Final report for GNE22-289
Project Information
Dairy production systems rely on perennial forage legumes for their high nutritional value and ability to supply biological nitrogen (N) to the soil. However, some forage legumes concentrate phytoestrogens in their tissues. Phytoestrogens are secondary metabolites that mimic the mammalian sex hormone estrogen and, if consumed in high quantities, may impair the reproductive performance of dairy cows and other livestock. Previous research has reported that legume species vary in their production of phytoestrogens and that a wide variety of environmental and management-related factors can affect their concentrations. Here, we report the results of three studies aimed at quantifying phytoestrogens across a variety of common forage legume species and cultivars, and the effects that forage management practices (cutting height and frequency) and climate change factors (elevated heat and CO2) have on their concentrations. Among the 12 legume cultivars grown in our common garden plots, total phytoestrogen concentrations varied widely, from over 4.0 mg/g dry matter (DM) in red clover (3 cultivars) to less than 0.02 mg/g DM in birdsfoot trefoil (2 cultivars) and alfalfa (1 cultivar). In our harvest intensity study, total phytoestrogen concentrations were 73 times higher in red clover than in white clover. Formononetin and biochanin A were the most abundant phytoestrogens in both species, cumulatively accounting for 85% (red clover) and 65% (white clover) of the total phytoestrogen concentration. Red clover cut five times in a growing season (5X) produced 35% more total phytoestrogens than red clover cut three times in a growing season (3X); however, white clover did not exhibit this difference. The concentration of phytoestrogens in both clover species varied among cutting events in the 5X treatment but not in the 3X treatment. Cutting height did not influence phytoestrogen concentrations in either species. In our growth chamber study, we found that after 10 days of exposure to elevated temperature, total phytoestrogen concentration in red clover decreased by 50% while neither temperature nor elevated CO2 affected phytoestrogen concentrations in cowpea (concentrations below the level of detection regardless of treatment). Results from these studies will be submitted to peer-reviewed journals and summarized in an extension publication. Overall, this project provides information to farmers and other stakeholders on phytoestrogen concentrations in forage legumes commonly used in dairy operations in the Northeast and how these concentrations can be influenced by cutting management practices and climate change factors.
The overall goal of this research is to investigate variation in phytoestrogen concentrations among forage legumes.
The specific objectives are to:
- Collect and process forage legume tissue samples from an existing field experiment.
- Quantify effects of cutting frequency and cutting height on variation in phytoestrogen concentration in red clover, white clover, alfalfa, and birdsfoot trefoil.
- Disseminate phytoestrogen data to stakeholders.
The Northeastern organic dairy industry is critical to meeting the increasing demand for organic milk [1–3]. Organic dairies in the region rely on perennial legume forages such as red clover (Trifolium pratense), alfalfa (Medicago sativa), and white clover (Trifolium repens) to make up a significant portion of cow diets due to their high protein concentration and palatability [4,5]. Compared to grass alone, legume forages increase feed intake and milk production [6,7]. Forage legumes also contribute to soil fertility through their ability to fix atmospheric N2 [6,8,9].
Despite their benefits to organic dairy production systems, some tradeoffs must be considered when feeding forage legumes, particularly red clover. Previous research demonstrated that many forage legume species accumulate phytoestrogens in their tissues, and if consumed at concentrations of >3 mg/g of dry matter (DM) may impair animal reproductive performance in sheep [10–12]. Phytoestrogens are plant-derived nonsteroidal, secondary metabolites that are biochemically similar to estrogen, the primary female sex hormone [13]. Phytoestrogens in animal tissues have been associated with impaired reproductive systems and infertility [14]. Previous research has also demonstrated that phytoestrogens are transferred to the milk of dairy cows [15,16].
Phytoestrogen accumulation in plant tissues can be affected by a variety of factors, including genotype, environment, and management [10,15–19]. Most phytoestrogens belong to two categories of polyphenolic compounds: flavonoids and lignans. Flavonoids are divided into 3 subgroups: (i) isoflavones, (ii) flavones, and (iii) coumestans [20]. Generally, the types and concentrations of phytoestrogens vary from species to species and even different cultivars of the same species, but are often most abundant in legumes [21], [22–27]. For example, previous research, as well as our own work reported here, suggests that red clover may be particularly prone to accumulating high concentrations of phytoestrogens, while tissues of other forage legumes, such as birdsfoot trefoil, have much lower concentrations [10,27].
Forage harvest practices, including cutting frequency and cutting height can influence the nutritional quality of forage legumes [28], and possibly their concentrations of phytoestrogens. After each cutting, plants become stressed and alter their physiological and biochemical activity in response [29]. In a previous study with subterranean clover, a species known to be highly phytoestrogenic in some situations, total isoflavone concentrations increased in the remaining mature leaves following cutting [30]. As dairy farmers in the Northeast change their forage cutting schedules in response to growing season extension due to climate change, they need science based information about the phytoestrogen content of their legume forages and the potential to affect those concentrations through their management practices. This project aims to quantify concentrations of phytoestrogens in four common perennial forage legumes (red clover, white clover, alfalfa, and birdsfoot trefoil) and determine if and how phytoestrogens are influenced by cutting frequency and cutting height over the growing season. Data generated in the project will be used to provide management recommendations to dairy farmers interested in managing and/or minimizing the accumulation of phytoestrogens in their legume forage crops and mixtures.
Cooperators
- (Educator and Researcher)
Research
Objective 1: Collect and process forage legume tissue samples from an existing field experiment.
