Final report for GNE24-327
Project Information
Soil lead (Pb) contamination is often a barrier to safe urban agriculture in the Northeastern U.S., with health risks tied to the bioaccessible Pb fraction. This study evaluates whether low-temperature heat-treatment can significantly modify biochar for greater Pb sorption and therefore reduction of Pb bioaccessibility in contaminated soils. First, we characterized and evaluated the Pb sorption capacity of wood-chip biochar heated in air at 300°C. Then, using the EPA Method 1340 in-vitro bioaccessibility assay, we performed three experiments: (1) a trial 13-week study with heated and unheated biochars and one soil, (2) an expanded 2-month study with four biochar types and two soils, and (3) an investigation of moisture effects on unheated biochar performance in one soil kept at three soil water contents. Our results suggest that while heat treatment did significantly alter the physical and chemical properties of biochar and enhance its Pb sorption capacity, the modified biochar was not consistently effective in reducing Pb exposure risk within the soil environment.
In addition to biochar modification, this study assesses benchtop energy-dispersive X-ray fluorescence (EDXRF, hereafter XRF) spectroscopy as a lower-cost alternative for measuring Pb in liquid environmental samples. Liquid-phase extractions are widely used to assess Pb solubility and bioaccessibility in soils, but these solutions are typically analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, hereafter ICP), a sensitive yet costly technique requiring specialized facilities and expertise. Accurate quantification of lead (Pb) in environmental samples is essential for evaluating contamination risks and guiding remediation, especially in urban soils affected by legacy pollution.
This study assesses XRF spectroscopy for measuring Pb in three solution types:
- EPA Method 1340 extracts (in vitro gastric-soluble Pb)
- Pb(NO3)2 solutions (used in biochar-Pb sorption studies)
- Mehlich 3 extracts (for nutrient and trace metal screening).
Regression analyses comparing XRF and ICP showed strong agreement (r² = 0.9835 - 0.9985) across all matrices. XRF detection limits (the smallest amount of analyte detectable by the instrument) ranged from 0.17 to 0.25 mg L-1, with quantification limits (the smallest amount of analyte reliably quantified by the instrument) between 0.51 and 0.75 mg L-1. Average measurement reproducibility was high (standard deviation 0.055–1.50, depending on solution type). These findings indicate that XRF offers a reliable, rapid, and cost-effective approach for quantifying Pb in aqueous extracts, thus extending its applicability to community laboratories, field-based research, and contexts with limited analytical infrastructure. While XRF has traditionally been employed for solid matrices, its demonstrated performance with liquid extracts underscores its potential to broaden the scope of environmental testing beyond conventional applications.
1.1.
Our first objective is to heat-functionalize biochar, characterize its properties, and run Pb adsorption tests in simple aqueous solutions. We attempted to alter the surface properties of biochar by heating it under various experimental conditions in an air atmosphere. This process is designed to generate additional chemically active sites on the biochar’s surface which can bond with Pb. Such chemical bonds are generally considered non-reversible, significantly reducing the likelihood of Pb re-entering the solution and thereby immobilizing it within the soil. Next, we tested whether the physical and chemical properties of the biochar were significantly altered by measuring samples’ pH, surface area, pore development, and functional groups (by FT-IR spectroscopy). To then test the biochar’s reactivity towards Pb, we quantified the adsorption of Pb onto the biochar surface using a Langmuir isotherm model.
1.2.
Our second objective is to assess the efficacy of heat-functionalized biochar in immobilizing lead within soil. We hypothesized that due to its increased porosity and surface functional groups, heat-treated biochar would significantly reduce Pb bioaccessibility in amended soil compared to unamended soil or soil amended with untreated biochar. We addressed this hypothesis in three steps. Experiment 1 tested whether low-temperature air-heating (300 °C) of a wood-chip biochar changes soil Pb in-vitro bioaccessibility (IVBA) relative to unheated biochar, using a 5% w/w amendment in a Pb-contaminated urban soil and sampling over 13 weeks. Experiment 2 scaled and generalized the approach across two soils with contrasting total Pb (~260 and ~720 mg kg⁻¹), applied 2% w/w biochar heated for 1, 3, or 4 hours, and tracked IVBA dynamics from 1 day to 2 months to evaluate variability across matrices and treatments. Experiment 3 then isolated a key environmental control—moisture—by comparing IVBA below, at, and above field capacity (~10%, ~23%, ~47% gravimetric) with and without 2% biochar, investigating whether moisture/redox conditions mediate the efficacy of the amendment.
1.3.
Our third objective was to establish methods for analyzing EPA Method 1340 assays using XRF spectroscopy. We used benchtop XRF to measure Pb in three types of liquid samples: two bioaccessible Pb extracts, and one aqueous solution of pure lead nitrate. We evaluated the accuracy of XRF by comparing it to ICP, which is typically used for measuring bioaccessible Pb. Three replicates on the same liquid samples were used to assess precision of the method. We then developed regression equations for bioaccessible lead measured in the three different liquid solutions using ICP and XRF results. Establishing this relationship helps to determine whether the methods are statistically equivalent. Finally, to validate each established regression curve, we measured an additional set of samples. We plotted calculated ICP results (based on XRF measurements) against ICP measurements to evaluate the agreement between these data. The end results of this objective will help determine if XRF can be utilized as a cost-effective alternative to ICP for measuring bioaccessible Pb in liquids.
Urban agriculture and gardening are important for community well-being, offering clear social and economic advantages to low-income communities (Hanna & Oh, 2000; Horst et al., 2017). Urban agriculture fosters neighborhood engagement, stimulates local economies, and enhances access to fresh produce. However, a major challenge facing urban agriculture is soil contamination with heavy metals, particularly lead (Pb), which remains a legacy contaminant (Lusby et al., 2015; O’Shea et al., 2021). When assessing urban sites for agriculture, stakeholders must develop reliable risk assessment approaches and choose robust and sustainable mitigation strategies (Kim et al., 2014; Wagner & Payne, 2019; Wharton et al., 2012).
Conventional approaches to mitigating Pb pollution in soil include removal, physical encapsulation, dilution, chemical treatment, electrokinetic remediation, and, more recently, phytoremediation techniques (Priya et al., 2023). In comparison, the use of biochar as an agent for soil remediation has received less attention (Amalina et al., 2022; Bashir et al., 2020), likely due to the fact that biochars exhibit a tremendous variety in properties. It is important to note that not all biochars are equal, and some may have better capability to retain Pb than others (Sivaranjanee et al., 2023; Tan & Yu, 2023). The reactivity of biochar can be enhanced through activation procedures, which include physical, chemical, and thermal methods (Amalina et al., 2022; Bashir et al., 2020). Increases in biochar reactivity can improve their ability to remove heavy metals from solution. In urban agriculture communities, compost additions are a commonly used approach to reduce (“dilute”) Pb concentration in soil, but there are limitations. Compost additions may not be effective for soils with high concentrations of Pb. Moreover, previous research has indicated that compost may not efficiently retain Pb in the soil; instead, compost could increase Pb’s mobility, potentially leading to Pb discharge into groundwater during decomposition processes (Bolan et al., 2014). In fact, relying solely on compost as a remediation approach can lead to nutrient overapplication and potential runoff of excessive nutrients to nearby streams (Small et al., 2019).
As opposed to compost, biochar demonstrates promise as a remediation material, exhibiting high efficiency in Pb retention due to its abundant surface area and reactive functional groups (Yuan et al., 2019). Activation methods can be used to functionalize (i.e. add functional groups to) biochars to improve their reactivity. However, some of the procedures employed to manufacture commercial activated biochars may involve strong chemicals and should be conducted at a large commercial facility (Yuan et al., 2019). An alternative method, air oxidation, has shown promise for increasing biochar porosity and surface oxygenation of biochar without the use of chemical reagents (Sun et al., 2022).
