Report for GNE22-303 Version 2
Project Information
The brown marmorated stink bug (BMSB) is an invasive insect that is a major agricultural pest in the United States and other countries. In the Mid-Atlantic, BMSB reached outbreak levels in 2010 and has caused severe damage to fruit, vegetable, and field crops since. Several native stink bugs persist in agroecosystems as well, and BMSB has altered community dynamics of these species and their natural enemies. For instance, Trissolcus japonicus, a nonnative egg-parasitoid that is closely associated with BMSB in Asia, has established populations in 15 known US states and DC since 2014, and its establishment is likely due to availability of BMSB egg masses. The arrival of T. japonicus has community-wide effects, as the parasitoid also parasitizes other pentatomid eggs and competes with endemic parasitoids for reproductive resources.
In this project, we will evaluate how habitat diversity and background pentatomid abundances influence stink bug-parasitoid food-webs. We will survey four species BMSB and three native stink bugs (two pests and one beneficial) and their associated parasitoids in four different habitat-types in New Jersey—peach-dominated, soybean-dominated, mixed-crop with flowers, and forest. Egg parasitoids will be surveyed via field-deployed sentinel egg masses, and host-parasitoid interactions will be classified as successful (adult parasitoid emergence) and unsuccessful (partially developed parasitoid) parasitism. We will compare parasitoid abundance and species richness and food-web metrics, such as linkage density, to compare control of stink bug pests in different habitats and inform management strategies aimed to maximize ecological pest control.
- Survey and compare abundances of four pentatomid species—BMSB, BSB, HB, and SSB—in four habitat types in New Jersey—peach-dominated, soybean-dominated, mixed crop, and forest.
- Assess successful and unsuccessful parasitism and predation of sentinel eggs of BMSB, BSB, HB, and SSB in four habitat types in New Jersey—peach-dominated, soybean-dominated, mixed crop, and forest. Identify parasitoid species using species identification keys and molecular techniques such as DNA barcoding.
- Compare abundance and diversity of the parasitoid complex across habitats
- Compare incidence of parasitism in eggs between pentatomid species and habitat type
- Calculate food-web complexity matrices across habitats and compare them between habitat types
The purpose of this project is to understand how habitat impacts the abundance of pentatomids—or stink bugs—and parasitism of their eggs. This project is applicable to sustainable agriculture because it will inform management strategies to increase ecological pest control of stink bug pests, mainly Halyomorpha halys, the brown marmorated stink bug (BMSB). BMSB is an invasive insect from Southeast Asia that was first detected in Pennsylvania in the 1990s. It is a major agricultural pest of fruit, vegetable, and row crops, including peach and soybean, and causes agricultural and nuisance problems in 9 of the 13 states and territory incorporated in Northeast SARE.1,2
In our study, we will monitor abundances of BMSB, two endemic stink bug pests—Euschistus servus (the brown stink bug, BSB) and Murgantia histrionica (the harlequin bug, HB)—and an endemic beneficial predator, Podisus maculiventris (the spined soldier bug, SSB). These four species are commonly found in Mid-Atlantic agroecosystems and their abundances can be monitored with pheromone-baited traps. We will monitor pentatomid population levels while simultaneously monitoring their egg-parasitoids with sentinel egg masses laid by lab-maintained colonies. We will retrieve sentinels from the field and monitor for successful (adult parasitoid emerges from egg) and unsuccessful (partially developed parasitoid in egg) parasitism, as both result in the death of the host. We will identify parasitoid species with identification keys and molecular forensics. Additionally, we will note evidence of egg predation in sentinel egg masses, although it will not be possible to ascertain which predator species is responsible for damage, so our analyses will focus primarily on parasitoids. We will conduct our surveys in August 2022, as August coincides with peaks in pentatomid and parasitoid abundances.3,4
We expect greatest parasitoid abundance and diversity at sites with high stink bug abundance and diversity. Additionally, we expect habitat to influence the composition of the parasitoid complex. We will look at two low-diversity agricultural habitat types (peach-dominated and soybean-dominated), a high-diversity agricultural habitat type (mixed-crop with flowers), and a high-diversity forest habitat type. We expect greatest levels of parasitism in the mixed-crop with flowers habitat because parasitoid wasps feed on nectar, and if our research supports this hypothesis, then our findings will provide farmers an economic incentive to diversify their crops.
Furthermore, we expect relatively high parasitism and the highest numbers of the nonnative parasitoid Trissolcus japonicus (TJ) to occur in the forest based on past sentinel studies.5–7 TJ is BMSB’s primary parasitoid in Southeast Asia; there, in the species’ native range, it parasitizes an average of 50% and up to 85% of BMSB eggs.8 This species had been studied in quarantine facilities since 2007, but has been detected in 15 states and DC since 2014.2,9,10 If we do detect parasitism by TJ in the forested habitats as we predict, this would indicate the importance of maintaining woody habitat adjacent to farmland to maximizing ecological pest control.