Experimental setup and design: This project leveraged two field studies, a common garden plot study of 12 individual forage legume cultivars and a field experiment examining the effects of cutting management (cutting frequency and height) on forage legume productivity and stand persistence. The common garden plots were established at the UNH Kingman Research Farm (Madbury, NH) in 2021, while the cutting management experiment was established in 2018 prior to this grant period. Compost was applied to the site of the cutting experiment in summer and treatments were sown in early September 2018. Four species of perennial forage legume red clover (‘Freedom’), white clover (‘Alice’), alfalfa (‘406AP2’), and birdsfoot trefoil (‘Bruce’) were grown as bicultures with orchardgrass (‘Latar’) (Dactylis glomerata L.). The proportion of sown legume and orchardgrass seed was 70:30. Whole-plot factors are factorial combinations of cutting frequency (3 vs. 5 times over the growing season (3X vs. 5X)) and cutting height (5 vs. 10 cm residual forage height) in a randomized complete block design. The sub-plot (2.3 m x 7.6 m) factor is legume species which is randomized within each whole-plot, and treatments were replicated 5 times. Cutting frequency and cutting height treatments were first initiated in early June 2019, and the legume samples for phytoestrogen analysis were collected between late May and late September 2022. The monthly mean air temperature was 9.6°C and rainfall was 9.9 cm in the experimental site in 2022 (Fig. 1). The forage legume tissue samples were collected immediately prior to each cutting event and analyzed in the laboratory (see objective 2).
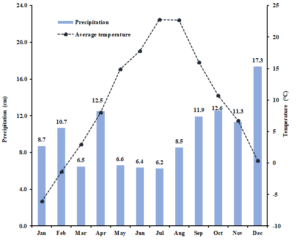 Figure 1. Monthly average temperature (°C) and precipitation (cm) in Kingman Farm at University of New Hampshire in 2022 growing season.
Figure 1. Monthly average temperature (°C) and precipitation (cm) in Kingman Farm at University of New Hampshire in 2022 growing season.
Sample collection: Tissue samples were collected in the 2022 growing season before each cutting event from each plot subjected to two different harvest frequency treatments (3X and 5X). A 0.5-m2 quadrat was placed randomly in three locations in each plot and all legume herbage was clipped from a height of either 5 or 10 cm (depending on cutting height treatment assigned to that plot). Immediately after harvesting, plant material was mixed, and a representative portion was placed in a plastic 50 mL centrifuge tube and immediately transferred to coolers with ice until transported to the laboratory, where they were stored at -80°C. After that, samples were freeze-dried at approximately -50°C and stored at -20°C. This step ensures that the samples remain stable for subsequent analyses.
For the 2023 growing season, we were unable to collect legume tissue samples due to insufficient legume abundance in all experimental plots. However, as an alternative, I conducted a growth chamber experiment from July 2022 to October 2023 at the Macfarlane Greenhouses at the University of New Hampshire to evaluate the influence of elevated temperature and CO2 on phytoestrogen concentration in two forage legume species. We collected forage legume samples from the legume garden established at Kingman Farm in 2022 and 2023 to establish the baseline of phytoestrogens in different forage legume species growing in the New England weather.
We conducted a pot experiment in a growth chamber (CMP6050, Conviron Inc., Canada) from July 2022 to October 2023. Experimental treatments included two factors: legume species red clover (cv ‘Freedom) and cowpea (cv ‘Red Ripper’) and elevated temperature (eT), and elevated CO2 (eCO2) stress. Seeds were sown in 3.8 L pots in a Promix Bx potting media. Pots were placed in the growth chamber under 16 h light at 24°C and 8 h dark at 18°C with ambient CO2 (~400 mmol mol-1). After one week of germination, seedlings were thinned to 8 and 4 plants per pot for red clover and cowpea, respectively. Red clover and cowpea plants were subjected to the following conditions for ten days: (i) control [24/18°C (D/N) and ~ 400 ppm CO2], (ii) eT [35/26°C (D/N) and ~ 400 ppm CO2], (iii) eCO2 [24/18°C (D/N) and ~ 750 ppm CO2], and (iv) eT+eCO2 [35/26°C (D/N) and ~ 750 ppm CO2]. The growth chamber average relative humidity was 65% and light intensity was 350 mmol m-2 s-1. Pots were watered each day, once with fresh water and once with water fertigated with 150 ppm N. We facilitated artificial CO2 supply to one of the growth chambers from a compressed cylinder by CO2 controller (Autopilot APC8200) and maintained desired CO2 level (set point ± 50 μmol mol−1) according to the treatment. Growth chamber temperature was checked periodically to make sure that it was maintained at the desired level. For phytoestrogen analysis samples were collected into a 50 mL conical centrifuge tube and immediately stored in a cooler box until being flash frozen in liquid N and stored at -80°C until biochemical analysis.
Objective 2: Quantify effects of cutting frequency and cutting height on variation in phytoestrogen content in red clover, white clover, alfalfa, and birdsfoot trefoil.
This component of the project proceeded according to plan with one minor modification. Due to problems with the HPLC I had planned to use in-house, we had to send the legume tissue samples to an outside lab (Virginia Tech Chromatography Center) for phytoestrogen analysis. This resulted in an unanticipated added expense; therefore, I only sent the samples of red clover and white clover from four replications of the cutting experiment for phytoestrogen analysis. Previous research suggests these species represent the highest risk for elevated phytoestrogen levels. Legume tissue samples collected from the common garden plots and the growth chamber study were also sent to the Virginia Tech Chromatography Center for analysis of phytoestrogens following the protocol below:
Phytoestrogen analysis: Phytoestrogen concentrations (isoflavones formononetin, biochanin A, genistein, daidzein, glycitein, prunetin, and coumestrol) were determined by high-performance liquid chromatography (HPLC) [31]. Freeze-dried samples were ground into powder before solvent extraction. Approximately 100 mg of ground sample were placed in 15 mL conical centrifuge tubes and 4,750 µL of 80% aqueous methanol plus 250 µL of flavone (400 ng/µL as internal standard) was added and vortexed for 5 s. Next, samples were sonicated for 10 m at room temperature and placed in a shaker at 400 rpm for 2 h. After that samples were left for soaking overnight before being spun down to sediment biomass. Then, samples were centrifuged for 2 min at 4,500 rpm to sediment biomass. Finally, 1.5 mL supernatant was filtered through 0.45 µm PVDF syringe filter into a 2 mL HPLC vial and stored at 4°C until analysis within next 24 h. An HPLC system (Agilent Technology 1100 Series, Palo Alto, CA) was used to identify and quantify phytoestrogens in the samples. Phytoestrogens were detected at 260 nm. Individual phytoestrogens in the samples were identified by comparing their retention times and UV-visible spectra with analytical standards such as biochanin A, formononetin, genistein, daidzein, prunetin, and coumestrol. Calibration curves constructed with these standard compounds were utilized to quantify the concentration of these compounds in the experimental samples. Finally, individual phytoestrogens concentration was determined on a dry matter basis of the samples.