In this study, we evaluate an inexpensive method to functionalize biochar and increase its reactivity towards Pb: heating biochar at 300°C under an air environment. We hypothesize that heating biochar in the presence of oxygen for several hours will activate its surface, creating more surface functional groups and making it more reactive towards Pb adsorption. We will test this hypothesis in a laboratory setting by characterizing physical and chemical changes to the biochar, performing aqueous Pb sorption experiments, and finally testing bioaccessible Pb in soil amended with the biochar. If the heated biochar does show superior Pb-sorbing properties, it follows that such a product would provide significant economic and health benefits as a soil amendment for urban gardeners.
To evaluate the efficacy of biochar amendment, we will assess whether functionalized biochar reduces bioaccessible Pb levels. While the EPA regulates total Pb concentration, this measure may not accurately reflect the potential health risks related only to the Pb readily available for absorption by the human body. An in vitro bioaccessibility method, such as EPA Method 1340 (US EPA, 2017), will allow us to estimate the portion of Pb that can be absorbed by humans and therefore provide a better understanding of its risk.
Moreover, to enhance the accessibility and affordability of this analysis, we seek to develop a procedure for analyzing EPA Method 1340 assays using X-ray fluorescence (XRF) spectroscopy. Unlike the costly and labor-intensive Inductively Coupled Plasma (ICP) analysis, XRF offers a more accessible and rapid alternative, aligning with our goal to make bioaccessibility assays more rapid, feasible and affordable, especially for laboratories serving underprivileged urban communities.
To summarize, if functionalized biochar proves to considerably decrease Pb bioaccessibility in soil (preferably, to below 60% bioaccessibility (US EPA, 2007)), its use could be beneficial for urban gardeners to mitigate the risk of Pb poisoning for themselves, their children, and their entire communities. In alignment with this objective, we are also validating a novel, rapid and cost-effective analytical method for quantifying the amount of Pb in liquid soil extracts.
References:
Amalina, F., Razak, A. S. A., Krishnan, S., Sulaiman, H., Zularisam, A. W., & Nasrullah, M. (2022). Biochar production techniques utilizing biomass waste-derived materials and environmental applications – A review. Journal of Hazardous Materials Advances, 7, 100134. https://doi.org/10.1016/j.hazadv.2022.100134
Bashir, S., Hussain, Q., Zhu, J., Fu, Q., Houben, D., & Hu, H. (2020). Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere, 30(6), 874–882. https://doi.org/10.1016/S1002-0160(20)60043-1
Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., Kirkham, M. B., & Scheckel, K. (2014). Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize? Journal of Hazardous Materials, 266, 141–166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Hanna, A. K., & Oh, P. (2000). Rethinking Urban Poverty: A Look at Community Gardens. Bulletin of Science, Technology & Society, 20(3), 207–216. https://doi.org/10.1177/027046760002000308
Horst, M., McClintock, N., & Hoey, L. (2017). The Intersection of Planning, Urban Agriculture, and Food Justice: A Review of the Literature. Journal of the American Planning Association, 83(3), 277–295. https://doi.org/10.1080/01944363.2017.1322914
Kim, B. F., Poulsen, M. N., Margulies, J. D., Dix, K. L., Palmer, A. M., & Nachman, K. E. (2014). Urban Community Gardeners’ Knowledge and Perceptions of Soil Contaminant Risks. PLoS ONE, 9(2), e87913. https://doi.org/10.1371/journal.pone.0087913
Lusby, G., Hall, C., & Reiners, J. (2015). Lead Contamination of Surface Soils in Philadelphia from Lead Smelters and Urbanization. Environmental Justice, 8(1), 6–14. https://doi.org/10.1089/env.2014.0008
O’Shea, M. J., Krekeler, M. P. S., Vann, D. R., & Gieré, R. (2021). Investigation of Pb-contaminated soil and road dust in a polluted area of Philadelphia. Environmental Monitoring and Assessment, 193(7), 440. https://doi.org/10.1007/s10661-021-09213-9
Priya, A. K., Muruganandam, M., Ali, S. S., & Kornaros, M. (2023). Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics, 11(5), 422. https://doi.org/10.3390/toxics11050422
Sivaranjanee, R., Kumar, P. S., & Rangasamy, G. (2023). A critical review on biochar for environmental applications. Carbon Letters, 33(5), 1407–1432. https://doi.org/10.1007/s42823-023-00527-x
Small, G., Shrestha, P., Metson, G. S., Polsky, K., Jimenez, I., & Kay, A. (2019). Excess phosphorus from compost applications in urban gardens creates potential pollution hotspots. Environmental Research Communications, 1(9), 091007. https://doi.org/10.1088/2515-7620/ab3b8c
Sun, Z., Dai, L., Lai, P., Shen, Feng, Shen, Fei, & Zhu, W. (2022). Air oxidation in surface engineering of biochar-based materials: A critical review. Carbon Research, 1(1), 32. https://doi.org/10.1007/s44246-022-00031-3
Tan, G., & Yu, H. Q. (2023). Rethinking biochar: Black gold or not? Nature Reviews Materials, 9(1), 4–5. https://doi.org/10.1038/s41578-023-00634-1
US EPA. (2007). Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. US Environmental Protection Agency. https://semspub.epa.gov/work/11/175416.pdf
US EPA. (2017). METHOD 1340 IN VITRO BIOACCESSIBILITY ASSAY FOR LEAD IN SOIL. US Environmental Protection Agency. https://www.epa.gov/sites/default/files/2017-03/documents/method_1340_update_vi_final_3-22-17.pdf
Wagner, F., & Payne, L. (2019). Growing Local: The Role of Urban Gardening in Fostering Food Security, Sustainability, and Community. Purdue Journal of Service Learning and International Engagement, 6(1). https://doi.org/10.5703/1288284316991
Wharton, S. E., Shayler, H. A., Spliethoff, H. M., Marquez-Bravo, L. G., Ribaudo, L., & McBride, M. B. (2012). A Comparison of Screening Tests for Soil Pb. Soil Science, 177(11), 650–654. https://doi.org/10.1097/SS.0b013e318277718b
Yuan, P., Wang, J., Pan, Y., Shen, B., & Wu, C. (2019). Review of biochar for the management of contaminated soil: Preparation, application and prospect. Science of The Total Environment, 659, 473–490. https://doi.org/10.1016/j.scitotenv.2018.12.400
Cooperators
- (Researcher)
- (Researcher)
Research
- 1. Thermal Oxidation of Biochar for Enhancing Pb Sorption
Functionalized Biochar Preparation Procedure
We chose to use sustainable wood-based biochar from a company in central Pennsylvania, Metzler Biochar, due to their products being certified according to International Biochar Initiative (IBI) requirements. Metzler Biochar provided us with 1 cubic foot of their PureCHAR biochar to use in our experiments free of charge. This biochar is produced from mixed hardwood (a common feedstock) with pyrolysis conditions of 704.4˚C for 30 min in the absence of oxygen. The biochar was used as-received.
First, we functionalized the biochar by heating it in a muffle furnace in the presence of oxygen. Approximately 4 cups of the biochar was heated on 30 cm x 30.5 cm x 4 cm cast iron trays in a Thermolyne tabletop muffle furnace (model type: F30400) in air at 300°C for varying lengths of time (1 hour, 2 hours, 3 hours, and 4 hours, excluding preheating time) at a 40°C/min heating rate. The trays were covered with perforated aluminum foil to prevent displacement of the biochar but allow air flow.
