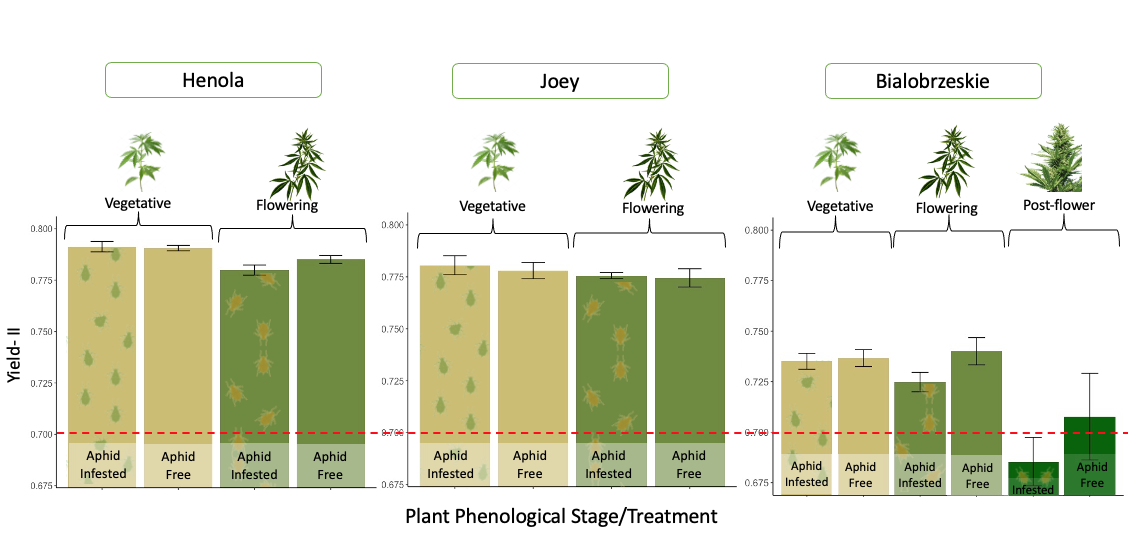
Aphid development is affected by the plant's phenological stage.
Aphids’ reproductive capacity is affected by the plant’s phenological stage. This effect was consistently observed between the aphid-infested treatment plants across all the fiber varieties during greenhouse evaluations. Fifteen days after we induced aphid herbivory stress by resealing five cannabis aphid adults in vegetative, flowering, and post-flowering plants, significant differences were observed in the aphid populations that grew at each phenological stage. In vegetative plants, the number of aphids was twice as high as the aphid population in flowering plants. In the Bialobrzeskie cultivar, we were able to include post-flowering plants, and the number of aphids in these plants was significantly smaller than in flowering and vegetative plants (Figure 26).
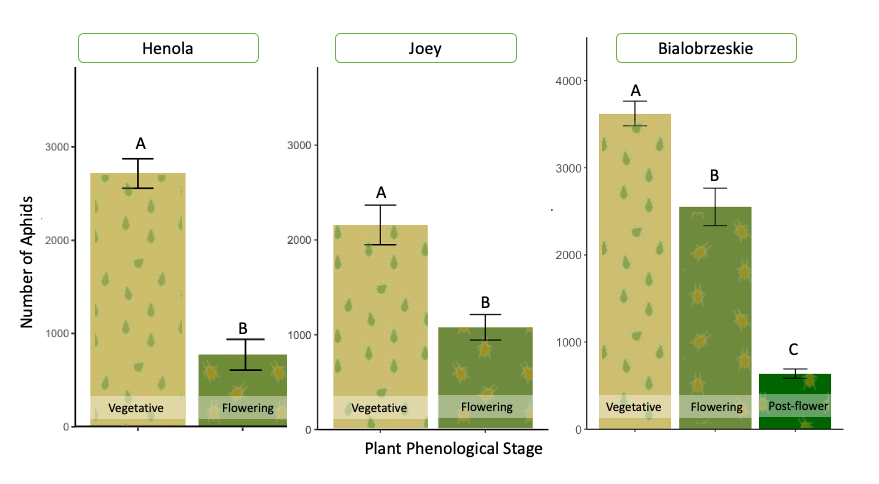
In only two weeks, five single aphids were able to form a colony with up to 3,800 aphids in vegetative plants—depending on the variety—highlighting the fast reproductive potential of P. cannabis in the absence of natural enemies, low cannabinoid compound concentrations in plants, competitors, and stable environmental conditions. In flowering and post-flowering plants, the same five aphids raised colonies with populations between 800 and 2,800—still quite a large population for a single plant. However, this indicates that older plants offer less favorable conditions for aphid reproduction. This could be due to chemical changes during plant maturation, combined with leaf toughness, which may prevent aphids from reaching their full potential.
Aphid herbivory seems not to affect the photosynthetic potential of hemp plants
The yield of photosystem II (PSII) reflects plant health by assessing how effectively the plant converts sunlight into the energy needed for growth and achieving maximum productivity. A low PSII yield indicates that plants are under stress due to water and nutrient deficiencies or other environmental issues that may hinder their photosynthetic capacity. Given the significant reproductive capacity of cannabis aphids, if large populations of aphids are feeding on the nutrients and sugars from the phloem of hemp plants, a disruption in the normal nutrient flow is expected, which could indirectly affect the plants' photosynthetic potential.