Statistical analysis: The effect of the treatments on plant response variables was assessed using analysis of variance (ANOVA) with partial (type II) sums of squares using R Studio version 2024.04.0. In both the cutting experiment and the growth chamber experiment, species were analyzed separately due to large differences in phytoestrogen concentration between species. Homogeneity of variances and normal distribution of residuals were analyzed using residual plots and the Levene (‘car’ R package) and Shapiro–Wilk tests, respectively. For variables that did not satisfy variance and distributional assumptions of ANOVA even after transformation, the nonparametric Kruskal-Wallis test was used to analyze these variables. The Tukey HSD test (α = 0.05) was used for all other variables to compare differences among means. Statistical analysis was not conducted on the data from the common garden plot study, as only two replicates of each cultivar were available each of the two years that tissues were sampled.
Cutting frequency and cutting height study
Research results
The total phytoestrogen concentration in red clover varied based on cutting frequency (3X vs 5X) but not cutting height (ANOVA, frequency (F): p<0.01, height (H): p=0.69). Total phytoestrogen concentration in red clover was higher in 5X than in 3X cutting (Fig. 2A). In white clover, total phytoestrogen concentration was not influenced by either cutting frequency or cutting height (ANOVA, F: p=0.06; H: p=0.17, Fig. 2B). 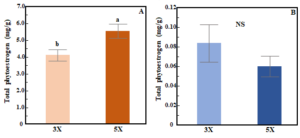 Figure 2. Bar chart showing effects of cutting frequency on total phytoestrogen concentration in red clover (A) and white clover (B). Whiskers indicate ±1 SE of the mean (n = 24 for 3X and 40 for 5X). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test. NS indicates no significant differences.
Figure 2. Bar chart showing effects of cutting frequency on total phytoestrogen concentration in red clover (A) and white clover (B). Whiskers indicate ±1 SE of the mean (n = 24 for 3X and 40 for 5X). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test. NS indicates no significant differences.
The concentration of total phytoestrogens in red clover was approximately 73 times higher compared to white clover. Formononetin and biochanin A were the most abundant phytoestrogens in both species, accounting for 85% (red clover) and 65% (white clover) of the total (Fig. 3). In red clover, formononetin alone accounted for nearly half of the total phytoestrogen content. Prunetin concentration was low in both clover species, while coumestrol was only reported in white clover and made up just 4% of the total phytoestrogen content.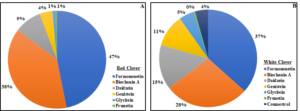 Figure 3. Pie chart showing the percentage of individual phytoestrogen compounds in red clover (A) and white clover (B).
Figure 3. Pie chart showing the percentage of individual phytoestrogen compounds in red clover (A) and white clover (B).
Concentrations of individual phytoestrogen compounds in red clover showed contrasting responses to cutting frequency. Formononetin concentration was 56% higher in 5X compared to 3X (ANOVA, F: p<0.01, Fig. 4A). In contrast, concentrations of daidzein (ANOVA, F: p<0.01, Fig. 4D) and glycitein (ANOVA, F: p<0.05, Fig. 4F) were 19.8% and 19.4% higher, respectively in 3X compared to 5X. Cutting frequency did not influence the concentration of biochanin A, genistein, or prunetin in red clover (ANOVA, F: p>0.05, Fig. 4B, C, and E). Concentrations of individual phytoestrogen compounds in red clover did not differ by cutting height (ANOVA, H: p>0.05) and there were no interactions between cutting frequency and height (ANOVA, F×H: p>0.05). There was no effect of cutting frequency and cutting height on individual phytoestrogen concentrations in white clover (ANOVA, F: p>0.05, H: p>0.05, data not shown).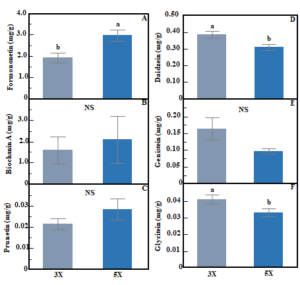 Figure 4. Bar graph showing effect of cutting frequency on individual phytoestrogen concentration in red clover; formononetin (A), biochanin A (B), prunetin (C), daidzein (D), genistein (E), and glycitein (F). Whiskers indicate ±1 SE of the mean (n = 24 for 3X and 40 for 5X). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test. NS indicates no significant differences.
Figure 4. Bar graph showing effect of cutting frequency on individual phytoestrogen concentration in red clover; formononetin (A), biochanin A (B), prunetin (C), daidzein (D), genistein (E), and glycitein (F). Whiskers indicate ±1 SE of the mean (n = 24 for 3X and 40 for 5X). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test. NS indicates no significant differences.
Phytoestrogen concentration over the harvest season
In both red clover and white clover, total phytoestrogen concentrations in the 5X treatment varied dramatically over the growing season. In red clover, the total phytoestrogen concentration increased by 89.7% in second cutting compared to the first cutting. The highest concentrations of total phytoestrogens in red clover were observed in the second and third cuttings (ANOVA, cutting time: p<0.01, Fig. 5A).
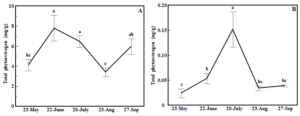 Figure 5. Line graph showing effects of cutting time on total phytoestrogen concentration in red clover (A) and white clover (B) in the 5X treatment. Whiskers indicate ±1 SE of the mean (n = 8). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test.
Figure 5. Line graph showing effects of cutting time on total phytoestrogen concentration in red clover (A) and white clover (B) in the 5X treatment. Whiskers indicate ±1 SE of the mean (n = 8). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test.
A somewhat similar pattern was observed in white (ANOVA, cutting time: p<0.01, Fig. 5B). Total phytoestrogen concentration at the third cutting time was more than eight times higher than the first cutting and nearly three times higher compared to the second cutting but dropped dramatically in the fourth and fifth cuttings (Fig. 5B). Total phytoestrogen concentrations did not differ among cutting times in the 3X treatment in either red clover or white clover (ANOVA, cutting time: p>0.05, data not shown).