Biochar pH was measured following IBI’s recommended protocol (Rajkovich et al., 2012). Biochar surface area and pore size distribution were analyzed by the Brunauer-Emmet-Teller (BET) and Density Functional Theory (DFT) methods (Thommes et al., 2015), using a Micromeritics 3Flex gas sorption analyzer. Biochar samples were outgassed at 150˚C and analyzed by nitrogen sorption at 77 K. Functional groups were determined by Fourier Transform Infrared Spectroscopy (FTIR) using a Thermo Scientific spectrometer with an attenuated total reflectance (ATR) accessory and OMNIC software for spectrum analysis. Functional groups selection and assignments were based on data from (Leon y Leon & Radovic, 1993).
Based on the characterization results, we decided to omit the 2-hour heated biochar from further experiments, as it did not show large differences from the 3-hour heated biochar. The 3-hour heated biochar was chosen for further experiments as it showed that large differences from the unheated biochar could be achieved at a moderate heating time, therefore reducing energy and time requirements for production.
Sorption experiments were conducted using unheated and 3-hour heated biochars. Approximately 0.1 g of biochar (with 3 experimental replicates) was added to 50 mL of solution, corresponding to a solid:solution ratio equal to 2 g L-1 Solutions consisted of ASTM Type I water with varying concentrations of Pb(NO3)2: 0, 50, 100, 150, 200, 300, 400, 500, 600, and 700 mg L-1 made using Sigma Aldrich lead (II) nitrate (ACS reagent grade, ≥ 99.0%).
The pH of each mixture was checked after 1.5 hours of shaking and remained between 5 and 6. The samples were then shaken overnight (18 h) at room temperature before filtration through 1) a Whatman 2 filter paper to remove large solids and 2) a 0.45 µm syringe filter to remove particulates. The filtered solutions were then stored at 4°C until their analysis by XRF.
Sorption isotherms were analyzed using the Langmuir model (Thommes et al., 2015):
V = (Vm*Keq*Ceq)/(1+Keq*Ceq)
where Ceq is the equilibrium Pb concentration in solution (mg L-1), V is the Pb adsorbed to the biochar at equilibrium (mg g-1), Vm is the monolayer adsorption saturation capacity (mg g-1), and Keq (L mg-1) is the Langmuir model constant. Triplicate data were averaged for the final isotherm graphs.
- 2. Establishing the effectiveness of heat-functionalized biochar for immobilizing lead in soil
Experiment 1: Heated biochar trial experiment
3-hour heated Metzler biochar was used to amend soil in this experiment. Soil was collected from a community garden site in Philadelphia, PA, in 2022 (Dadio, S., 2024). The soil was dried and sieved to 2 mm. It was a sandy loam texture (hydrometer method with sand sieving from (Gee & Bauder, 1986; Gee & Or, 2018) with a pH of 7.44 (1:1 w/w water:soil) and 6.5% organic matter (loss on ignition method, Schulte & Hoskins, 2011). The total lead concentration was measured as 510.78 mg kg-1 following EPA Methods 3050B and 6010B (US EPA, 1996a, 1996b).
32 g of soil was placed into each of 36 plastic 50-mL beakers. The beakers were divided into three treatments: control soil, soil with 5% w/w unheated biochar, and soil with 5% w/w heated biochar. Biochar was mixed into the dry soil until evenly distributed. The total lead in the unheated and heated biochar mixtures was estimated to be 486.46 mg kg-1, assuming negligible Pb in the 5% biochar. Each beaker was covered in perforated parafilm and maintained at 40% moisture by mass with weekly checks.
At approximately 0, 2, 8, and 13 weeks, one beaker of each of the three treatments was removed for bioaccessibility and pH tests. The EPA Method 1340: In-Vitro Bioaccessibility (IVBA) Assay for Lead in Soil procedure was used (following the 2013 revision, soil was sieved to 250 μm) (US EPA, 2017). The resulting extracts were tested by EPA Method 6010B on ICP-OES (MDL = 0.005 and LOQ = 0.025) (US EPA, 1996b). IVBA calculations were carried out as follows: (Extracted Pb*100)/(Total Pb * Mass of Sample). Note that due to experimental error and the nature of the IVBA calculation (the total Pb value is also derived from a soil extraction), some IVBA percentages can exceed 100%. Nonparametric statistical analyses (Kruskal-Wallis and Dunn’s tests) were used due to small sample sizes, and statistical significance was determined at the 5% level. Graphs were constructed in R (version 4.4.1) using the ggplot2 package (Kassambara, 2025).
Experiment 2: Exploration of the effects of heated biochar on soil Pb bioaccessibility
After completing trial work with Philadelphia soil (≈500 mg kg⁻¹ Pb; 5% biochar) in Experiment 1, we redesigned the study to better reflect practical remediation constraints and to probe early-time dynamics. Specifically, we (i) reduced the biochar dose to improve remediation cost, (ii) expanded thermal treatments to include 1, 3, and 4 h at 300°C, (iii) tested two Pb contamination levels (Low and High) to bracket common regulatory thresholds, (iv) shortened the incubation to 2 months but added more frequent sampling to resolve short-term changes, and (v) held similar initial moisture but did not maintain moisture thereafter, allowing soils to dry naturally over time. Together, these modifications were intended to evaluate whether lower-dose, thermally modified biochars can meaningfully alter Pb IVBA under field-leaning moisture regimes and across relevant concentration ranges. Soil was sourced from another location as a larger quantity was needed than was collected for Experiment 1.
Soil samples were taken in 2024 along the drip line of a row home located in Lancaster, PA, by Darren Parmer, Housing Intervention Manager for the Green & Healthy Homes Initiative. The size of the draw was 0.76 m by 6.71 m on the dripline of the gable end side of the house facing east.
The soil texture was classified following Gee & Bauder, 1986. The percent sand was determined by wet sieving the soil to 0.075 mm and weighing the sand following the hydrometer measurements (Gee & Or, 2018).
After drying at 105°C for two hours, the percent organic matter was determined using loss on ignition (Schulte & Hoskins, 2011). Three replicates were run and averaged for a final percent OM.
The soil Pb content was approximately measured using portable XRF (Vanta Element, Geochem) to be 2948 mg kg-1. Two dilutions were then prepared using uncontaminated soil. The first dilution to ~200 mg kg-1 Pb (referred to hereafter as “Low Pb” soil) was made using 2.8 kg of Morrison soil mixed with 0.7 kg of the contaminated soil. The second dilution to ~600 mg kg-1 Pb (referred to hereafter as “High Pb” soil) used 3.267 kg Morrison soil and 0.233 kg contaminated soil. Samples of the soils were sieved to < 150 µm (as specified in EPA Method 1340) before sending to Agricultural Analytical Services Laboratory for EPA Method 3050B total Pb extraction followed by EPA Method 6010 ICP analysis (US EPA, 1996a, 1996b). The texture class and percent organic matter for each dilution were determined following the same procedures as described above.
Texture class and organic matter of the diluted soils were determined as above. Gravimetric field capacity was estimated by saturating air-dried (< 2 mm) soil in metal rings lined with cheesecloth, allowing 24 hours of saturation and 48 hours of drainage at room temperature, then oven-drying at 105°C to constant weight.
Wood chip biochar was again sourced from Metzler Biochar. Biochar was heated to 300°C for one-, two-, three-, and four-hour treatments. Biochar pH measurements were taken using the procedure recommended by the International Biochar Initiative (Rajkovich et al., 2012), and surface properties were characterized by IR spectroscopy and BET/FTR analyses.
We chose the 1-, 3-, and 4-hour heated biochars for use in our experiment as these biochars showed large differences in pH and functional groups as compared to the unheated sample. 2-hour heated biochar was not used as it did not appear to be significantly different from 3-hour heated biochar.
For both contaminated soil mixtures, we set up the following conditions in 250-mL Nalgene bottles with 3 replicates each:
- Unamended soil mixture
- Soil mixture with 2% by mass unheated biochar
- Soil mixture with 2% by mass 1-hour heated biochar
- Soil mixture with 2% by mass 3-hour heated biochar
- Soil mixture with 2% by mass 4-hour heated biochar.