We didn’t find significant differences in the PSII yield between aphid-infested plants and aphid-free plants at any of the phenological stages that could demonstrate detrimental effects of aphid herbivory on the photosynthetic potential of the fiber varieties. While bialobrzeskie exhibited, in general, lower PSII yields than the rest, none of the vegetative and flowering plants displayed values that would indicate plant stress (which would be lower than 0.7). The lower photosynthetic potential observed in post-flowering plants could be attributed to the natural senescence of plants at this stage (see Figure 27).
Figure 27. Effect of cannabis aphid herbivory on the plant photosynthetic potential of three industrial hemp varieties.
Hemp varieties responded differently to the effect of aphid herbivory on plant yield.
The impacts of aphid herbivory on plant biomass and the ability of the plants to withstand high aphid populations varied across the three fiber varieties assessed in the greenhouse. These differences highlight the different levels of resistance and tolerance among hemp varieties to aphid damage.
In Joey, no differences were observed between infested and non-infested plants at the vegetative and flowering stages. This suggests that aphid feeding did not affect plant biomass, indicating that this variety could exhibit higher tolerance to aphid infestations (Figure 28).
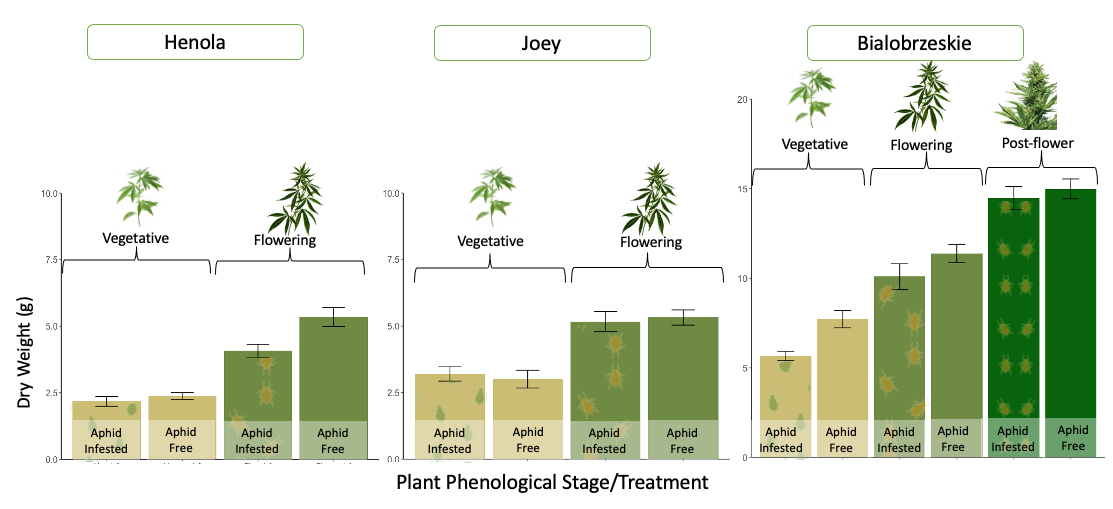
In Bialobrzeskie, while aphid-infested plants tended to reduce biomass compared to aphid-free plants at all phenological stages, these differences were not significant (Figure 28). This suggests that this variety might have a moderate tolerance to aphids, with plants possibly showing a slight decrease in growth, but not to the extent of impairing plant development.
In contrast, Henola showed a significant reduction in dry weight when flowering plants were exposed to aphid infestations for two weeks (Figure 28). This suggests that Henola had a lower tolerance for aphid attack during the reproductive stage. It is possible that aphid infestations during the flowering stage impact plant processes such as nutrient allocation and reproductive energy.
The greenhouse experiment provided valuable insights into the impact of aphids on plant biomass in a controlled environment. However, it’s important to recognize that these results may not fully reflect the interactions between aphids and plants in natural settings. While we can carefully regulate factors such as temperature, humidity, and light in the greenhouse, these conditions may not accurately represent the variability and challenges present outside. In our study, Joey demonstrated remarkable tolerance to aphid infestations, whereas Henola experienced a significant decline in biomass during the flowering phase. Nonetheless, it’s crucial to assess these effects in field conditions, where real-world elements like weather fluctuations, soil health, pest-predator dynamics, and competition from surrounding plants could significantly influence how plants respond to aphid herbivory.
Plant defenses against aphids: Phytohormones, Plant Volatiles and Cannabinoid compounds
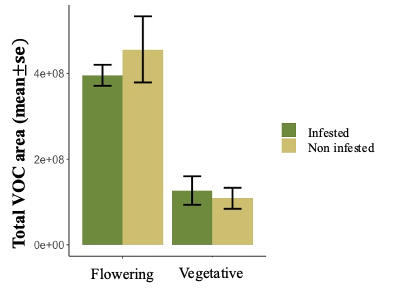
We are making significant progress in identifying plant chemical compounds that play a crucial role in defending against key pests like aphids, as well as compounds that may influence the attraction of natural enemies to hemp crops. However, conducting experiments with a new crop like hemp has presented several challenges, including adapting methods, the need for proper infrastructure for growing hemp, and dealing with insect and pathogen contamination in plants grown for experimentation, which can delay the expected outcomes. Despite these obstacles, we have successfully collected volatiles from about 400 samples from Henola, Bialobrzeskie, Joey, Abacus, and Auto-flower varieties in our greenhouse experiments, and these samples are currently being processed for further analysis.