Discussion
These results reveal large differences in phytoestrogen concentrations between two commonly grown clover species. Phytoestrogen concentrations were 73 times higher in red clover compared to white clover and this result is in line with previous studies [26,32]. The total phytoestrogen concentration (5.01 mg/g) and relative proportion of different individual phytoestrogen compounds in red clover are comparable with those observed in a previous greenhouse study conducted on the same cultivar [33]. Formononetin and biochanin A are two most abundant phytoestrogens in both clover species, and this finding is consistent with other studies [32,34,35]. We observed 34.6% higher total phytoestrogen concentration in red clover cut more frequently (5X) compared to when it was cut less frequently (3X). After each cutting, plants regrow their leaves to restore their photosynthetic area. In the 5X treatment, aboveground biomass was cut more frequently, and plants had less time to recover, which likely altered the physiological activity of plants by interrupting their energy supply and triggering the re-distribution of assimilates and mobilization of carbohydrate reserves [36]. Thus, the frequent cutting likely results in a stress response in red clover that may trigger upregulation of phytoestrogen production. In the case of white clover, total phytoestrogen concentration was not influenced by cutting frequency. Variation between the two clover species in their phytoestrogen response to cutting frequency could be due to variation in the capacity to utilize root reserves as well as their growth pattern [37,38]. White clover grows laterally and is shorter compared to red clover, and so it may be less stressed by cutting than is red clover because more leaf area remains undamaged [39]. Previous studies have reported that isoflavone concentrations in red clover can increase soon after defoliation [40,41]. After a cutting event, both species rely on their remaining leaf area and root reserves to recover [42,43]; however, in red clover less leaf area and stem material remains undamaged compared to white clover. Interestingly, however, we did not find an effect of cutting height on phytoestrogen accumulation in either species. It is possible that the difference in cutting height between our treatments (5cm vs 10cm residual height) was not great enough to result in substantive differences residual leaf area or root reserves, and therefore a stress response in terms of their production of phytoestrogens. Unfortunately, we did not measure residual leaf area (or root reserves) after any of the harvests.
We observed striking differences in phytoestrogen concentrations at different cutting times in the 5X treatment. The variation in isoflavone concentration at different cutting times over the growing season may have been associated with variation in the weather [44]. In our previous research on red clover, we found that drought stress more than doubled the phytoestrogen levels in red clover [33]. During the second and third cutting times, air temperatures were relatively high, and there was relatively low precipitation, which could have led to short-term drought conditions that increased the phytoestrogen levels in the plants. It is also possible that concentrations of some phytoestrogen compounds are naturally dynamic. In previous research on red clover, genistein and daidzein content were observed to increase from June to July, while biochanin A and formononetin concentrations peaked in early September [45].
Elevated temperature and CO2study
Phytoestrogen concentrations
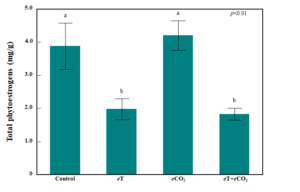
Effects of the temperature and CO2 treatments were visually apparent in both legume species (Figure 6). Phytoestrogen concentrations in cowpea were below the detection level regardless of treatment (data not shown). In contrast, phytoestrogen concentrations in red clover were well above the detection level and influenced by treatment (ANOVA, treatment: p<0.01; Figure 7). Compared to red clover grown under the ambient control conditions, elevated temperature (eT), both when applied alone and in combination with elevated CO2 (eCO2), resulted in a 50% and 53% reduction in total phytoestrogen concentration, respectively. Total phytoestrogen concentration in red clover did not differ compared to the ambient control when eCO2 was applied alone. Figure 6. Representative photographs of cowpea (upper row) and red clover (lower row) in each temperature and CO2 treatment following 10 days of exposure.
Figure 6. Representative photographs of cowpea (upper row) and red clover (lower row) in each temperature and CO2 treatment following 10 days of exposure.
Figure 7. Bar graph showing total phytoestrogen concentration in red clover (mg/g DM) after 10 days of exposure to eT and eCO2. Bars indicate standard errors of the mean (n = 4). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test.
In red clover, individual phytoestrogen compounds varied in their responses to the temperature and CO2 treatments (Figure 8). Compared to the ambient control, concentrations of formononetin were 49% and 47% lower, respectively, in the eT and eT+eCO2 conditions (ANOVA, treatment: p<0.05, Figure 8a). Similarly, biochanin A concentrations were 59% lower in the eT and 63% lower in eT+eCO2 compared to the control (ANOVA, treatment: p<0.05, Figure 8b). The concentration of daidzein was reduced by 43% in eCO2 and 42% in eT+eCO2 condition (ANOVA, treatment: p<0.01, Figure 8c). Genistein, glycitein and prunetin concentrations were not influenced by the treatments.
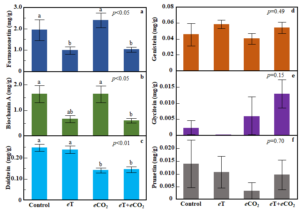 Figure 8. Effects of exposure to eT and eCO2 on concentrations (mg/g DM) of individual isoflavones formononetin (a), biochanin A (b), daidzein (c), genistein (d), glycitein (e), and prunetin (f) in red clover. Bars indicate standard errors of the mean (n = 4). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test.
Figure 8. Effects of exposure to eT and eCO2 on concentrations (mg/g DM) of individual isoflavones formononetin (a), biochanin A (b), daidzein (c), genistein (d), glycitein (e), and prunetin (f) in red clover. Bars indicate standard errors of the mean (n = 4). Treatments sharing the same letter are not significantly different (α = 0.05) as determined by Tukey’s HSD test.