Subsamples of each soil were taken for measuring total Pb: digestion by EPA method 3050B followed by ICP-OES testing using EPA Method 6010B. The total Pb measurements were averaged and used in calculating in-vitro bioaccessible (IVBA) Pb. One outlier with an unusually high total Pb (Grubb’s outlier test p-value < 0.001) was removed from the Low Pb dataset.
The soils were wetted to approximately 38% moisture by mass (slightly lower than in Experiment 1 to account for lower organic matter content in the new soils) and each container was covered with perforated parafilm. Water was not added following the initial moistening. At the following timepoints, 25 g of soil was removed and dried at 35°C:
- T1: 1 day
- T2: 3 days
- T3: 1 week
- T4: 2 weeks
- T5: 1 month
- T6: 2 months.
A portion of each sample was then sieved to obtain 1 g of the < 150 μm fraction required for the EPA Method 1340 in-vitro bioaccessible Pb assay (2017 revision) (US EPA, 2017). The assay was carried out on the sieved samples and resulting solutions were sent to Penn State University’s Agricultural Analytical Services Laboratory for ICP analysis (EPA Method 6010B) (US EPA, 1996b). For quality control, NIST soil standard 2711a was extracted and tested with each batch of samples; in all cases, its resulting IVBA was within the acceptable range listed in the EPA Method 1340 procedure.
Pb IVBA was calculated using the following formula: (Extracted Pb*100)/(Total Pb * Mass of Sample). Nonparametric statistical analyses (Kruskal-Wallis and Dunn’s tests) were used, and statistical significance was determined using α = 0.05.
Experiment 3: Effects of soil moisture on Pb bioaccessibility
The previous two experiments both used high moisture contents (40% and 38% by mass) and showed significant variation in Pb bioaccessibility over time. These findings raised the question of how soil moisture might affect biochar amendments and extractable Pb. While researching biochar effects on bioaccessible Pb in wetland soils, Plunkett et al. found that in situ redox conditions of samples from saturated environments influence Pb bioaccessibility (Plunkett et al., 2022). This experiment aimed to answer the question of how soil moisture may have affected the previous two experiments’ results by comparing Pb IVBA from control and biochar-amended soil below, at, and above field capacity.
The High Pb soil mixture (721.48 mg Pb kg-1) from Experiment 2 was used in this experiment. The soil was incubated with and without the addition of 2% by mass unheated Metzler wood chip biochar at three different moisture levels: low (10% gravimetric moisture content—below saturation), medium (23% gravimetric moisture content—near field capacity), and high (46.9% gravimetric moisture content—at saturation, calculated from soil bulk density). Each sample of 18 g oven-dry soil was brought to the appropriate mass by the addition of DI water. Each condition was replicated four times.
Following a 2-day incubation, samples were freeze-dried in an attempt to preserve redox-sensitive species prior to extraction (Furman et al., 2007). Bioaccessible Pb was then extracted following EPA Method 1340. The extraction solutions were measured with a benchtop EDXRF calibrated by regression analysis against ICP-OES.
- 3. Establishing a procedure for analyzing Pb in liquid samples by XRF spectroscopy
Liquid EPA Method 1340 Extractions
This method involves subjecting soil samples to simulated physiological conditions, mimicking the acidic environment of the human stomach, to determine the portion of lead that can potentially be absorbed by the body. The extraction entails dissolving 1 g of soil in 0.4 M glycine solution at pH 1.5, shaking at 30 rpm and 37˚C for 1.5 hours, and finally filtering the solution to remove any soil particles. The amount of lead dissolved in the solution is then quantified, typically by Inductively Coupled Plasma (ICP) spectroscopy. The amount of lead released from the soil under these conditions provides an estimate of its bioaccessibility. In this work, instead of using ICP, we will develop an XRF-based approach as a faster and more affordable method to measure bioaccessible Pb via EPA 1340 extractions.
37 contaminated soil samples were collected from multiple locations in Philadelphia, PA, in 2022 (Dadio, S., 2024) and digested following EPA Method 1340. Alongside each batch of samples, NIST soil standard 2711a was extracted and tested for quality control purposes; in all cases, it was within the acceptable range listed in the EPA Method 1340 procedure.
Aqueous Pb(NO3)2 solutions
Additionally, simple aqueous Pb(NO3)2 solutions, commonly used in soil spiking for laboratory experiments, were chosen for XRF method development.
30 solutions of varying concentrations of Pb were prepared using Pb(NO3)2 (ACS reagent-grade, ≥ 99.0%) dissolved in Type I water. A drop of reagent-grade concentrated nitric acid was used to acidify each solution to below pH 2. The approximate concentrations were made starting at 1 mg Pb L-1 and then in increments of 25 mg Pb L-1 (1, 25, 50, … , 700, 725 mg Pb L-1). 6 additional solutions of concentrations within this range were created for model validation.
Mehlich 3 Bioaccessible Pb Extractions
Mehlich 3 is a standard soil nutrient and trace metal extraction that has gained popularity as a proxy for estimating bioaccessible Pb (Minca et al., 2013). Briefly, 20 ml of Mehlich 3 solution (0.2 N CH3COOH + 0.25 N NH4NO3 + 0.015 N NH4F + 0.013 N HNO3 + 0.001 M EDTA) is added to 2 g of soil, placed on a reciprocating shaker for 5 minutes at 180 oscillations per minute, and filtered. Mehlich 3 extractants are typically measured by ICP-OES. Given the widespread use of this extraction method in soil testing, we chose to include this solution type as well for XRF testing.
A subset of soils previously characterized as part of the Northeast Coordinating Committee project NECC-1812 (Hamel et al., 2003) was used to assess Pb content and XRF response. These soils represent a range of management histories and physicochemical properties relevant to urban and agricultural contexts.
XRF and ICP
X-ray fluorescence (XRF) is a non-destructive method of elemental analysis that provides concentrations of elements ranging from sodium to uranium. Samples are most commonly prepared as powders (for lower quality results), flattened pellets, or fused beads (for highest quality results). X-rays strike the sample, causing electrons to be ejected from the atoms’ inner orbitals. Electrons from energetically higher orbitals must then replace the ejected electrons, and energy is released in the form of measurable x-ray fluorescence. Because the amount of energy released is different for different elements, we can efficiently identify metals and quantify their concentrations. In this work, we aim to investigate the potential utility of XRF for measuring heavy metals in liquid samples rather than solid samples. In this project, we tested the precision and accuracy of XRF as compared to ICP.
Pb concentrations in EPA Method 1340 and Pb(NO3)2 solutions were analyzed by ICP-OES (Varian 730-ES Axial ICP Spectrometer, now Agilent Technologies, Santa Clara, CA) following EPA Method 6010B. For Mehlich 3 solutions, quality assurance included analysis of an independent calibration verification standard before sample measurement and a continuing calibration verification standard every 10 samples, and at the end of each run, with results required to be within 10% of expected values. A method blank and laboratory quality control sample were also run with each batch of 28 samples.
Analyses were performed on a Rigaku NEX CG II benchtop EDXRF spectrometer equipped with a Pd X-ray tube, a silicon drift detector, and a 15-position autosampler (referred to here simply as “XRF”) (“Energy Dispersive X-Ray Fluorescence Spectrometer,” 2023).
For each batch of extracted samples, a blank sample (either 0.4 M glycine, type I water, or Mehlich 3 extract, depending on the measured solution type) was tested first. This “background spectrum” was subtracted from subsequent sample measurements. We found that when many analytes were selected, interference increased, so Pb was selected as the only analyte. Additionally, to account for the matrix effects we used glycine as the “balance” in the software for EPA Method 1340, and water for both Pb-nitrate solutions and Mehlich 3 extracts. The “balance” setting allows the XRF software to quantify the detectable components in a sample while accounting for unmeasurable components (i.e., light elements).