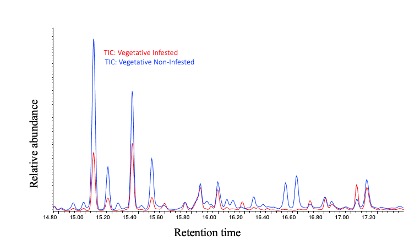
We have found that, in general, flowering plants release approximately three times more volatiles than vegetative plants, as observed in Figure 29. In the chromatogram of volatiles analysis, we have consistently identified peaks corresponding to around 16 chemical compounds, including green leaf volatiles, terpenes, and sesquiterpenes, present in both phenological stages. Some of these volatiles include alpha- and beta-pinene, myrcene, limonene, ocimene, caryophyllene, beta-farnesene, and humulene. Among vegetative plants, particularly within the sesquiterpene group, we observed lower concentration peaks in aphid-infested plants (Figure 30), suggesting the potential suppression of these compounds. We are currently conducting further analyses to determine whether aphids suppress specific chemical defenses in the plants to facilitate colonization. Additionally, flowering plants exhibited significant variability in the concentration of the same sesquiterpenes between aphid-infested and aphid-free plants, highlighting a dynamic chemical response to aphid herbivory (Figure 31).
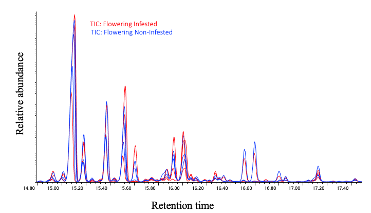
In addition to the volatile compound analysis, we have collected the same number of samples for phytohormone analysis and cannabinoid profiling, both of which are currently in the chemical analysis process. These analyses will provide a comprehensive understanding of the chemical interactions between hemp plants, pests, and natural predators, which are important for developing sustainable pest management strategies. We are working to make these results available to farmers to provide valuable insights into the management of important pests like cannabis aphids in hemp crops.
Works Cited:
- Bragard C, Dehnen-Schmutz K, Serio FD, et al (2020) Pest categorisation of Diabrotica undecimpunctata 18:. https://doi.org/10.2903/j.efsa.2020.6358
- Britt KE, Pagani MK, Kuhar TP (2019) First Report of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Associated With Cannabis sativa (Rosales: Cannabaceae) in the United States. J Integr Pest Manag 10:17. https://doi.org/10.1093/jipm/pmz014
- Chen Y, Li C-D (2016). Three new species and new distributional data for five rare species of Aphelinus (Hymenoptera: Aphelinidae) from China. Zootaxa 4092:258–272. https://doi.org/10.11646/zootaxa.4092.2.8
- Cranshaw WS, Halbert SE, Favret C, et al (2018) Phorodon cannabis Passerini (Hemiptera: Aphididae), a newly recognized pest in North America found on industrial hemp. Insecta Mundi
- Giladi Y, Hadad L, Luria N, et al (2020) First Report of Beet Curly Top Virus Infecting Cannabis sativa in Western Colorado. Plant Disease 104:999–999. https://doi.org/10.1094/PDIS-08-19-1656-PDN
- Gill HK, Garg H, Gill AK, et al (2015) Onion Thrips (Thysanoptera: Thripidae) Biology, Ecology, and Management in Onion Production Systems. Journal of Integrated Pest Management 6:6. https://doi.org/10.1093/jipm/pmv006
- Hu J, Masson R, Dickey L (2021) First Report of Beet curly top virus Infecting Industrial Hemp ( Cannabis sativa ) in Arizona. Plant Disease 105:1233–1233. https://doi.org/10.1094/PDIS-11-20-2330-PDN
- Stats AK, Sweat KG, Masson RN, et al (2023) The Desert Whale: the boom and bust of hemp in Arizona. J Cannabis Res 5:19. https://doi.org/10.1186/s42238-023-00187-8
- Yasnosh VA (1981) Aphelinus maculatus. (Hymenoptera, Aphelinidae) - a parasite of aphids in the south of the Maritime Territory. Tr Vsesoyuznogo Entomol Obshchestva 61:164–165
This survey of insect pests and their natural enemies on Pennsylvania hemp farms was funded by the Northeast Sustainable Agriculture Research and Education (SARE) program and the Pennsylvania Department of Agriculture. The SARE Graduate Student Grant was vital for the first phase of a two-year insect sampling initiative, and it allowed us to expand our efforts across hemp farms in six counties in central and southeastern Pennsylvania. The findings from the 2022 survey provide a baseline of the insect communities inhabiting hemp crops that could be used to determine priorities and support decisions in pest management
Prevalent insect pests:
Based on our sampling methods, Herbivorous insects dominated the insect community in hemp crops. Despite their higher abundance, herbivore communities were less diverse, predominantly composed of several key pest families, including aphids (Aphididae), thrips (Thripidae), and leafhoppers (Cicadellidae). Although less prevalent, leaf beetles (Chrysomelidae), plant bugs (Miridae), and stink bugs (Pentatomidae) were also observed to play a critical role during certain stages of crop growth.