Research
Site selection
To address both objectives, we will collect data in August 2022 from the same sites. We will choose two habitat sites of each of the four habitat types—peach-dominated, soybean-dominated, mixed crops, and forest. The sites will be based at Rutgers agricultural research farms in blocks that will not receive insecticide sprays and at Rutgers forested field stations. Habitat sites will be at least 5 km apart.
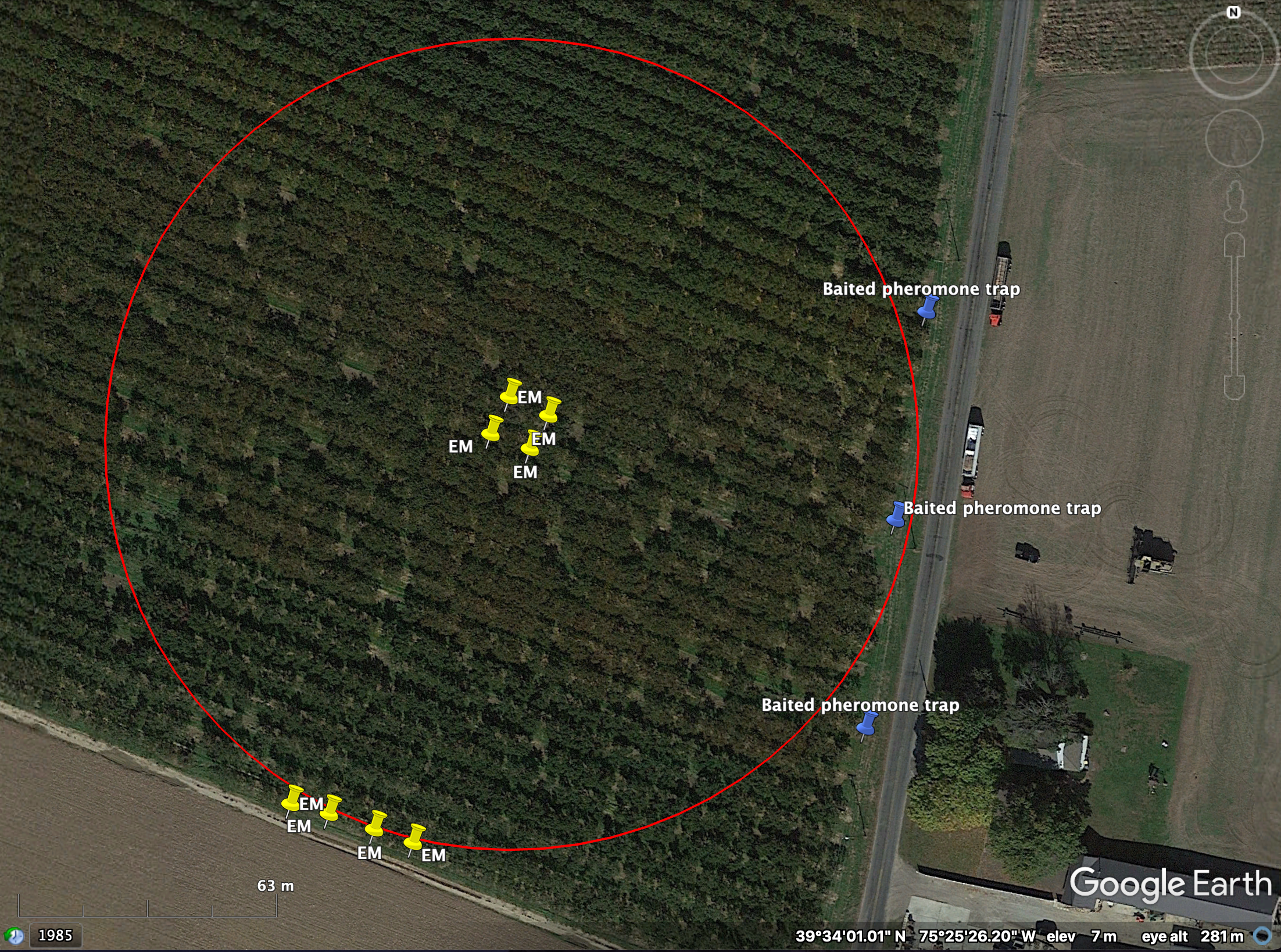
Each habitat site will have two sample sites. Sample sites are defined by a circle with a radius of 100m with an area of 3.14 ha2, and the two sample sites at one habitat site should be within 1km of each other. In the peach- and soybean-dominated habitats, at least 90% of the area in the circle will be continuous peach or soybean monoculture. In the mixed-crop habitat, the 3.14 ha2 plot will be at least 90% covered with crops but will feature a patchwork of mixed crops with at least one flowering crop present (buckwheat, marigold, cut flowers, etc.) and a total crop diversity between 4-6 crops. Forested sample sites will be continuous deciduous forest. In the agricultural sample sites, the plot should intersect with forested and/or manmade structures along the edges of the agricultural land when possible. Location of sample sites will be assigned using Google Earth satellite images and habitat makeup will be ground-truthed in the field to check accuracy of satellite images.
