Progress report for GNE22-287
Project Information
Spotted lanternfly (Lycorma delicatula, SLF) is an invasive sap-feeding insect that poses a serious threat to several agricultural and ornamental industries, especially grapes, fruit and walnut trees, and hops. The invaded range that started in one county in PA in 2014 has expanded to 17 states in the Northeast, Midwest and Mid-Atlantic regions. To prevent losses from this pest, it is necessary to find control methods that are sustainable and cost effective, for which biocontrol shows great promise. We explored predatory insects that are endemic to the U.S. as potential source of biocontrol agents in this project. Using field surveys and a community science project, I was able to determine that generalist predatory insects in the orders Mantodea, Hymenoptera, and Hemiptera were feeding on SLF in the U.S. I also assessed the predation efficiency of commercially available or easily collected predators in enclosures, and found that spined soldier bugs, Chinese mantises, and Carolina mantises were effective at reducing nymphal and adult SLF populations. I also explored if there were functional response relationships, where the rate of predation increases with increasing prey density, between these predators and increasing SLF density, though there did not appear to be from my work. My findings have been shared through several presentations and will continue to be through direct interactions with stakeholders by our PSU Extension collaborator and PSU Extension Educators, posts on the Penn State Extension webpages (https://extension.psu.edu/spotted-lanternfly; https://extension.psu.edu/spotted-lanternfly), Penn State News, social media, and presentations I will give at grower meetings (e.g., PA Wine Marketing and Research Board, Ag Progress Days, and the PA Farm Show). I also expect to publish at least one peer-reviewed paper from this project.
- Survey for insect predators that are consuming different SLF life stages in the field.
Justification: While we have a great deal of data on predators of adult SLF from a citizen science project, we did not receive many reports of predators of earlier life stages, including eggs and nymphs, likely due to their inconspicuous nature. Identifying wild predators that feed on different life stages will indicate which species to encourage through conservation biocontrol or release for augmentation biocontrol.
- Identify which insect predators have the highest rate of SLF consumption by life stage over time.
Justification: By identifying which insect predators already present in the introduced range have the highest rate of SLF consumption, I will collect information that is useful for the development of a biocontrol program for the sustainable management of SLF. Predators that feed at higher rates than others will have the most promise and should be the foci for future work. I will test species that are commercially available or can easily be collected and reared for use in augmentation or supported through conservation biocontrol. For this project, I will test convergent ladybugs (Hippodamia convergens), harlequin ladybugs (Harmonia axyridis), brown lacewings (Micromus variegatus), and green lacewings (Chrysoperla rufilabris) to determine if they will feed on SLF eggs and early instar nymphs (1sts and 2nds) since they are generalist predators that feed on insect eggs and small soft-bodied insects. We will also test Chinese praying mantises (Tenodera sinensis), Carolina mantises (Stagmomantis carolina), wheel bugs (Arilus cristatus) and spined soldier bugs (Podisus maculiventris) against the nymphs and adults since these predators feed on larger mobile insects. Wheel bugs are not commercially available but frequently attack SLF in the field (Johnson pers. obs). We have also observed wheel bug egg masses laid next to SLF egg masses and received reports of nymphal wheel bugs feeding on SLF nymphs and adults.
- Determine which predators display a functional response to SLF density.
Justification: The most effective biocontrol agents typically show a functional response to their prey, increasing their consumption rate as prey density increases (Jeschke et al., 2004). Determining which of the predators tested in Objective 2 show a functional response to SLF is useful for the selection of the most effective biocontrol agents for further evaluation. This information will also guide initial predator to prey ratios to test for efficacy in a crop setting for control of SLF (such as in an orchard or vineyard).