The Cannabis aphid (P. cannabis) was found to be the most abundant and widely distributed pest in Pennsylvania hemp farms, standing out as the only specialist insect among the most abundant herbivores. This aphid has evolved to exploit hemp plants as its primary resource, contributing to its high abundance and successful establishment. In contrast, thrips, leafhoppers, and leaf beetles—the other most abundant herbivores observed—are polyphagous insects. They can feed opportunistically on hemp but do not have the same level of host specialization as P. cannabis. The effects of these prevalent insects on hemp could be significant, not only due to their capacity to damage plant tissues, but most of them can also transmit pathogens or aid in their entry into the plant.
Other notable hemp pests, including the Eurasian hemp borer (Grapholita delineana) and the hemp russet mite (Aculops cannabicola), were not found in our survey despite being reported in different areas. It is possible that these pests are not established in the hemp farms where our samplings were conducted, or local conditions may limit their occurrence, highlighting the importance of continued monitoring to detect pest arrivals and adapt pest management strategies considering new pest threats. The corn earworm, a major generalist pest affecting hemp, was not a threat to crops in 2022 due to its reduced numbers. Nonetheless, ongoing monitoring is recommended to identify potential outbreaks, which can inflict serious harm because of their migratory habits and the possibility of rising populations in years with warmer temperatures.
Variation across hemp types and hemp farms:
The insect communities associated with CBD and fiber hemp crops showed significant differences in composition. For herbivore communities, the group of dominant insects remains the same across both CBD and fiber crops; however, their relative abundance differs notably between crop types. This suggests that while the same pests are prevalent in both systems, their impact varies depending on the type of hemp. Beyond hemp type, insect community composition also varies among farms. Although the insect communities are more consistent across CBD hemp farms, distinct factors—such as crop variety, surrounding vegetation, previous crops, and farming practices—contribute to particular community structures on individual fiber hemp farms.
In addition, this phase of the insect survey allowed us to monitor the population dynamics of these prevalent herbivores, identifying their occurrence in the growing cycle of each type of hemp and determining when their populations could become harmful. Understanding the differences in insect community composition between CBD and fiber hemp, as well as the variations across different farms and throughout the plant’s phenological stages, has significant implications for pest management strategies.
This knowledge allows hemp farmers to identify which insect species are most likely to pose problems in their specific crop systems, whether growing CBD or fiber hemp. By understanding the unique pest pressures associated with each type of hemp, farmers can tailor their monitoring efforts to the specific needs of their crops, resulting in more effective pest management. For example, pest populations may vary between CBD and fiber hemp varieties due to differences in plant structure, chemical composition, or growth stages. Additionally, pest pressure can change throughout the plant's phenological stages (such as during the vegetative growth phase versus the flowering phase), highlighting the need for farmers to create and adjust their monitoring schedules and pest management approaches accordingly. This deeper understanding enables farmers to implement more precise and timely pest control measures, reducing crop damage, minimizing the need for broad-spectrum pesticides, and ultimately improving both crop yield and quality.
Natural predators play a significant role in reducing crop damage caused by herbivores.
Natural enemies were less abundant, but their diversity was significantly higher than that of herbivores. The most commonly observed groups were lady beetles (Coccinellidae), spiders, and minute pirate bugs (Anthocoridae). Predatory thrips (Aeolothripidae), ants (Formicidae), hoverflies (Syrphidae), and lacewings (Chrysopidae) were present in moderate abundance. Several families of parasitic wasps were also significant, particularly as captured by yellow sticky traps.
In our evaluation of insect damage symptoms in crops, we found that in 2022, CBD hemp crops experienced a higher incidence of insect damage than fiber hemp. These damages were primarily inflicted by chewing and piercing-sucking insects; however, the overall severity of these damages was low. The diversity index for each insect community in both types of hemp showed higher diversity values for beneficial insects throughout the entire season, showcasing a more stable and evenly distributed community with a great capacity to mitigate pest pressure. This diversity of natural enemies is crucial for maintaining lower pest levels in the crops, as these beneficial insects help control pest populations and prevent detrimental effects on crop health and yield.
Unveiling the Potential Impact of Cannabis Aphids on Hemp Varieties.
Our greenhouse experiments indicated that the performance of cannabis aphids declines when they are feeding on flowering and post-flowering plants, possibly due to changes in the chemical composition of older plants. However, in our controlled experimental conditions, we also observed evidence of their reproductive potential under conditions that support population growth, for example absence of predators, competitors, and plants with low cannabinoid concentrations. Furthermore, the influence of aphid feeding on the health of plants exhibited variability among different hemp varieties, with the Joey variety demonstrating a higher tolerance to aphid infestations without significant reductions in biomass or photosynthetic potential. In contrast, the Henola variety showed a decline in both dry weight and photosynthetic efficiency, especially when aphid infestations occurred during the flowering phase, highlighting a possible reduced tolerance to aphid herbivory. We are still validating those effects in field-cultivated hemp.