Each sample site will have two locations where sentinels of the four pentatomids will be deployed: at the middle of the 100m radius and at the edge of the radius, preferably at a habitat edge. In the middle of the plot, the sentinels will be placed 10m apart in a square formation. At the edge of the radius, the sentinels will be placed 10m apart along the edge of the agricultural block. The pheromone-baited pheromone traps for monitoring pentatomids will be placed 100m from the center of the sample site in a different direction from the second set of sentinels and will preferably be placed at an edge with woods or human-made structure. These traps will be placed 50m apart. Please see Figure 1 for a schematic of each sample site.
Objective 1
Pentatomid populations will be monitored by pheromone-baited sticky traps. Traps will be deployed at the edge of each of the eight 100m-radius sample sites. Three traps will be deployed to monitor for the four pentatomids and use the following lures: Dead-Inn MDD lure for Euschistus to monitor BSB, custom-ordered pheromone lures for SSB, and the Dead-Inn Xtra Combo Broad Spectrum lure to use to monitor numbers of both BMSB and HB, for both species are attracted by this lure. The lures will be attached via binder clips to the top of 5-ft wooden stakes hammered into the ground. Dead-Inn clear sticky traps will also be attached to the top of the wooden stakes to catch insects that are attracted to the trap. The lures will not need to be replaced for the entirety of the field experiment as per manufacturer guidelines. To ensure maximum capture of bugs, the clear sticky traps will be replaced each week. The numbers of stink bugs in the traps will be recorded each week.
To analyze if habitat type is a significant predictor of pentatomid abundance, we will run a multivariate analysis of variance with habitat type as the predictor variable and abundances of each of the four pentatomids as response variables.
Objective 2
Sentinel egg masses will be sourced from colonies maintained by Emma Waltman and an hourly worker at Rutgers Agricultural Research and Extension Center (RAREC). The BMSB colony was started in summer 2021 from BMSB eggs sourced from the Philip Alampi Beneficial Insect Laboratory in Trenton, NJ. The BSB, HB, and SSB colonies will be started in May-June 2022 from field-caught bugs at RAREC. Colonies of BMSB, BSB, and HB will be maintained in BugDorm insect rearing tents in a greenhouse kept at photoperiod 16:8 (L:D) and 25-30°C. Colonies will be maintained as detailed in Abrams et al. 2020; they will be supplied with black-eyed pea and sunflower plants as a food source, to maintain humidity in the tents, and as an oviposition substrate.16 The insects will also be supplied paper towels lining the tents as an oviposition substrate. Their diets will be supplemented with corn, sunflower seeds, and pumpkin seeds. The SSB colony will be kept in insect cages with black-eyed pea plants as a source of moisture and oviposition substrate. They will be fed with wax worm pupae and given paper towels as an additional oviposition substrate.
Sentinel egg masses will be prepared by carefully cutting fresh (<24 hours old) egg masses and about a dime-size area of the oviposition substrate from the plant or paper towel. They will then be attached via double-sided tape to a 3.5 cm x 3.5 cm piece of cardstock paper labeled with species, date, habitat and sample site, and other identifying characteristics. Egg masses and labels will be photographed and carried into the field via tight-fitting petri dishes. Egg masses will be attached via clothespins to the underside of host plants at the sites (peach, soybean, a known BMSB host plant such as pepper or eggplant at the mixed crop sites, and a known BMSB host plant such as black walnut at the forest sites). Each sentinel will be left in the field for 72 hours. Egg masses of all four pentatomids will be deployed in one peach, one soybean, one mixed-crop, and one forest site on Mondays and the other four habitat sites on Tuesdays of each week and retrieved on Thursdays and Fridays of each week. This will occur for three weeks in August.