The purpose of my project is to identify the most efficient native and naturalized predators of SLF in the mid-Atlantic and Northeastern regions of the U.S. as well as those that are commercially available or can be collected and reared for potential augmentative biocontrol of SLF. SLF was first found in the U.S. in 2014 in Berks County, Pennsylvania and now has established populations in 17 states in the region (NYS IPM, 2024). It is a highly polyphagous pest with the potential to damage many agricultural crops such as grapes, hops, and tree fruits. Grapes are currently the crop of greatest economic concern, as there have been reported losses of yield and fruit quality and occasionally vine death in vineyards with high levels of SLF, with one vineyard reporting a yield loss of 90% (Urban, 2020). This has led to increased pest management costs for growers, with some spray records showing that the average cost of insecticide treatments nearly tripled through increased applications (Urban, 2020), which can also have negative effects on beneficial arthropods, other wildlife, and human health (Bale et al., 2007). In addition to the detrimental effects on the ecosystem, SLF control in agriculture relies primarily on two classes of insecticides, neonicotinoids and pyrethroids (Leach et al. 2019), to which SLF could develop insecticide resistance, making it vital to find alternative control methods. Research into more sustainable control methods for SLF that can be applied in an agricultural setting is needed and biocontrol shows great promise.
Biocontrol is the management of a pest through its natural enemies, including pathogens, parasitoids, and predators. It can be more sustainable environmentally and economically than pesticide treatments and is divided into 3 types: classical, augmentation, and conservation biocontrol (Bale et al., 2007). Classical biocontrol is the introduction of a natural enemy from the native range of the pest, but assessments for environmental impacts, including non-target testing, to obtain approval for release usually takes over a decade (Borowy, 2021). This is not an issue for augmentation biocontrol, where natural enemies already found in the invaded range are released. Conservation biocontrol is often employed in combination with augmentation, which is where measures are taken to support natural enemy populations already present by modifying the environment, which might include intercropping or hedgerows of plants that provide shelter and/or sustenance for natural enemies. Thus, by focusing on endemic natural enemies, biocontrol can be implemented sooner by growers. However, not all native predators will be equally effective at controlling SLF. A natural enemy’s ability to control a pest is dependent on its functional and numerical responses to pest population levels; a functional response is the change in predation rate with changes in prey density and a numerical response is an increase in the predator population with increases in prey density (Lester & Harmsen, 2002). While both are important metrics in realizing the impact of predators on prey, assessing functional responses in this system will allow us to find which predators should be our priority in augmentative releases.
Research
Objective 1. To determine which predatory insects have the most potential for biocontrol of SLF, I began by conducting surveys in areas with high SLF populations to document which predators in the invaded range are already feeding on SLF, especially on eggs and nymphs. I targeted host plants infested with a large number of SLF and recorded all interactions between predators and prey for 3 hours, varying the time of day at which observations occurred to see predators that are active at different times. I surveyed once per week in the fall of 2022 (adults and eggs) and the spring, summer, and fall of 2023 (nymphs, adults, and eggs) for a total of approximately 60 observation sessions as SLF passed through each life stage. I recorded the species of the host plant, species and life stage of the predator, life stage of SLF it fed on, how many SLF were eaten, and feeding behavior of the predators. I varied what host plants I used to observe predation to see if this influences the composition of the predator community.
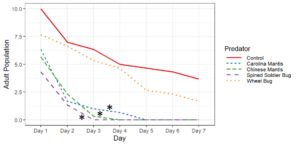
Objective 2. To determine which predatory insects are the most promising for control of SLF, I set up 3 enclosures for each predator species and a control treatment without predators at each SLF life stage. Enclosures were 12 cubic feet (0.3398 cubic meters) in size, containing a potted grape and a potted tree of heaven for the SLF. These plants are easy to grow, are preferred by SLF, and provide diet mixing, which maximizes SLF fitness (Nixon et al., 2021). Predator density per enclosure will be according to the recommendation of the seller or using the density for a similar commercially available predator. I will provide each predator with 10 egg masses, 25 nymphs, or 10 adult, field-collected SLF. The number of SLF eaten was recorded every day over 1 week. This allowed me to compare the average SLF populations overtime in the enclosures using a repeated measures ANOVA followed by a Tukey HSD post hoc analysis in the programming language R.