Discussion
To the best of our knowledge, ours is the first study to quantify phytoestrogen concentration in the vegetative component of cowpea. While previous research has demonstrated that cowpea seeds contain low concentrations of phytoestrogens [46,47], and that concurrent application of high temperatures and increased levels of CO2 can decrease isoflavone concentrations in seeds of other legumes such as soybean [48], we found phytoestrogen concentrations in cowpea vegetative tissues to be below the level of detection. Whether cowpea might upregulate phytoestrogen concentrations in its vegetative tissues under other types of environmental stress, such as drought or pest damage, or how eT or eCO2 may influence phytoestrogen concentrations in cowpea seeds, remains unknown.
In contrast to cowpea, red clover had relatively high total concentrations of phytoestrogens (3.9 mg/g in the ambient control) and these concentrations were sensitive to eT. Compared to the control treatment, eT alone and in combination with eCO2 reduced total phytoestrogen concentration by 50% (2.0 mg/g) and 53% (1.8 mg/g), respectively. Much of this total was made up of formononetin and biochanin A, which together accounted for 83-96% of the total phytoestrogen measured, depending on treatment, which is in line with previous studies conducted on red clover [33,44]. Baseline formononetin concentrations in red clover can vary depending on a range of factors, including cultivar, plant maturity, and growing conditions [49,50]. Formononetin concentrations we observed in red clover grown under our ambient control treatment conditions were lower than those observed in a previous greenhouse study conducted on the same cultivar at similar maturity (3.2 mg/g), possibly due to differences in the ambient growing conditions in the climate-controlled growth chamber compared to those in a greenhouse [33,44].
Our finding that eT depressed total phytoestrogen production in red clover vegetative tissues is congruent with previous research examining the effects of temperature on phytoestrogen contents of soybean seeds and pods [48,51,52]. The decrease in isoflavone synthesis in some legumes under heat stress may occur as plants prioritize resources to synthesize heat shock proteins and to activate other protective mechanisms at the expense of isoflavone synthesis [53]. Determining whether this mechanism explains our observations in red clover requires further research. Isoflavone synthesis through the phenylpropanoid pathway requires carbon, with about 20% of photosynthetic carbon going toward making phenolic compounds, including flavonoids [54,55]. In soybean, increased levels of CO2 has been shown to increase isoflavone concentrations in seeds and can offset the declines in concentrations due to high temperature [48,52]. We observed that red clover exposed to eCO2 either alone or in combination with eT did not appreciably increase formononetin and biochanin A concentrations, suggesting that synthesis of these compounds was not carbon limited.
Objective 3: Disseminate phytoestrogen data to stakeholders.
We have prepared two manuscripts from this project for submission to peer-reviewed journals. One manuscript, which we plan to submit to the journal Crop Science, reports on the effects of cutting frequency and height phytoestrogen levels in red clover and white clover. The other manuscript, which reports the effects of elevated temperature and CO2 on phytoestrogens in red clover and cowpea, will be submitted to Scientific Reports. We are also working with UNH Extension to publish an extension article reporting the phytoestrogen concentrations of the 12 forage legume cultivars grown in our common garden plots (Table 1).
Table 1: Concentrations of phytoestrogen compounds (mg/g DM) and total phytoestrogens measured in different forage legume species and cultivars grown for two seasons in Madbury, NH. Concentrations were measured each August in 2022 and 2023. The values are means (1 standard deviation), n = 4.
|
Species |
Cultivar |
Formn. |
BioA. |
Daid. |
Gen. |
Gly. |
Prn. |
Kaemp. |
TotalP |
|
Red clover |
Freedom |
2.0 (0.83) |
1.69 (0.89) |
0.31 (0.08) |
0.05 (0.04) |
0.03 (0.01) |
0.02 (0.01) |
nd |
4.10 (1.74) |
|
Red clover |
Ruby Red |
3.1 (0.65) |
1.45 (0.73) |
0.38 (0.14) |
0.07 (0.04) |
0.04 (0.01) |
0.03 (0.01)
|
nd |
5.07 (1.07) |
|
Red clover |
Blaze |
3.1 (2.77) |
2.14 (2.08) |
0.40 (0.14) |
0.07 (0.05) |
0.04 (0.02) |
0.04 (0.03)
|
nd |
5.75 (4.90) |
|
Alfalfa |
Viking 3800 |
nd |
nd |
nd |
nd |
nd |
nd |
|
nd |
|
3White clover |
Alice |
0.1 (0.1) |
0.054 (0.09) |
nd |
0.01 (0.1) |
nd |
nd |
nd |
0.13 (0.22) |
|
2White clover |
Ladino |
0.15 (0.14) |
0.131 (0.16) |
nd |
0.011 (0) |
nd |
nd |
nd |
0.29 (0.29) |
|
Birdsfoot Trefoil |
VNS (ALS) |
0.015 (0.03) |
nd |
nd |
nd |
nd |
nd |
nd |
0.015 (0.03) |
|
Birdsfoot Trefoil |
Bull |
nd |
0.004 (0.01) |
0.001 (0) |
0.001 (0) |
0.004 (0.01) |
nd |
nd |
0.01 (0.02) |
|
3Kura clover |
VNS (ALS) |
0.04 (0.02) |
0.1 (0.01) |
0.01 (0.001) |
nd |
0.004 (0) |
nd |
nd |
0.06 (0.02) |
|
Kura clover |
VNS (WSH) |
0.31 (0.41) |
0.2 (0.28) |
0.02 (0.02) |
0.01 (0.01) |
0.002 (0.004) |
0.01 (0.01) |
nd |
0.55 (0.70) |
|
Aberlasting |
- |
0.09 (0.07) |
0.04 (0.04) |
0.01 (0.01) |
0.01 (0.01) |
0.01 (0.01) |
nd |
0.07 (0.12) |
0.22 (0.17) |
|
2Alsike clover |
VNS |
0.01 (0.01) |
0.01 (0.01) |
0.01 (0.1) |
0.004 (0.01) |
0.012 (0.02) |
nd |
nd |
0.038 (0.05) |
|
*Subterranean clover |
Variety not stated |
1.0 (0.42) |
1.1 (0.48) |
0.09 (0.01) |
0.93 (0.27) |
0.04 (0.01) |
nd |
nd |
3.13 (0.96) |
Data are based on two replicates sampled over two years (n=4), except 2, 2-two replicates and 3, 3-three replicates; * grown in greenhouse conditions; Phytoestrogen compounds are Formn.-Formononetin, BioA.-Biochanin A, Daid.-Daidzein, Gen.-Genistein, Gly.-Glycitein, Prn.-Prunetin, Kaemp.-Kaempferol, and TotalP.-Total Phytoestrogens.