Sentinels will remain on their labeled card and be brought back to RAREC. They will be placed in tight-fitting petri dishes and placed in 25°C incubators. They will be monitored every weekday for emergence of pentatomid nymphs and parasitoids. When pentatomid nymphs emerge at approximately 5 days post initial deployment, nymphs will be carefully counted and removed with a paintbrush to ensure that they do not inhibit emergence of parasitoids. The number of missing eggs will also be recorded along with evidence of predation following the egg-fate categories defined by Morrison et al. 2016.17 These categories are: complete chew, incomplete chew, sucking predation with stylet sheath present, sucking predation with puncture injury but no stylet present. After making these classifications, we will put egg masses back into the incubator for 6 weeks to allow for emergence of parasitoids from egg masses. After 6 weeks, egg masses will be frozen to kill parasitoids for identification and preserve unemerged eggs for molecular analysis.
After emergence of parasitoids, emerged parasitoids will be sexed and identified to species using identification keys.18 Remaining eggs that did not have emergence of a stink bug nymph or parasitoid or evidence of predation will be carefully separated. Each unemerged egg will be placed on a clean piece of double-sided tape and carefully dissected with sterilized equipment. If an unemerged parasitoid can be identified to species, then this will be recorded. If the egg fits into any of the following categories—unemerged parasitoid (unknown species), white/light goo, dark goo, or residue—then the egg and its contents will be placed in a singular microcentrifuge tube and frozen. DNA will be extracted from these samples using Qiagen blood & tissue kits. PCR and sequencing will be performed as defined in Gariepy et al. 2019.19 PCR will be run with Scelionidae-specific primers reported in Gariepy et al. 2019 as well as more general specific primers (associated with the CO1 gene locus) to generate sequences for non-Scelionid parasitoids. We will get samples sequenced and will compare sequences to sequences in the Nearctic Trissolcus species DNA barcode database and other databases on Barcode of Life Data System (BOLD).
Once we have species identifications of parasitoids, we will statistically determine in habitat type is a significant predictor of abundance and diversity of parasitoids and if pentatomid species experience significantly different levels of parasitism. We will also quantify food-web linkage using metrics defined by Bersier at al.20 These metrics include generality (mean number of host species per parasitoid species), vulnerability (mean number of parasitoids per host species), and linkage density (mean number of links per species). We will calculate these metrics using only successful parasitism as links and using all parasitism as links and compare them. We will then run an analysis of variance to see if habitat type is a significant predictor of food-web complexity of pentatomid-parasitoid food-webs.
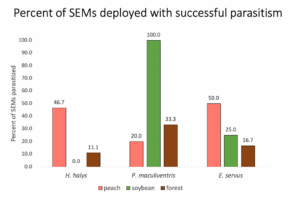
I completed a pilot study in the summer of 2022. For this study, I deployed egg masses of Halymorpha halys, Euschistus servus, and Podisus maculiventris egg masses laid by colony-reared stink bugs in three different habitats in southern New Jersey: peach monoculture, soybean monoculture, and forest. I deployed eggs at two sites per the three habitat types. Across all sites, 16.3% of H. halys egg masses (N=49), 31.3% of E. servus egg masses (N=16), and 50% of P. maculiventris egg masses (N=12) had parasitism present.
Fig 1. Percent of egg masses with successful parasitism across species and habitats.
Different stink bug species had different levels of parasitism in different habitats. P. maculiventris experienced highest overall parasitism rates, had 100% parasitism in soybean (N=4), but had no parasitism in peach habitats (N=5). There was highest parasitism for H. halys in peach, while low rates occurred in forest and none in soy. Euschistus servus experienced low-to-moderate egg parasitism rates in all three habitats.
This pilot study is promising, although sample sizes were quite low. I look forward to continuing this work in the 2023 in the spring, summer, and fall with more egg masses and in more sites.
Education & outreach activities and participation summary
Participation summary:
Results obtained from this study will be shared with reserach scientists at the Entomological Society of American Eastern Branch conference in March 2023, a talk at the Ecology & Evolution Graduate Program student seminar, and as a published peer-reviewed article. Additionally, the results of the study will be shared with growers electronically as a blog post on Rutgers Plant & Pest Advisory Bulletin. To engage with the public, Emma Waltman has committed to run an informational booth and prepare educational materials about the importance of and opportunities in conservation biological control at the 2022 Watershed Butterfly Festival, a family-friendly event to fundraise for The Watershed Institute, a non-profit environmental organization in central NJ. These materials will also be distributed at Rutgers Day 2023, a public, university-wide event with thousands of attendees, and will be shared at venues such as the Highland Park Farmers Market, other farmers markets, and/or at farms during pick-your-own season. As a scout for Rutgers Cooperative Extension, Emma Waltman maintained relationships with farmers operating large pick-your-own operations (Gary Mount at Terhune Orchards, Bradley Burke at Longmeadow Farm, and John Melick at Melick’s Town Farm), and will reach out to these farmers about distributing educational materials to customers.