Statistical Methods
The “Statistical error” output on the Rigaku EDXRF instrument is the ±1σ uncertainty from counting noise: the software integrates the element’s net peak (after background), where N is the photon counts collected (≈ intensity I in cps/mA × live time t in s); the Poisson 1σ is √N, which is then propagated through the calibration and Fundamental Parameters model to give the uncertainty in mg L-1.
Minitab software (Minitab, LLC, 2023) was used for regression analyses and checks of model assumptions. For each dataset, we assessed model assumptions including linearity, normality of residuals (Anderson–Darling test), and homogeneity of variance (F-tests, Breusch–Pagan test). Where assumptions were violated (Mehlich 3 dataset), data were log–log transformed, and high-leverage points were evaluated prior to refitting the model. Regression outputs included the model equation, 95% confidence intervals for slope and intercept, coefficient of determination (r2), root mean square error (RMSE), p-values, and residual plots. For EPA Method 1340 and Mehlich 3 data, model validation was conducted by randomly splitting the dataset into approximately 80% for training and 20% for validation. For the Pb(NO3)2 model, we separately mixed solutions of varying concentration for the validation set. The measured XRF values were entered into the regression equations to calculate predicted ICP values, which were then plotted against the corresponding measured ICP values, with predicted values on the x-axis and measured values on the y-axis.
References:
Dadio, S. (2024). An Investigation of Elemental Soil Composition Testing Methods and Site Assessment Procedure for Determining Trace Elemental Pollutant Concentrations in Urban Soils [The Pennsylvania State University]. https://etda.libraries.psu.edu/catalog/27842sqd5560
Energy Dispersive X-ray Fluorescence Spectrometer. (2023). Rigaku Journal, 39(1), 35–37.
Furman, O., Strawn, D. G., & McGeehan, S. (2007). Sample Drying Effects on Lead Bioaccessibility in Reduced Soil. Journal of Environmental Quality, 36(3), 899–903. https://doi.org/10.2134/jeq2006.0340
Gee, G. W., & Bauder, J. W. (1986). Particle-Size Analysis. In Methods of Soil Analysis (2nd ed., pp. 383–411). Soil Science Society of America. https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1898399
Gee, G. W., & Or, D. (2018). Particle-Size Analysis. In J. H. Dane & G. Clarke Topp (Eds.), SSSA Book Series (pp. 255–293). Soil Science Society of America. https://doi.org/10.2136/sssabookser5.4.c12
Hamel, S. C., Heckman, J. R., Shilke‐Gartley, K. L., & Hoskins, B. (2003). Lead Extraction Using Three Soil Fertility Tests and Environmental Protection Agency Method 3050. Communications in Soil Science and Plant Analysis, 34(19–20), 2853–2873. https://doi.org/10.1081/CSS-120025211
Kassambara, A. (2025). Ggplot2 package (Version 0.6.2) [Computer software]. https://rpkgs.datanovia.com/ggpubr/
Leon y Leon, C., & Radovic, L. R. (1993). Interfacial Chemistry and Electrochemistry of Carbon Surfaces. In Chemistry & Physics of Carbon (1st ed., Vol. 24, pp. 213–292). CRC Press.
Minca, K. K., Basta, N. T., & Scheckel, K. G. (2013). Using the Mehlich-3 Soil Test as an Inexpensive Screening Tool to Estimate Total and Bioaccessible Lead in Urban Soils. Journal of Environmental Quality, 42(5), 1518–1526. https://doi.org/10.2134/jeq2012.0450
Minitab, LLC. (2023). Minitab Statistical Software (Version 21) [Computer software]. www.minitab.com
Plunkett, S. A., Eckley, C. S., Luxton, T. P., & Johnson, M. G. (2022). The effects of biochar and redox conditions on soil Pb bioaccessibility to people and waterfowl. Chemosphere, 294, 133675. https://doi.org/10.1016/j.chemosphere.2022.133675
Rajkovich, S., Enders, A., Hanley, K., Hyland, C., Zimmerman, A. R., & Lehmann, J. (2012). Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biology and Fertility of Soils, 48(3), 271–284. https://doi.org/10.1007/s00374-011-0624-7
Schulte, E. E., & Hoskins, B. (2011). Recommended Soil Organic Matter Tests. In J. T. Sims & A. Wolf (Eds.), Recommended Soil Testing Procedures for the Northeastern United States (3rd ed., pp. 63–74).
Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., & Sing, K. S. W. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87(9–10), 1051–1069. https://doi.org/doi:10.1515/pac-2014-1117
US EPA. (1996a, December). METHOD 3050B ACID DIGESTION OF SEDIMENTS, SLUDGES, AND SOILS. US Environmental Protection Agency. http://epa.gov/hw-sw846/sw-846-test-method-3050b-acid-digestion-sediments-sludges-and-soils
US EPA. (1996b, December). METHOD 6010B INDUCTIVELY COUPLED PLASMA-ATOMIC EMISSION SPECTROMETRY. US Environmental Protection Agency. https://www.epa.gov/sites/default/files/documents/6010b.pdf
US EPA. (2017). METHOD 1340 IN VITRO BIOACCESSIBILITY ASSAY FOR LEAD IN SOIL. US Environmental Protection Agency. https://19january2017snapshot.epa.gov/sites/production/files/2015-12/documents/1340.pdf
3.1. Thermal Oxidation of Biochar for Enhancing Pb Sorption
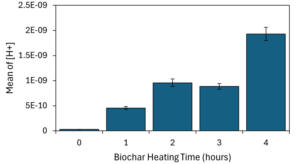
Biochar average pH decreased significantly with heating time: from pH 10.51 ± 0.01 for unheated biochar, pH 9.34 ± 0.03 for 1 hour of heating, pH 9.02 ± 0.03 for 2 hours of heating, pH 9.06 ± 0.03 for 3 hours of heating, and pH 8.72 ± 0.03 for 4 hours of heating (Figure 2-1).
The total surface area of the biochar also changed with heating time, increasing from 503.18 m2g-1 in unheated biochar to 633.4 m2g-1 in 4-hour heated biochar.
Figure 2-2 shows a gas adsorption isotherm plotting nitrogen adsorbed vs. relative pressure for unheated biochar and biochars heated for 1, 2, and 3 hours. The height of the curve on the y axis indicates more gas adsorbed, while the shape of the curve on the x axis is associated with the sizes of slit-shape pores typical of carbon materials. The first plateau shows the range of micropores, the second plateau shows mesopores, and finally macropores towards 0.95 P/Po (Thommes et al., 2015). At any given P/Po, a higher curve means more gas adsorbed (greater accessible surface/pore volume); here the samples rank 3h = 2h > 1h ≫ 0h.
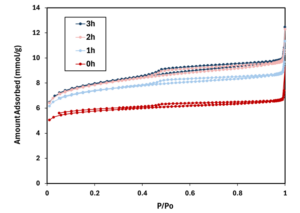
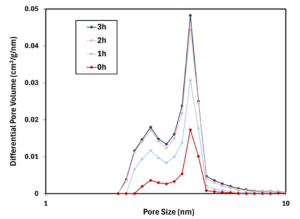
Figure 2-3 plots pore volume vs. pore size for unheated biochar and biochars heated for 1, 2, and 3 hours. While pore size remained consistent, pore volume was increased steadily by heating.
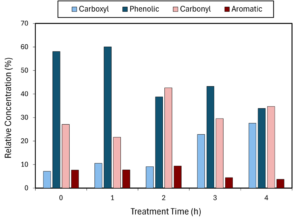
IR spectroscopy showed that, with the exception of the 2-hour heated biochar, heating tended to decrease the proportion of phenolic and aromatic groups (wavenumbers 1079 cm-1 and 874 cm-1) and increase the more acidic carboxyl and carbonyl groups (wavenumbers 1584 cm-1 and 1422 cm-1) (Figure 2-4).