References
- Greene, C.; McBride, W. Consumer Demand for Organic Milk Continues to Expand—Can the US Dairy Sector Catch Up? Choices 2015, 30, 1–6.
- Pereira, A.B.D.; Brito, A.F.; Townson, L.L.; Townson, D.H. Assessing the Research and Education Needs of the Organic Dairy Industry in the Northeastern United States. J. Dairy Sci. 2013, 96, 7340–7348.
- Li, X.; Peterson, H.H.; Xia, T. Demand for Organic Fluid Milk across Marketing Channels. Agric. Resour. Econ. Rev. 2018, 47, 505–532.
- Paulson, J.; Jung, H.; Raeth-Knight, M.; Linn, J. Grass vs. Legume Forages for Dairy Cattle. In Proceedings of the Proceedings of the 69th Annual Minnesota Nutrition Conference, Owatonna, MN. 16–17 Sept.; University of Minnesota. Minnesota Extension Service: Owatonna, MN, 2008; pp. 119–133.
- Kuhnen, S.; Stibuski, R.B.; Honorato, L.A. Farm Management in Organic and Conventional Dairy Production Systems Based on Pasture in Southern Brazil and Its Consequences on Production and Milk Quality. Animals 2015, 5, 479–494.
- Steinshamn, H. Effect of Forage Legumes on Feed Intake, Milk Production and Milk Quality–a Review. Anim. Sci. Pap. reports 2010, 28, 195–206.
- Brito, A.F.; Lange, M.J.; Lange, M.J. The Key Role of Forage Legumes in Organic Dairy Diets: Effects on Your Bottom Line Available online: https://nodpa.com/n/945/The-Key-Role-of-Forage-Legumes-in-Organic-Dairy-Diets-Effects-on-Your-Bottom-Line.
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing Soil Fertility in Organic Farming Systems. Soil use Manag. 2002, 18, 239–247.
- Carlsson, G.; Huss-Danell, K. Nitrogen Fixation in Perennial Forage Legumes in the Field. Plant Soil 2003, 253, 353–372.
- Sarelli, L.; Tuori, M.; Saastamoinen, I.; Syrjälä-Qvist, L.; Saloniemi, H. Phytoestrogen Content of Birdsfoot Trefoil and Red Clover: Effects of Growth Stage and Ensiling Method. Acta Agric. Scand. - Sect. A Anim. Sci. 2003, 53, 58–63.
- Woclawek-Potocka, I.; Piskula, M.K.; Bah, M.M.; Siemieniuch, M.J.; Korzekwa, A.; Brzezicka, E.; Skarzynski, D.J. Concentrations of Isoflavones and Their Metabolites in the Blood of Pregnant and Non-Pregnant Heifers Fed Soy Bean. J. Reprod. Dev. 2008, 54, 807300086.
- Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago Sativa L.) Populations. Agronomy 2020, 10, 87.
- Hashem, N.M.; Soltan, Y.A. Impacts of Phytoestrogens on Livestock Production: A Review. Egypt. J. Nutr. Feed. 2016, 19, 81–89.
- Retana-Márquez, S.; Hernández, H.; Flores, J.A.; Muñoz-Gutiérrez, M.; Duarte, G.; Vielma, J.; Fitz-RodrÃguez, G.; Fernández, I.G.; Keller, M.; Delgadillo, J.A. Effects of Phytoestrogens on Mammalian Reproductive Physiology. Trop. Subtrop. Agroecosystems 2011, 15, S129–S145.
- Tucker, H.A.; Knowlton, K.F.; Meyer, M.T.; Khunjar, W.O.; Love, N.G. Effect of Diet on Fecal and Urinary Estrogenic Activity. J. Dairy Sci. 2010, 93, 2088–2094.
- Gierus, M.; Koch, M.; Schulz, H. Phytoestrogen Carryover into Cow’s Milk from Legumes-an Overview along the Food Chain. Berichte über Landwirtschaft 2012, 90, 354–379.
- De Rijke, E.; Aardenburg, L.; Van Dijk, J.; Ariese, F.; Ernst, W.H.O.; Gooijer, C.; Brinkman, U.A.T. Changed Isoflavone Levels in Red Clover (Trifolium Pratense L.) Leaves with Disturbed Root Nodulation in Response to Waterlogging. J. Chem. Ecol. 2005, 31, 1285–1298.
- Gerard, P.J.; Crush, J.R.; Hackell, D.L. Interaction between Sitona Lepidus and Red Clover Lines Selected for Formononetin Content. Ann. Appl. Biol. 2005, 147, 173–181.
- Carlsen, S.C.K.; Understrup, A.; Fomsgaard, I.S.; Mortensen, A.G.; Ravnskov, S. Flavonoids in Roots of White Clover: Interaction of Arbuscular Mycorrhizal Fungi and a Pathogenic Fungus. Plant Soil 2008, 302, 33–43.
- Strauss, L.; Santti, R.; Saarinen, N.; Streng, T.; Joshi, S.; Mäkelä, S. Dietary Phytoestrogens and Their Role in Hormonally Dependent Disease. Toxicol. Lett. 1998, 102, 349–354.
- Reed, K.F.M. Fertility of Herbivores Consuming Phytoestrogen-Containing Medicago and Trifolium Species. Agriculture 2016, 6, 35.
- Hoeck, J.A.; Fehr, W.R.; Murphy, P.A.; Welke, G.A. Influence of Genotype and Environment on Isoflavone Contents of Soybean. Crop Sci. 2000, 40, 48–51.
- Tsao, R.; Papadopoulos, Y.; Yang, R.; Chris Young, J.; Mcrae, K. Isoflavone Profiles of Red Clovers and Their Distribution in Different Parts Harvested at Different Growing Stages. J. Agric. Food Chem. 2006, 54, 5797–5805.