The identification of volatile compounds indicated that flowering plants emitted substantially higher concentrations of these compounds compared to their vegetative counterparts, with terpenes and sesquiterpenes consistently detected across both growth stages. Infestations of aphids resulted in alterations within the profiles of these compounds, suggesting that aphids may have an impact on the chemical defenses of the plants. These observations indicate that a comprehensive understanding of the timing of aphid infestations, in conjunction with the chemical responses exhibited by various hemp varieties, could enhance the development of targeted pest management strategies, ultimately benefiting both plant health and crop yield. We will provide further insights as we advance in our hemp research.
Drawing from our research findings, we created this factsheet to assist farmers in recognizing the most common pests identified during the initial year of our insect survey. The prevalence rates for CBD and fiber hemp are estimated based on the average relative abundance of these herbivores: Very high: > 40 %, High: 20 % - 39%, Moderate: 10% - 19 %, Low: 2 - 9%, Very low: < 2%.
Fact sheet 1. Prevalent and potentially harmful pests for CBD and fiber hemp crops in Pennsylvania
|
Insect Family/ Species |
Feeding Habit / Host Range |
Affected Plant Organ |
Damage in Crop |
Potential Risk |
Natural Enemies |
Prevalence in CBD Hemp |
Prevalence in Fiber Hemp |
|
Aphids (Aphididae): Cannabis aphid, Phorodon cannabis Passerini |
Piercing-Sucking/ Specialist |
Leaves, stems |
Leaf curling, stunting, Production of honeydew and sooty mold, Virus Vector |
High risk: rapid reproduction and potential for virus transmission |
Lady beetles, lacewings, parasitoid wasps |
Very high (43.4%) |
Medium (16.9 %) |
|
Thrips (Thripidae): Onion thrips (Thrips tabaci), western flower thrips (Frankliniella occidentalis), and soybean thrips (Neohydratothrips variabilis) |
Scraping and Piercing-Sucking/ Generalist |
Leaves, stems, flowers |
Scraping, discoloration, facilitate the entrance of pathogens |
Moderate risk: direct damage and disease transmission |
Predatory thrips, minute pirate bugs |
Moderate (16.8%) |
High (24.5%) |
|
Leafhoppers (Cicadellidae) |
Piercing-Sucking / Generalist |
Leaves |
Hopper burn, necrosis; virus vector |
Moderate risk: vectoring diseases and reducing plant vigor |
Spiders, predatory beetles |
Low (8.7 %) |
Low (7.9%) |
|
Leaf Beetles (Chrysomelidae): Flea beetles (Altica sp., Systena sp., and Crepidodera sp.) and Spotted Cucumber Beetle (Diabrotica undecimpunctata |
Chewing/ Generalist |
Leaves |
Holes in leaves, defoliation |
Low risk: can cause significant leaf loss if populations are high |
Parasitic wasps, predatory bugs |
Moderate (14.8%) |
Moderate (13%) |
|
Plant Bugs (Miridae): tarnished plant bugs (Lygus spp.) |
Piercing-Sucking / Generalist |
Flowers, stems, seeds |
Punctures, deformities in growing tips, flower abortion |
Moderate risk: direct damage affecting plant development and yield |
Predatory bugs, spiders |
Very Low (1.8%) |
Low (8%) |
|
Stink Bugs (Pentatomidae) |
Piercing-Sucking/ Generalist |
Flowers, seeds, delicate plant tissues |
Seed damage, deformation, transmitting pathogens |
Moderate risk: Affects seed quality and can transmit diseases |
Tachinid flies, parasitic wasps |
Low (2.9%) |
Low (5.2%) |
|
Corn Earworm (Noctuidae): Helicoverpa zea |
Chewing/ Generalist |
Flowers, buds, leaves |
Bore into flowers and buds, causing direct damage |
High risk: can cause severe damage to reproductive parts of the plant |
Parasitic wasps, predatory beetles |
Very Low (0.2%) |
Very Low (0.7%) |
|
Grasshoppers (Orthoptera) |
Chewing/ Generalist |
Leaves, stems |
Extensive defoliation, cutting young seedlings |
High risk: potential for extensive crop destruction |
Birds, rodents, parasitic flies |
Low (2.2%) |
Very Low (0.2%) |
|
Japanese Beetles (Scarabaeidae ): Popillia japonica |
Chewing / Generalist |
Leaves, flowers |
Skeletonization of leaves, damage to flowers |
Moderate to high risk depending on population density |
Tachinid flies, parasitic wasps |
Very Low (0%) |
Low (2.1%) |
We developed a pest occurrence calendar for CBD and fiber hemp crops by analyzing the population’s relative abundance of key herbivores during different phenological stages. This calendar can be updated annually based on new monitoring data and serves as a useful resource to direct monitoring efforts. It also prepares us to implement other management strategies if these pests prove to be significantly detrimental to the crops.