In the sorption experiment, a paired t-test comparing the amount of Pb adsorbed to the biochars gave a p-value of >0.001 for a mean difference of 16.46 (19.90, 13.03). At the 5% significance level, we rejected the null hypothesis that the mean difference was equal to 0 and, from the positive confidence interval, concluded that heated biochar had a higher sorption of Pb than unheated biochar in each Pb(NO3)2 solution.
A sorption isotherm using the Langmuir model showed a Keq (the Langmuir equilibrium constant, an estimate of the affinity of the sorbate to the sorbent) of 0.34 L mg-1 and a Vm (monolayer adsorption saturation capacity) of 55.07 g kg-1 Pb for unheated biochar (Figure 2-5). The isotherm for 3-hour heated biochar had a Keq of 4.58 L mg-1 and Vm of 72.96 g kg-1 Pb (Figure 2-5). Adsorption values were more variable in the last 2-3 samples. Variability and higher Pb adsorption values could potentially be due to underestimation of Pb concentrations if Pb fell out of solution in the more concentrated samples.
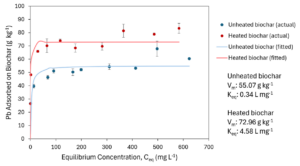
Oxidizing a wood chip-based biochar in air at 300˚C produced a measurable transformation of surface chemistry and texture, lowering suspension pH, increasing specific surface area and pore development, and shifting FTIR signatures toward more oxygenated functionalities. These modifications were accompanied by systematically greater Pb sorption capacity and apparent affinity in batch tests, with conventional isotherm models sufficiently capturing the data. This evidence supports a mechanistic interpretation in which added acidic sites (e.g., –COOH/–C=O) strengthen specific interactions with Pb(II) beyond what can be attributed to solution pH alone.
Collectively, these results indicate that low-temperature oxidative functionalization is a practical strategy to enhance the Pb-binding performance of biochar for environmental applications. Industrial-scale implementation of this biochar modification appears feasible; however, future work will evaluate whether the added costs are justified by the net gains in Pb immobilization efficiency.
3.2. Establishing the effectiveness of heat-functionalized biochar for immobilizing lead in soil
Experiment 1: Heated biochar trial experiment
The Kruskal-Wallis test was used with the null hypothesis that the median Pb IVBA values at each sampling timepoint were equal. In each treatment, the Kruskal-Wallis p-value was less than 0.05, so the null hypothesis was rejected and a Dunn’s post hoc test was used to determine which timepoints differed. In all three treatments, Pb IVBA was significantly lower at Time 1 (1 day) than at Time 2 (2 weeks) (see Figure 3-1 panels A-C). After 8 weeks (Times 3-4), Pb IVBA seemed to stabilize and did not change significantly.
Because the data from 8 and 13 weeks (times t3 and t4 in Figure 3-1 panels A-C) show all samples remaining between about 93% and 98% bioaccessibility, we chose to investigate differences in Pb IVBA between treatments using only the last two timepoints. The Kruskal-Wallis test was used to test the null hypothesis that the median Pb IVBA values for each treatment were equal. The p-value was 0.003889, indicating that at the 5% significance level, the three medians were not equal. Dunn’s post-hoc test was conducted to determine which medians differed. At the 5% significance level, median Pb IVBA was significantly higher in the control soil than in either the unheated biochar treatment (p = 0.0110) or the heated biochar treatment (p = 0.001711). However, the biochar treatments did not have significantly different median Pb IVBA values when compared to each other (p = 0.5520).
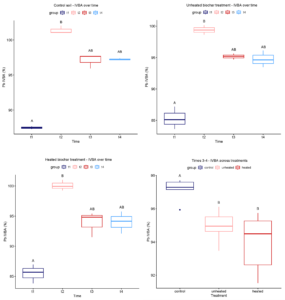
Heat treatment in air decreased pH and increased the surface area and pore volume of biochar, suggesting potentially higher reactivity of the treated material. Non-parametric tests showed that both unheated and heated biochar treatments significantly reduced median Pb bioaccessibility after 8 weeks (p < 0.05). Heated biochar did not show a statistically significant improvement over unheated biochar in lowering Pb bioaccessibility. To further investigate these results, we continued with a larger-scale experiment using two different contaminated soils, biochar heated for different times, and a longer timeframe.
Experiment 2: Exploration of the effects of heated biochar on soil Pb bioaccessibility
The hydrometer analysis showed a loam texture for the original soil, with the three LOI replicates giving an average of 6.15% organic matter. The average total Pb measurement of the Low Pb mixture was 259.84 mg kg-1 Pb, while that of the High Pb mixture was 721.48 mg kg-1 Pb. Both diluted soils were classified as sandy loams. For the Low Pb soil, three replicates averaged to 1.73% organic matter, and for the High Pb soil, three replicates averaged to 2.29% organic matter. Low Pb soil had an approximate gravimetric field capacity of 23.64%, while that of the High Pb soil was 23.24%.
In the low Pb soil, across all timepoints no significant differences were seen between treatments (Kruskal-Wallis p-value > 0.05). In the high Pb soil, significant differences between treatments were seen only in Time 1 (one day; H-statistic = 9.633 and p = 0.0471) and Time 3 (one week; H-statistic = 9.767 and p = 0.0445), so Dunn’s post hoc test was run on those data. At Time 1, median IVBA Pb in soil treated with biochar heated for either 1 or 4 hours (88.07% and 90.83% IVBA, respectively) was significantly higher than in the control soil (82.96% IVBA; see Figure 3-2 panel A). At Time 3, median IVBA Pb in soil treated with unheated biochar or 1 hour-heated biochar (97.61% and 97.47% IVBA, respectively) was higher than in the control soil (86.45%; see Figure 3-2 panel B).

Looking at individual treatments over time, the Low Pb soil again did not show any significant variation (Kruskal-Wallis p-value > 0.05). In high Pb soil, unheated biochar, 3-hour heated biochar, and 4-hour heated biochar treatments showed variation over time, with Kruskal-Wallis tests giving H-statistics of 13.09, 13.68, and 14.05, and p-values of 0.0225, 0.0178, and 0.0153, respectively. Dunn’s post hoc test was conducted on these groups.
In the unheated biochar treatment, Pb IVBA increased from Time 1 to Time 3, decreased at Time 4, and finally increased at Time 6 (Figure 3-3). A similar trend was observed in both 3- and 4-hour heated biochar treatments (Figures 3-4 and 3-5). In all three cases, IVBA tended to initially spike and then decrease before increasing again, similar to the pattern observed in Experiment 1 (Figure 3-1 panels A-C).
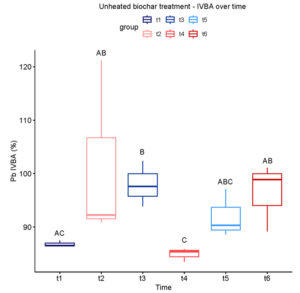
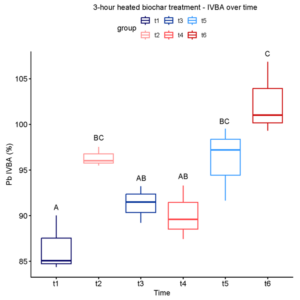
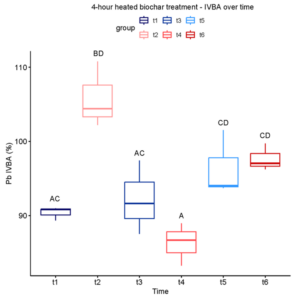
After our initial trial experiment showed a significant, albeit small, lowering of Pb IVBA in 5% (w/w) biochar-amended soil, we redesigned the study to better reflect practical remediation constraints and to probe early-time dynamics. Specifically, we reduced biochar dose, expanded thermal biochar treatments to include 1, 3, and 4 h at 300°C, tested two Pb contamination levels (low and high), and added more frequent sampling to resolve short-term changes. Fluctuations over time were observed, likely corresponding to background effects of soil conditions on the behavior of Pb species. Significant differences in treatments were only seen the high Pb soil at time 1 (one day) and time 3 (one week), where some biochar treatments raised Pb IVBA rather than lowering it as hypothesized. In conclusion, this variety of wood-chip biochar at 2% (unheated or heated 1-4 h at 300°C) did not prove to be an effective immobilization amendment for these soil conditions.