- Saviranta, N.M.M.; Anttonen, M.J.; Von Wright, A.; Karjalainen, R.O. Red Clover (Trifolium Pratense L.) Isoflavones: Determination of Concentrations by Plant Stage, Flower Colour, Plant Part and Cultivar. J. Sci. Food Agric. 2008, 88, 125–132.
- Butkutė, B.; Lemežienė, N.; Dabkevičienė, G.; Jakštas, V.; Vilčinskas, E.; Janulis, V. Source of Variation of Isoflavone Concentrations in Perennial Clover Species. Pharmacogn. Mag. 2014, 10, S181–S188.
- Saloniemi, H.; Kallela, K.; Saastamoinen, I. Study of the Phytoestrogen Content of Goat’s Rue (Galega Orientalis), Alfalfa (Medicago Sutiva) and White Clover (Trifolium Repens). Agric. Food Sci. 1993, 2, 517–524.
- Höjer, A.; Adler, S.; Purup, S.; Hansen-Møller, J.; Martinsson, K.; Steinshamn, H.; Gustavsson, A.-M. Effects of Feeding Dairy Cows Different Legume-Grass Silages on Milk Phytoestrogen Concentration. J. Dairy Sci. 2012, 95, 4526–4540.
- Gierus, M.; Kleen, J.; Loges, R.; Taube, F. Forage Legume Species Determine the Nutritional Quality of Binary Mixtures with Perennial Ryegrass in the First Production Year. Anim. Feed Sci. Technol. 2012, 172, 150–161.
- Sanderson, M.A.; Stair, D.W.; Hussey, M.A. Of Perennial Forages to Stress. Adv. Agron. 1997, 59, 171.
- Rossiter, R.C. Physiological and Ecological Studies on the Oestrogenic Isoflavones in Subterranean Clover (T. Subterraneum L.). VI. Effects of Defoliation Including Grazing. Aust. J. Agric. Res. 1969, 20, 25–35.
- Payette, M.; Lima, M.R.M.; Coleman, W.M.; Ashraf-Khorassani, M. Separation Optimization and Quantitative Analysis of Phytoestrogens Employing Reverse-Phase High-Performance Liquid Chromatography with UV-VIS Detection. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 888–896.
- Andersen, C.; Weisbjerg, M.R.; Hansen-Mller, J.; Sejrsen, K. Effect of Forage on the Content of Phyto-Oestrogens in Bovine Milk. Animal 2009, 3, 617–622.
- Mandal, P.; Mortensen, D.A.; Brito, A.F.; Wallingford, A.K.; Lima, M.R.M.; Warren, N.D.; Smith, R.G. Water Stress Influences Phytoestrogen Levels in Red Clover (Trifolium Pratense) but Not Kura Clover (T. Ambiguum). J. Agric. Food Chem. 2024, 72, 10247–10256.
- Mustonen, E.; Tuori, M.; Kurki, P.; Isolahti, M.; Taponen, J.; Vanhatalo, A. Variety, Time of Harvest and Conditions during Growing Season Have Impact on Red Clover Isoflavone Content. Agric. Food Sci. 2018, 27, 102–109.
- Tucak, M.; Popović, S.; Horvat, D.; Čupić, T.; Krizmanić, G.; Viljevac Vuletić, M.; Ravlić, M. The Characterization of Isoflavone Content in the Croatian Red Clover Collection. Poljoprivreda 2019, 25, 3–11.
- Briske, D.D.; Richards, J.H. Physiology of Plants Recovering from Defoliation. In Proceedings of the Proceedings of the XVII international grassland congress; 1993; p. 85.
- Li, R.; Volenec, J.J.; Joern, B.C.; Cunningham, S.M. Seasonal Changes in Nonstructural Carbohydrates, Protein, and Macronutrients in Roots of Alfalfa, Red Clover, Sweetclover, and Birdsfoot Trefoil. Crop Sci. 1996, 36, 617–623.
- Moot, D.; Black, A.; Lyons, E.M.; Egan, L.M.; Hofmann, R.W. Pasture Resilience Reflects Differences in Root and Shoot Responses to Defoliation, and Water and Nitrogen Deficits.; New Zealand Grassland Association, 2021.
- Patterson, J.D.; Laidlaw, A.S.; McBride, J. The Influence of Autumn Management and Companion Grass on the Development of White Clover over Winter in Mixed Swards. Grass Forage Sci. 1995, 50, 345–352.
- McMurray, C.H.; Laidlaw, A.S.; McElroy, M. The Effect of Plant Development and Environment on Formononetin Concentration in Red Clover (Trifolium Pratense L.). J. Sci. Food Agric. 1986, 37, 333–340.
- Mustonen, E.A.; Tuori, M.; Saastamoinen, I.; Taponen, J.; Wähälä, K.; Saloniemi, H.; Vanhatalo, A. Equol in Milk of Dairy Cows Is Derived from Forage Legumes Such as Red Clover. Br. J. Nutr. 2009, 102, 1552–1556.
- Meuriot, F.; Avice, J.-C.; Simon, J.-C.; Laine, P.; Decau, M.-L.; Ourry, A. Influence of Initial Organic N Reserves and Residual Leaf Area on Growth, N Uptake, N Partitioning and N Storage in Alfalfa (Medicago Sativa) during Post-Cutting Regrowth. Ann. Bot. 2004, 94, 311–321.
- Teixeira, E.I.; Moot, D.J.; Brown, H.E.; Pollock, K.M. How Does Defoliation Management Impact on Yield, Canopy Forming Processes and Light Interception of Lucerne (Medicago Sativa L.) Crops? Eur. J. Agron. 2007, 27, 154–164.
- Sivesind, E.; Seguin, P. Effects of the Environment, Cultivar, Maturity, and Preservation Method on Red Clover Isoflavone Concentration. J. Agric. Food Chem. 2005, 53, 6397–6402.
- Booth, N.L.; Overk, C.R.; Yao, P.; Totura, S.; Deng, Y.; Hedayat, A.S.; Bolton, J.L.; Pauli, G.F.; Farnsworth, N.R. Seasonal Variation of Red Clover (Trifolium Pratense L., Fabaceae) Isoflavones and Estrogenic Activity. J. Agric. Food Chem. 2006, 54, 1277–1282.