Calendar of occurrences for the most prevalent insect pests affecting CBD and fiber crops in Pennsylvania.
|
CBD HEMP |
|||
|---|---|---|---|
|
Crop Stage |
Vegetative |
Flowering |
Post-Flowering |
|
Jun- July - Aug |
July- Aug - Sep |
Sep-Oct |
|
|
Aphids (Aphididae): Cannabis aphid, Phorodon cannabis Passerini |
Very High |
High |
Very High |
|
Thrips (Thripidae): Onion thrips (Thrips tabaci), western flower thrips (Frankliniella occidentalis), and soybean thrips (Neohydratothrips variabilis) |
High |
High |
Medium |
|
Leafhoppers (Cicadellidae) |
low |
Medium |
Low |
|
Leaf Beetles (Chrysomelidae): Flea beetles (Altica sp., Systena sp., and Crepidodera sp.) and Spotted Cucumber Beetle (Diabrotica undecimpunctata |
Low |
Medium |
Low |
|
Plant Bugs (Miridae): tarnished plant bugs (Lygus spp.) |
Low |
Low |
Low |
|
Stink Bugs (Pentatomidae) |
None |
Low |
Medium |
|
FIBER HEMP |
|||
|
Crop Stage |
Vegetative |
Flowering |
Post-Flowering |
|
June-July |
July-Aug |
Aug-Sep |
|
|
Aphids (Aphididae): Cannabis aphid, Phorodon cannabis Passerini |
Medium |
Medium |
High |
|
Thrips (Thripidae): Onion thrips (Thrips tabaci), western flower thrips (Frankliniella occidentalis), and soybean thrips (Neohydratothrips variabilis) |
Very High |
Medium |
Medium |
|
Leafhoppers (Cicadellidae) |
Low |
Medium |
Very Low |
|
Leaf Beetles (Chrysomelidae): Flea beetles (Altica sp., Systena sp., and Crepidodera sp.) and Spotted Cucumber Beetle (Diabrotica undecimpunctata) |
Medium |
Low |
Low |
|
Plant Bugs (Miridae): tarnished plant bugs (Lygus spp.) |
Low |
Medium |
Medium |
|
Pentatomidae (Stink Bugs) |
Very Low |
Medium |
Medium |
Recommendations for farmers
Evaluate Pest Risks According to Hemp Varieties and Farm Location: Assess the specific pest risks associated with the type of hemp being cultivated (e.g., CBD or fiber) and the characteristics of the farm. It is essential to prioritize monitoring the pests that are most prevalent and potentially detrimental to the crop system. Maintain a comprehensive list of pests while observing population trends and symptoms indicative of pathogen-related damage. Utilize this fact sheet as a reference for identifying potential threats and tracking pest dynamics over time.
Establish and Refine a Monitoring Calendar: Create a calendar for monitoring pests and diseases, updating it each year according to your abundance observations. Tailor the monitoring frequency to suit the specific requirements of your crops, taking into account environmental changes, plant growth stages, and levels of pest activity. Consistent monitoring is essential for early detection and timely intervention.
Monitor Aphid Populations During Vulnerable Periods: Pay special attention to aphid populations during the vegetative phase of your crops. This period is critical for plant growth and the development of chemical defenses. Aphids can rapidly proliferate during this stage, and early monitoring can help mitigate their impact before they cause significant damage to crop health. During this monitoring, verify the presence of natural enemies like lady beetle larvae before deciding on measures that could diminish their efforts to feed on aphids. Post-flowering was also important for aphid populations.
Watch for New Pests and Disease Symptoms: Stay vigilant for the arrival of new pest species, especially those that may be introduced through nearby crops or environmental factors. Additionally, monitor for symptoms of diseases transmitted by insect vectors, such as aphids or whiteflies, which can spread viruses or other pathogens that threaten your crops.
Minimize Pesticide Use to Preserve Beneficial Insects: Low pesticide use typically benefits hemp crops, helping maintain the balance of beneficial insects that naturally regulate pest populations. Given the high diversity and stability of natural enemies observed in this study, it is recommended to focus on biological control methods, which can be highly effective in maintaining pest control without harming beneficial species.
Conservation Biological Control: The conservation of natural enemies (predators, parasitoids, etc.) provides natural habitats and food sources, shelter, and nesting sites for beneficial insects; farmers can support these populations and reduce the need for chemical interventions. This approach helps preserve the ecological functions of natural enemies, ensuring a more sustainable and resilient pest management system.
Prepare for Potential Pest Outbreaks: Be prepared for possible pest outbreaks, particularly from species like the corn earworm, which can cause significant damage. Have an emergency plan in place, and work closely with your local extension agents to access expertise and resources for rapid intervention when necessary.
Consult Biopesticide Options for Hemp: Familiarize yourself with the list of biopesticides that are approved for use in hemp production. These environmentally friendly options can help manage pests while minimizing the impact on beneficial organisms and the surrounding ecosystem.
These recommendations aim to promote sustainable pest control practices, ensuring the long-term success of hemp cultivation in the region. This project represents the first step toward expanding the fundamental understanding of hemp crops in the Northeast. Moving forward, we will continue to provide valuable insights and updates through ongoing research conducted at the Department of Entomology at Pennsylvania State University, further supporting farmers with the latest knowledge and strategies for managing pests and enhancing crop health
Education & Outreach Activities and Participation Summary
Participation Summary:
Completed Outreach Activities:
1. Field Days and Demonstrations:
Multiple field days were conducted at the Southeast Agricultural Research and Extension Center, alongside collaborations with hemp farms across Central and Southeastern Pennsylvania. These events gathered farmers, researchers, and extension agents to observe ongoing research on insect pests and biological control in hemp crops. Demonstrations included sampling techniques such as vacuum sampling, yellow sticky traps, and visual observations.