Our results highlight the importance of careful testing of individual biochar types in specific soil conditions prior to recommendation of use in the field. Additionally, trusted and low-cost methods of creating safe gardening environments—such as practicing good hygiene and using raised beds filled with clean soil—cannot be replaced by amendments.
Experiment 3: Effects of soil moisture on Pb bioaccessibility
In the control soil, Pb IVBA decreased steadily from 104.19 ± 2.95% to 72.95 ± 3.43% with increasing soil moisture content (Figure 3-6). In the amended soil, Pb IVBA peaked with medium moisture content (114.97 ± 4.82%) and was lowest with high moisture content (85.21 ± 1.44%).
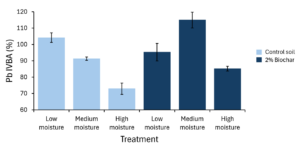
Pb in-vitro bioaccessibility (IVBA) in the control soil showed a steady decrease as gravimetric moisture increased, from 104.19 ± 2.95% at low moisture (~10%) to 91.31 ± 0.98% at medium moisture (~23%, near field capacity) and ~73.95 ± 3.43% at high moisture (~47%, near saturation) (n = 4). This monotonic decrease could be explained with a shift from oxic toward suboxic/anaerobic conditions at higher water contents, which (1) can promote microbial sulfate reduction and sulfide production, driving precipitation of sparingly soluble PbS that may be less extractable by EPA Method 1340; (2) tends to raise pH/alkalinity during reductive processes, favoring Pb sorption and formation of carbonate/hydroxyl-carbonate solids; and (3) alters Fe/Mn redox cycling, generating Fe(II) phases and FeS surfaces that (re)sequester dissolved Pb (Pignatello et al., 2024).
In contrast, the 2% biochar treatment showed a hump-shaped response: IVBA increased from 95.32 ± 5.34% (low moisture) to 114.97 ± 4.82% (medium moisture) and then dropped to 85.21 ± 1.44% (high moisture), converging with the control at near-saturation. The mid-moisture peak likely reflects conditions optimal for microbial activity and solute diffusion that enhance desorption/complexation (e.g., higher DOC), whereas at high moisture the same mechanisms (1–3) dominate, lowering IVBA in both treatments. Specifically, at intermediate moisture (≈ field capacity), soils have the best balance of water and oxygen for microbes and solute transport, which can temporarily raise Pb IVBA. First, aerobic microbial activity and extracellular enzyme diffusion peak near field capacity, accelerating decomposition and releasing dissolved organic carbon (DOC) (Birch, 1958; Skopp et al., 1990). Second, DOC—especially low-molecular-mass acids and fulvic/humic ligands—complexes Pb in solution and can compete with mineral and organic sorption sites, shifting Pb from surface-bound to dissolved/ligand-bound forms (McBride et al., 1997; Sauvé et al., 1998; Tipping, 2002). Third, higher moisture improves diffusive transport, helping DOC and inorganic ligands reach sorption domains and mobilize surface-associated Pb before stronger reducing conditions develop at near-saturation; once pores become water-filled and O₂ is limited, sulfide formation and Fe(II)/FeS surfaces re-immobilize Pb, so IVBA drops again.
The contrast between the control soil and the biochar-amended soil can be explained by fundamental redox processes. Biochar contains redox-active carbon matrices with measurable electron-donor capacity (EDC) and electron-acceptor capacity (EAC)—quantities (meq g⁻¹) describing how much charge the material can reversibly donate or accept (Klüpfel et al., 2014; Sun et al., 2017; Yuan et al., 2017). Quinone/hydroquinone moieties, aromatic sheets, and persistent free radicals in biochar act as electron-storage and shuttle sites, enabling rapid electron transfer to or from surrounding minerals, solutes, and microorganisms (Klüpfel et al., 2014; Sun et al., 2017). Through these coupled half-reactions, biochar can reduce electron acceptors such as Fe(III), Mn(IV), or Cr(VI), or oxidize reduced species when oxygen or nitrate is present, thereby altering soil redox potential (Eh) and the speciation of metals such as Pb (Husson, 2013; Stumm & Morgan, 1996).
During these electron exchanges, reactive oxygen species (ROS) such as hydrogen peroxide (H₂O₂), superoxide (O₂⁻), and hydroxyl radicals (•OH) can form. These short-lived oxidants originate when O₂ accepts electrons supplied by biochar EDC, or when H₂O₂ decomposes on redox-active surfaces (Yuan et al., 2017). ROS can further transform Pb and Fe minerals, shifting Pb from more soluble to less soluble phases depending on moisture and redox status (Antić-Mladenović et al., 2017).
In our soils, the control (soil SOM only, no biochar) showed steadily lower Pb IVBA as moisture increased, consistent with decreasing Eh and precipitation of sulfide or carbonate phases under reducing conditions after nitrate and ferric iron depletion (Husson, 2013; Stumm & Morgan, 1996). By contrast, the biochar-amended soil had higher Pb IVBA at low to medium moisture, where its strong EDC and surface alkalinity favored transient Pb mobilization, but converged with the control at high moisture when strongly reducing conditions immobilized Pb. This behavior mirrors field evidence: Plunkett et al. (2022) found that biochar modestly lowered Pb bioaccessibility in upland soils but had little effect in periodically flooded soils where oxidation of Pb sulfides during drying nearly doubled bioaccessibility. Likewise, a meta-analysis of biochar aging shows that O-functionalization (↑EAC, ↓EDC) and microbial activity gradually reshape biochar’s redox behavior and its control over metal solubility (Yuan et al., 2021).
Together, these findings show that biochar may act as a long-lived redox capacitor: it stores and releases electrons (high EDC/EAC), generates ROS, and buffers Eh. However, its impact on Pb mobility ultimately depends on the prevailing moisture-redox regime and on longer-term chemical and microbial aging.
3.3. Establishing a procedure for analyzing Pb in liquid samples by XRF spectroscopy
The average values of statistical errors, limits of detection, and limits of quantification for each regression solution are shown in Table 4-1.
Table 4-1. Statistical error, detection limit, and quantification limit for Pb measurements by benchtop EDXRF across three solution types
| Average value (mg L-1) | ||||
|
Solution |
Standard deviation |
Statistical error |
Detection limit |
Quantification limit |
|
EPA Method 1340 |
0.055 |
0.0856 |
0.170 |
0.509 |
|
Pb(NO3)2 |
1.501 |
0.8466 |
0.250 |
0.751 |
|
Mehlich 3 |
0.094 |
0.1566 |
0.236 |
0.709 |
Summary of Regression Models
Table 4-2 summarizes simple linear regression analysis between ICP and XRF for the 3 solutions used in this study. Regression models demonstrated strong agreement between XRF and ICP measurements across all three solution matrices. All models had high coefficients of determination (r2 > 0.98), confirming that XRF values are highly predictive of ICP results. Nevertheless, the negative intercepts (with the exception of the Mehlich 3 model intercept) and slopes greater than unity indicate systematic biases, with XRF tending to underestimate Pb relative to ICP at higher concentrations. RMSE was lowest for EPA Method 1340 (0.2811 mg L-1) and highest for Pb(NO3)2 (11.96 mg L-1). The validation MAE values followed this trend, with the EPA Method 1340 model having better predictive ability than the Pb(NO3)2 model. Mehlich 3 solutions were intermediate in both RMSE and validation MAE values.