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. Maryl. US Dep. Agric. 2008, 15, 21.
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and Lignans in Legumes: Nutritional and Health Aspects in Humans. J. Nutr. Biochem. 1998, 9, 193–200.
- Caldwell, C.R.; Britz, S.J.; Mirecki, R.M. Effect of Temperature, Elevated Carbon Dioxide, and Drought during Seed Development on the Isoflavone Content of Dwarf Soybean [Glycine Max (L.) Merrill] Grown in Controlled Environments. J. Agric. Food Chem. 2005, 53, 1125–1129.
- Petrović, M.; Sokolović, D.; Babić, S.; Vymyslický, T.; Marković, J.; Zornić, V.; Dajić-Stevanović, Z. Isoflavones of the Red and Hungarian Clover and Possible Impact on Animal Diet. Czech J. Food Sci. 2021, 39, 169–175.
- Papadopoulos, Y.A.; Tsao, R.; McRae, K.B.; Mellish, A.E.; Fillmore, S.A.E. Genetic Variability of Principal Isoflavones in Red Clover. Can. J. Plant Sci. 2006, 86, 1345–1347.
- Chennupati, P.; Seguin, P.; Chamoun, R.; Jabaji, S. Effects of High-Temperature Stress on Soybean Isoflavone Concentration and Expression of Key Genes Involved in Isoflavone Synthesis. J. Agric. Food Chem. 2012, 60, 12421–12427.
- Kim, S.; Jung, W.; Ahn, J.; Kim, J.; Chung, I. Quantitative Analysis of the Isoflavone Content and Biological Growth of Soybean (Glycine Max L.) at Elevated Temperature, CO2 Level and N Application. J. Sci. Food Agric. 2005, 85, 2557–2566.
- Reddy, P.S.; Chakradhar, T.; Reddy, R.A.; Nitnavare, R.B.; Mahanty, S.; Reddy, M.K. Role of Heat Shock Proteins in Improving Heat Stress Tolerance in Crop Plants BT - Heat Shock Proteins and Plants. In; Asea, A.A.A., Kaur, P., Calderwood, S.K., Eds.; Springer International Publishing: Cham, 2016; pp. 283–307 ISBN 978-3-319-46340-7.
- Tsai, C.; Harding, S.A.; Tschaplinski, T.J.; Lindroth, R.L.; Yuan, Y. Genome‐wide Analysis of the Structural Genes Regulating Defense Phenylpropanoid Metabolism in Populus. New Phytol. 2006, 172, 47–62.
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid Biosynthesis and Its Regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257.
- Red clover has the highest phytoestrogen concentrations among the perennial forage legumes we investigated.
- When red clover was cut more frequently (5X/growing season), total phytoestrogen concentration increased by 35% compared to when it was cut less frequently (3X/growing season).
- Cutting frequency did not influence phytoestrogen concentration in white clover.
- Cutting height (5 cm or 10 cm) did not influence phytoestrogens concentrations in either red clover or white clover.
- Total phytoestrogen concentration in red clover was around 73 times higher compared to white clover.
- Formononetin and biochanin A were the most abundant phytoestrogens in red clover and white clover, accounting for 85% and 65% of their total phytoestrogen content, respectively.
- Concentrations of total phytoestrogens in red clover were reduced by 50% under conditions of elevated temperature and in the absence of additional stress factors such as drought.
In summary, our results show that under the conditions of our study, red clover contains much higher phytoestrogen levels than white clover, and adjusting defoliation frequency could help manage livestock intake of these compounds. In addition, heat waves may lower phytoestrogen levels in red clover. Lastly, our study provides the only evidence we are aware of that cowpeas used as forage are unlikely to expose livestock to phytoestrogens.
Education & outreach activities and participation summary
Participation summary:
We presented our research results at the University of New Hampshire (UNH) Graduate Research Conference in May 2024 and at the UNH Department of Agriculture, Nutrition, and Food Systems departmental seminar in November 2023. An abstract from this research was accepted for a presentation at the Tri-societies annual meeting in 2024 in San Antonio, Texas. In addition, an abstract reporting results from the elevated temperature and CO2 study was also submitted to the American Forage and Grassland Conference that will be held in 2025 in Kissimmee, Florida. Manuscripts in-review or in preparation with the support of this grant include:
- Mandal P, Lima MRM, Wallingford AK, Mortensen DA, Warren ND, Brito AF, & Smith RG. Influence of defoliation frequencies and intensity on phytoestrogens concentration in red clover and white clover (In preparation).
- Mandal P, Lima MRM, Wallingford AK, Mortensen DA, Warren ND, Brito AF, & Smith RG. Assessment of the interactive effects of elevated temperature and CO2 on phytoestrogen concentrations in red clover and cowpea (In preparation).
Project Outcomes
This research provided data on phytoestrogen levels in different forage legume species grown in New England. It also explored how forage management practices and climate change factors, including higher temperature and elevated CO2, affect these levels. The findings will help dairy and livestock producers in the region manage phytoestrogen exposure from forage legumes more effectively for sustainable livestock production.
This project generated data and understanding on how forage harvesting practices influence the phytoestrogen concentrations of red clover and white clover, two commonly grown perennial forage legumes. We also determined that cowpea vegetative tissues are non-phytoestrogenic. Our study sheds light on how climate change factors (elevated temperature and CO2) may influence the phytoestrogen levels of red clover. Lastly, this research reports the range of phytoestrogen concentrations in a variety of perennial forage legume species and cultivars commonly grown in the Northeast region. Overall, this project provides farmers and other stakeholders with valuable information on phytoestrogen concentrations in forage legumes common to the Northeast and elsewhere so they can better anticipate and manage risks to livestock.
A challenge/limitation of this study was that all the data come from a single site (the UNH Kingman Research Farm in Madbury, NH). Future research could examine the effects of cutting frequency and cutting height across multiple sites to illuminate potential interactions between environmental factors and management practices. We also do not fully understand the effects of phytoestrogens on cow reproductive health. Effects likely vary based on many factors, on both the animal side as well as the forage side, which have yet to be elucidated.