Convergent ladybugs, harlequin ladybugs, and green lacewings were tested against SLF eggs and 1st and 2nd instar nymphs, while wheel bugs, spined soldier bugs, Chinese mantises, and Carolina mantises were tested against all nymphal stages and adults. Brown lacewings were originally considered for testing, but preliminary results showed that they would not feed on offered SLF eggs or nymphs. Both species of ladybug larvae and green lacewing larvae are more voracious predators than the adults and thus larvae were used in experiments. For the remaining insect predators, the life stage was determined by what was available at the time of the test.
Objective 3. To determine if predators showed a functional response to SLF population density, I compared the average reduction in population over 24 hours based on the population of SLF in the enclosures for Carolina mantises, Chinese mantises, spined soldier bugs, and wheel bugs based on the data collected for Objective 2. I will set up 3 enclosures for Carolina mantises, Chinese mantises, spined soldier bugs, and wheel bugs and SLF life stage, 12 cubic feet (0.3398 cubic meters) in size, containing a potted grape and a potted tree of heaven for the SLF. Each enclosure will contain the number of predators recommended by the seller recommendation or using the recommendations for the most similar commercially available predator. I will add 1st instar nymphs, 4th instar nymphs, or adults that are field collected at three densities: 10, 25, or 40 nymphs, and 5, 10, or 15 adults. The number of SLF eaten will be recorded every day for a week, adding additional prey as needed to maintain these densities. I will compare the daily and weekly rate of predation with increasing prey density using multiple regression with predator species and time as explanatory variables and number of prey consumed per day (and per week) as the dependent variable.
Problems and Solutions: Our biggest issue with this project came down to a lack of time and personnel. In order to get results, much of the work had to be scaled back, such as reducing the number of replicates and SLF populations within the enclosures, as collecting the SLF needed to do the project as originally outlined would not have been feasible without more people helping. We also saved on time by using premade popup enclosures instead of building our own.
Objective 1. During my observations, I saw many insects such as flies, ants, yellow jackets, and red paper wasps feeding on the honeydew produced by SLF on multiple host plants, including tree of heaven, red maple, silver maple, and black walnut. Red paper wasps were also seen attempting to grab adult SLF during an observation period on September 29th, 2022, which may have been attempts at predation, but the wasps would leave the SLF alone as soon as they moved. This may also be an indication that the wasps were more interested in feeding on the honeydew produced by the SLF than the insect itself, and were perhaps looking for easier access to the source of honeydew. In 2023, I observed an adult bald faced hornet sting and capture an adult SLF off a tree of heaven plant on September 7th which it then carried away presumably to feed itself and its colony. On September 14th, 2023, I observed an adult praying mantis feeding on an adult SLF on tree of heaven, which consumed all but the wings.
These targeted observations were not very effective at identifying new predators of SLF, contrary to what we expected. Both of the observed predators were previously reported in a community science project we performed and were only observed at the adult stage. One reason that this may not have worked was that we were only able to observe a limited area during any observation period, so it would be easy for us to miss predation events outside of this area. Additionally, our presence may have discouraged predators from hunting the SLF we were watching, as they may have viewed us as a threat. Fortunately, in our community science project, we received reports of a wide array of predators, with arthropods and birds frequently being observed feeding on SLF. This has been published as an open access paper and can be found at https://doi.org/10.1017/S0007485323000317
Objective 2. Preliminary testing done on convergent ladybugs, harlequin ladybugs, brown lacewings, and green lacewings showed them to be ineffective predators of SLF as they did not feed on the eggs or nymphs offered to them. Thus, when setting up enclosures, I did not test brown lacewings and did test Chinese and Carolina mantises, as based on results from the community science project and lab testing, these were far more likely to be effective predators. Egg masses were not fed upon by any of the tested predators, despite testing with both fresh egg masses and those that had overwintered. Chinese mantises and spined soldier bugs significantly reduced populations of all nymphal and adult SLF life stages relative to the control and Carolina mantises significantly reduced populations of adults and all nymphal life stages except 1st instars. Wheel bugs significantly reduced the populations of 4th instars relative to the control. Spined soldier bugs consistently did the best in these tests, fully eliminating the SLF within the enclosures within a week at all mobile life stages. This was frequently achieved through communal feeding, where the spined soldier bugs would mass attack an SLF and feed on it together, allowing them to prey upon SLF that were much larger than them.