At the Hemp Twilight Field Days, we presented our survey research focused on hemp pests and management strategies involving natural predators. We also shared insights from our laboratory studies, emphasizing that some hemp cultivars may possess natural resistance to herbivorous pests. During the networking event "A Bio-based Future" in Lancaster County, Pennsylvania, we highlighted our findings on how significant insect pests, like the cannabis aphid, affect two fiber varieties cultivars.
These events fostered engagement with a diverse group of stakeholders, including farmers, researchers, extensionists, entrepreneurs, and organizations within the hemp industry. This collaboration provided valuable opportunities to share experiences and reinforced our commitment to developing novel, sustainable pest management solutions in this emerging cropping system.
2. Workshops and Presentations:
We delivered a workshop on "Integrated Pest Management in Hemp Crops" at the Pennsylvania State Extension Conference. The workshop provided practical knowledge on identifying prevalent pests and their natural enemies, implementing effective monitoring techniques such as visual inspections and trap-based sampling, and integrating sustainable pest management practices. Participants, including growers and extension agents, gained insights into strategies to reduce reliance on chemical controls while maintaining crop health and yield.
In addition to the workshop, we presented our research findings at prominent regional, national, and international agricultural conferences, including the Entomological Society of America Annual Meetings, the International Biological Control Congress, and the International Society of Chemical Ecology. Our presentations emphasized the significance of minimal pesticide use, the critical role of natural predators in pest regulation, and the need to harness ecological interactions for effective pest control. Additionally, we emphasized our research on the role of plant chemical compounds against insect pests like the cannabis aphid, illustrating how these compounds enhance the plant's response to aphid infestations.
Through these outreach and dissemination efforts, we actively engaged with diverse audiences, fostering discussions on advancing pest management strategies that align with sustainability goals and address the unique challenges in hemp crop production.
3. Educational Materials:
We developed a factsheet summarizing key findings from our insect survey, including pest occurrence calendars and actionable recommendations for monitoring and managing pests in hemp crops. Additionally, we designed a poster highlighting the effects of cannabis aphid herbivory on fiber hemp yield and plant defenses. To engage a broad audience of stakeholders, we shared these contents during field days, workshops, and networking events. To ensure continued dissemination and practical application of our research insights, they will also be made accessible through the Penn State Extension website.
4. Collaboration with Farmers:
We actively engaged with farmers to discuss pest pressures and monitoring strategies tailored to their specific hemp types, including CBD and fiber cultivars. Through these discussions, we gained valuable insights into the unique challenges faced by growers, ensuring our recommendations were practical and relevant. Feedback from these interactions directly informed the development of our factsheets, workshops, and outreach materials, enabling us to provide targeted and actionable guidance that addressed the diverse needs of the hemp farming community.
Ongoing Outreach Activities:
1. Data Dissemination:
We are preparing two peer-reviewed articles to publish the results of our two-year insect survey, highlighting its implications for hemp pest management in Pennsylvania. These publications aim to serve as accessible resources for both the scientific community and agricultural stakeholders, fostering a deeper understanding of pest dynamics and sustainable management strategies.
In addition to these articles, we will present further findings at upcoming conferences, including the Entomological Society of America’s Annual Meeting. These presentations will provide opportunities to engage with researchers, extension agents, and industry professionals, further amplifying the impact of our research and its application in hemp production systems.
2. Digital Outreach:
We will collaborate with the Penn State Extension team to create video tutorials that provide practical guidance on pest identification, sampling methods, and biological control strategies for hemp crops. These videos will be designed to be user-friendly and accessible, ensuring that farmers, extension agents, and other stakeholders can easily implement the techniques. The videos will be hosted on the Penn State Extension’s online platform, expanding their reach and serving as a valuable resource for the agricultural community to promote sustainable pest management practices.
3. Farmer Outreach Surveys:
We are conducting surveys with hemp farmers across Pennsylvania to evaluate the adoption of monitoring techniques and pest management strategies introduced through this project. Survey results will guide future outreach and research efforts.
This section reflects the comprehensive efforts made to engage with the agricultural community, share findings, and support the adoption of sustainable pest management practices in hemp cultivation. Future outreach will focus on expanding accessibility to educational resources and incorporating stakeholder feedback to refine recommendations.
Project Outcomes
The primary contribution of this research lies in enhancing the pest management knowledge of growers who incorporate hemp into their farms. This enables them to succeed in a market offering diverse products. Furthermore, this research provides critical insights that can help farmers achieve economic, environmental, and social sustainability through more informed and sustainable practices.
By conducting weekly insect surveys in hemp plots across central and southeastern Pennsylvania, we not only identified pest challenges but also fostered direct engagement with farmers. These interactions facilitated an exchange of experiences, which helped us understand farmers’ constraints and expectations within this emerging system. Importantly, we raised awareness of the role of insect diversity in informing pest management strategies, a practice that will directly impact the quality, safety, and sustainability of future hemp production.Each participating farm offered unique contributions to agricultural sustainability:
- Shaffer and Lazy Moon Farms in Clearfield and Blair Counties cultivated CBG and CBD hemp varieties for medicinal purposes. These farms actively engaged with local communities through outreach, creating social value by promoting the benefits of hemp-based medicines.