These results show that there are several predators with the potential to be used in augmentation biocontrol, notably spined soldier bugs, Chinese mantises, and Carolina mantises which are all commercially available. However, much work remains to be done before implementing these predators through augmentation biocontrol. Predators and SLF were contained within a small area in these tests, which affects predation as neither the predators nor the SLF were able to disperse, affecting the SLF's ability to escape and ensuring that predators were present in the area, which may not occur if they were applied in the field and allowed to leave. There were also no alternative prey items in the enclosures, meaning the predators only options were to feed on the SLF or each other, whereas if different prey were available, the predators may prefer to feed on that.
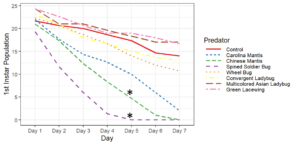
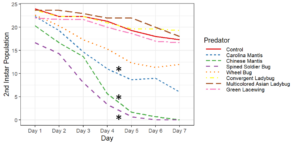
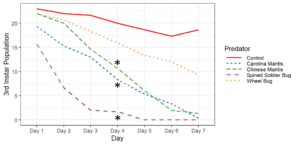
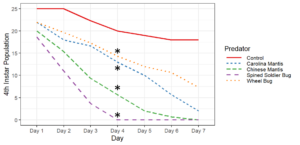

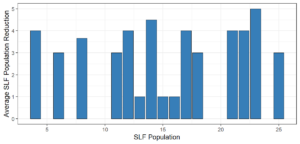
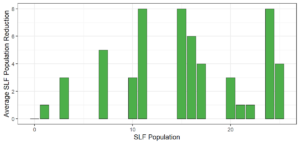
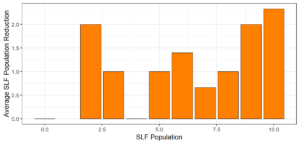
Objective 3. As Carolina mantises, Chinese mantises, spined soldier bugs, and wheel bugs had resulted in significant decreases of SLF populations, these were selected for a closer look into if they displayed a functional response. There were not clear patterns in the rate of consumption relative to the SLF population levels for any of these predators, potentially indicating that they do not display a functional response, though more work needs to be done.
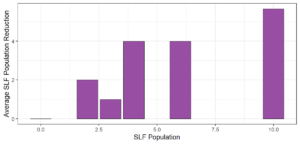
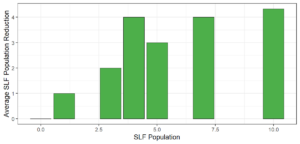
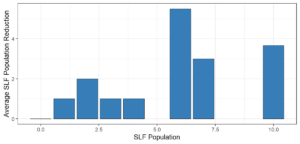
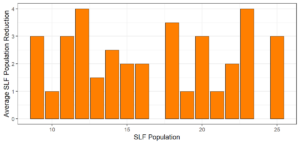
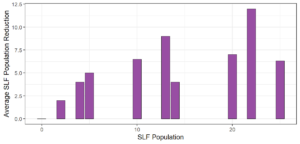
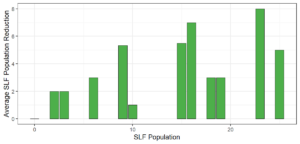
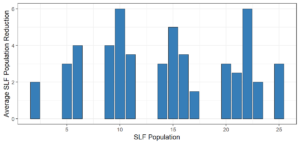
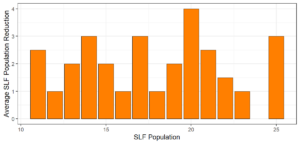
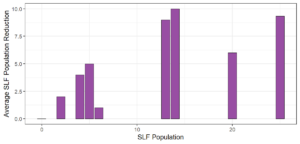
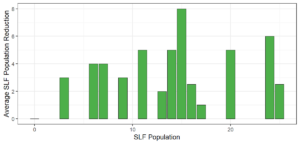
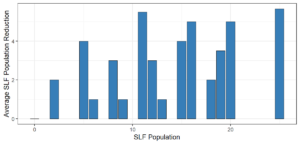
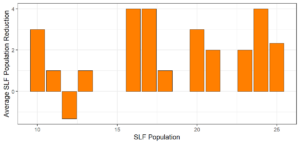
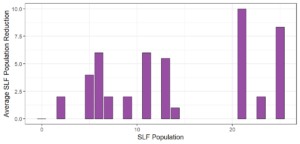
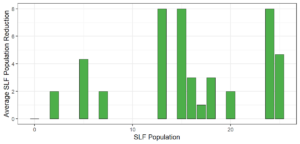
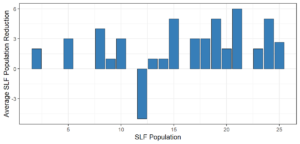
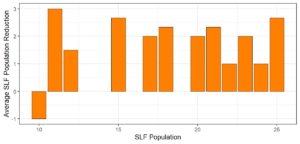
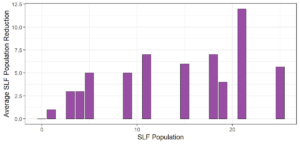
Objective 1. We found that a wide array of generalist predators feed on SLF, notably predatory arthropods. This is good news for farmers, as it suggests that taking steps to encourage predator populations through is conservation biocontrol could be implemented to help sustainably reduce SLF populations. Additionally, there are commercially available generalist predatory arthropods that were observed in the field feeding on SLF, making further research into use of these predators in augmentation biocontrol easier.
Objective 2. Several of the predators we tested were effective at reducing SLF populations within the enclosures, with spined soldier bugs, Chinese mantises, and Carolina mantises performing particularly well. More work remains to be done to see how these predators would perform in the field in the presence of other prey species.
Objective 3. There was no clear functional response in the predators we tested, though more testing should be done to make certain this is the case. This means that we have no clear indications if any of the predators would be more effective in this regard as biocontrol agents. Further study could reveal a functional response in one of our predatory species, which could indicate it would be more effective at reducing SLF populations.
Education & Outreach Activities and Participation Summary
Participation Summary:
I have conveyed my results through presentations to academics and members of the general public, such as Penn State’s Ag Progress Days, Entomological Society of Pennsylvania, and the USDA Annual Meeting on Spotted Lanternfly, and will continue to do so at outreach and Extension events as they occur. The outcomes of this project have been shared with members of PSU Extension, who will be able to inform the public of our results through Penn State Extension activities (webpages, trade journal publications, and social media posts). In addition, we have ongoing relationships with Penn State News’ journalists who continue to provide an avenue for conveying new information to a broad audience. I will post key findings on my Facebook page created for our citizen science project (Birds Biting Bad Bugs, http://facebook.com/birdsbitingbadbugs) very soon once graphics are finished, allowing people who have shown an interest in predators of SLF to see how this information may be applied for SLF management. I have also written a paper to publish my results as an open access journal article.
Project Outcomes
This project has taken the first step in identifying predatory insect biocontrol agents which can be used for the sustainable management of SLF. SLF has the potential to cause much economic harm to farmers, especially as it continues to spread, through both yield losses and increased control costs. Additionally, as the primary control method for SLF is currently the application of pesticides, developing an alternative control method like biocontrol can help reduce the negative environmental and health effects associated with this management strategy.
We learned much about the process of identifying good conservation and augmentation biocontrol agents, especially the potential for spined soldier bugs, Chinese mantises, and Carolina mantises as predators that would be worthwhile to explore further. We need to get a better idea of whether or not the predators show a functional response to SLF density, how alternative prey affects their feeding behaviors, and determine how efficient they are at searching for SLF prey. More generally, I would like to continue researching sustainable management tactics for invasive insects in my career. I also have discovered I like teaching and outreach, so finding a position that would continue to allow me to share my research with people in the future is now my goal.