- Rhodes Farm in Clinton County focused on producing high-quality CBD products using sustainable, pesticide-free practices, demonstrating an economic model that balances profitability with environmental stewardship.
- Cedar Meadow Farm in Lancaster County diversified its hemp production for fiber, seed, grain, and extracts, transforming hemp into cosmetics, medicinal, and veterinary products. This multi-functional approach showcases the economic versatility of hemp while emphasizing sustainability.
- Coexist Farm specializes in cultivating fiber hemp for eco-friendly construction materials, such as hemp concrete blocks, advancing the use of hemp in sustainable building practices.
Our research on these farms identified pest issues and explored biological control solutions, promoting reduced pesticide use and fostering environmental sustainability. By supporting farmers in adopting these practices, our project enhances economic resilience by reducing input costs, safeguards ecological health, and builds social capital by fostering farmer education and community engagement. These efforts collectively contribute to the development of a more sustainable and profitable hemp production system that benefits both growers and their communities.
Over the course of the project, we have learned about crop attributes for sustainability in three unique types of hemp agricultural production systems: seed/oil, fiber, and medicinal extracts. Our initial research sites included 4 farms representing diverse cultivation practices. Importantly, we learned, firsthand, the potential for hemp-growing practices to contribute to environmental, social, and economic sustainability. Three additional sampling plots were established and monitored at Penn State Farm facilities across Pennsylvania.
The architecture of the plant and the crop management practices vary widely depending on the crop purpose, whether it is for fiber, grain, or medicinal extracts. Overall, hemp is a low-input crop in terms of pesticides due to the lack of registered, allowable options for growers. Most growers are working to manage their hemp with minimal disturbances and inputs and are excited to learn about the potential problems they could encounter in terms of insect herbivory. The growers expressed a keen curiosity about how the abundance and composition of beneficial natural enemies (predatory and parasitic insects) present in their farms were associated with the pressure of insect pests. Facilitated by this grant, our goal during the 2022 production cycle was to characterize the pests and natural enemies across hemp cultivars and crop purposes and to communicate that information to our various growers. In doing so, we gained grower interest in determining the potential of the biological control agents observed in reducing pest pressure and reducing issues of increased cannabinoid content associated with herbivory. One reason to monitor pest insects is due to their potential to increase cannabinoids, especially the federally regulated THC, to a level that renders their crop unusable and warrants total crop destruction. This devastating outcome of increased THC left one of our growers to destroy a $60,000 crop of hemp for CBD. We have also begun to explore the impact of insect pest herbivory on plant quality and ultimately yield in the lab. Through these communications and observations on farms, we are now more aware of the challenges these growers face as well as the scales and outputs of their operations.
We were also able to secure $ 58,000 in funding from the Pennsylvania Department of Agriculture to increase the impact of our project, including new objectives to support the sustainability of the hemp industry in pest management and we also developed cooperation with growers and researchers that have previous experience in the hemp system.
I appreciate the aims of sustainable agriculture and enjoy helping the growers move toward using those strategies. The role of hemp in relation to sustainable development contributes not only in terms of sustainable agriculture practices but also in terms of their applications to the industry as a whole. This encourages my desire to support and perform future research in sustainable systems that could have a positive impact on the achievement of sustainable development goals.
Assessment of Research and Educational Methods
One of the main constraints during the initial stage of this research was the dispersal of the sampling sites across the state of Pennsylvania, which limited the number of farms that could be covered weekly. With the additional funding we received, we were able to include new sites and more varieties during a second insect sampling during the 2023 hemp growing season to broaden our research questions. This funding allowed us to begin filling the gaps in pest management for the hemp industry in the Northeastern region of the USA.
A significant methodological challenge was the time-consuming process of analyzing the numerous insect samples collected during weekly surveys. This delay hindered our ability to provide timely and actionable information to farmers. Reflecting on this, we recognize the need for research that develops more effective and farmer-friendly sampling methods. These tools should be easy for farmers to use and capable of delivering faster and more accurate insights into pest and natural enemy populations. Such advancements would enable growers to make informed pest management decisions more quickly, especially in distinguishing between pest pressures in CBD and fiber hemp crops.
Lessons Learned and Future Research Recommendations
Moving forward, research should prioritize the development of innovative sampling techniques that save farmers time while offering precise information on both pests and natural enemies. These improvements would enhance decision-making related to biological control, enabling farmers to conserve or augment beneficial insect populations effectively.
Additionally, educational outreach played a crucial role in the project's success. Direct interaction with farmers during farm visits provided valuable insights into their constraints, expectations, and practical needs. Future educational efforts should maintain this collaborative approach, focusing on creating resources—such as video tutorials and factsheets—that closely align with growers’ real-world needs and challenges.
Looking back, better centralization of sampling efforts and an earlier focus on rapid processing methods might have enhanced the project’s timing and impact. Combining these changes with ongoing farmer engagement and funding for research into sustainable pest monitoring techniques will be essential for improving the effectiveness and accessibility of pest management in new cropping systems like hemp.