Table 4-2. Regression statistics summary predicting ICP by XRF for the three solution matrices.
|
Solution |
N |
Slope* |
Y Intercept* |
r2 |
Model p-value |
RMSE** |
Validation MAE*** |
|
EPA Method 1340 |
30 |
1.4412 (1.369, 1.513) |
-0.548 (-0.770, -0.320) |
0.9835 |
< 0.001 |
0.2811 |
0.0918 |
|
Pb (II) nitrate |
30 |
1.147 (1.128, 1.166) |
-16.98 (-25.70, -8.26) |
0.9982 |
< 0.001 |
11.96 |
4.0360 |
|
Mehlich 3**** |
41 |
12.87 (11.78, 14.06) |
0.6256 (0.5782, 0.6769) |
0.9885 |
< 0.001 |
1.099 |
1.0864 |
*Data is presented within a 95% confidence interval
** RMSE = root mean square error
*** MAE = mean absolute error
**** Slope, intercept, and RMSE are back-transformed
Across all matrices, XRF exhibited strong agreement with ICP results (r2 > 0.98), with validation confirming predictive reliability. Detection limits (0.17 - 0.25 mg L-1), quantification limits (0.509 - 0.751 mg L-1), and reproducibility (standard deviations as low as 0.055 mg L-1) indicate that XRF is suitable for aqueous Pb determinations within the concentration ranges typically encountered in environmental assessments.
Systematic biases were observed, including slopes greater than unity and negative intercepts, which suggest that raw XRF values tend to underestimate Pb concentrations relative to ICP, particularly at higher concentrations. These biases underscore the importance of regression calibration before applying XRF data in practice. Further, the magnitude of error (RMSE 0.2811 - 11.96 mg L-1) indicates the level of precision that must be acceptable to use XRF for Pb measurement.
Overall, calibrated XRF shows potential to provide a rapid, cost-effective, and accessible alternative for quantifying Pb in environmental solutions, with particular utility for community laboratories, field-based research, and resource-limited settings. By enabling rapid and affordable Pb measurements in soil extracts, XRF could expand environmental testing capacity for urban gardening, public health monitoring, and community-led remediation projects where access to conventional laboratory infrastructure is limited.
References:
Antić-Mladenović, S., Frohne, T., Kresović, M., Stärk, H.-J., Tomić, Z., Ličina, V., & Rinklebe, J. (2017). Biogeochemistry of Ni and Pb in a periodically flooded arable soil: Fractionation and redox-induced (im)mobilization. Journal of Environmental Management, 186, 141–150. https://doi.org/10.1016/j.jenvman.2016.06.005
Birch, H. F. (1958). The effect of soil drying on humus decomposition and nitrogen availability. Plant and Soil, 10(1), 9–31. https://doi.org/10.1007/BF01343734
Husson, O. (2013). Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant and Soil, 362(1–2), 389–417. https://doi.org/10.1007/s11104-012-1429-7
Klüpfel, L., Keiluweit, M., Kleber, M., & Sander, M. (2014). Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science & Technology, 48(10), 5601–5611. https://doi.org/10.1021/es500906d
McBride, M., Sauve, S., & Hendershot, W. (1997). Solubility control of Cu, Zn, Cd and Pb in contaminated soils. European Journal of Soil Science, 48(2), 337–346. https://doi.org/10.1111/j.1365-2389.1997.tb00554.x
Pignatello, J. J., Uchimiya, M., & Abiven, S. (2024). Aging of biochar in soils and its implications. In Biochar for Environmental Management (3rd ed.). Routledge.
Sauvé, S., McBride, M., & Hendershot, W. (1998). Soil Solution Speciation of Lead(II): Effects of Organic Matter and pH. Soil Science Society of America Journal, 62(3), 618–621. https://doi.org/10.2136/sssaj1998.03615995006200030010x
Skopp, J., Jawson, M. D., & Doran, J. W. (1990). Steady‐State Aerobic Microbial Activity as a Function of Soil Water Content. Soil Science Society of America Journal, 54(6), 1619–1625. https://doi.org/10.2136/sssaj1990.03615995005400060018x
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry: Chemical equilibria and rates in natural waters (Third edition). John Wiley & Sons, Inc.
Sun, T., Levin, B. D. A., Guzman, J. J. L., Enders, A., Muller, D. A., Angenent, L. T., & Lehmann, J. (2017). Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nature Communications, 8(1), 14873. https://doi.org/10.1038/ncomms14873
Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., & Sing, K. S. W. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87(9–10), 1051–1069. https://doi.org/doi:10.1515/pac-2014-1117
Tipping, E. (2002). Cation Binding by Humic Substances (1st ed.). Cambridge University Press. https://doi.org/10.1017/CBO9780511535598
Yuan, C., Gao, B., Peng, Y., Gao, X., Fan, B., & Chen, Q. (2021). A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Science of The Total Environment, 756, 144058. https://doi.org/10.1016/j.scitotenv.2020.144058
Yuan, Y., Bolan, N., Prévoteau, A., Vithanage, M., Biswas, J. K., Ok, Y. S., & Wang, H. (2017). Applications of biochar in redox-mediated reactions. Bioresource Technology, 246, 271–281. https://doi.org/10.1016/j.biortech.2017.06.154
This study presents an evaluation of biochar modification for lead (Pb) immobilization and the application of benchtop energy-dispersive X-ray fluorescence spectroscopy (XRF) for Pb analysis in liquid samples. Our findings demonstrate the potential for biochar-based materials and XRF methods to enhance environmental remediation and monitoring, while also highlighting the need for refinement to optimize their practical application.
The oxidative modification of wood chip-based biochar in air at 300°C led to significant physical and chemical changes, including an increase in specific surface area, pore development, and the introduction of oxygenated functional groups. In addition, enhanced Pb(II) sorption capacity was seen in biochar sorption experiments. The results suggest that the modification process creates additional acidic sites which facilitate stronger interactions with Pb(II).
However, when applied in soils under more realistic conditions, the impact of biochar on Pb immobilization was less pronounced. Three experiments revealed that biochar did not consistently reduce bioaccessible Pb in soil. In fact, at one sampling point, biochar amendments were found to increase bioaccessible Pb. The study included a variety of biochar treatments, ranging from unheated to thermally modified biochar (at 300°C for 1–4 hours), and tested different Pb contamination levels. The results indicated that biochar’s performance as a Pb immobilization agent was highly variable and influenced by soil conditions, with no significant differences observed in the majority of treatments. These findings underscore the complexity of biochar’s behavior in the soil environment and suggest that biochar's effectiveness may be limited by environmental factors.
In parallel, the performance of benchtop XRF as an alternative for quantifying Pb in aqueous extractants was evaluated. XRF provided reliable Pb measurements, with a high correlation to inductively coupled plasma (ICP) results (r2 > 0.98). The detection limits and quantification limits (ranging from 0.17–0.25 mg L-1 and 0.509–0.751 mg L-1, respectively) were found to be well-suited for environmental Pb concentrations typically encountered in soil extracts. Additionally, the precision of XRF was excellent, indicating that XRF could offer a rapid, cost-effective alternative to traditional laboratory techniques. However, systematic biases, including underestimation of Pb concentrations at higher levels, were observed, which suggests the need for careful calibration when using XRF for Pb quantification. Despite these biases, the technique’s reliability and accessibility make it an attractive option for field-based research, community laboratories, and low-resource settings where conventional laboratory infrastructure is limited. The ability of XRF to deliver fast and affordable Pb measurements could enhance environmental testing capacities, particularly for urban gardening, public health monitoring, and community-led remediation projects.
In conclusion, the results of this study highlight the potential of heat-modified biochar as a tool for Pb immobilization in environmental remediation efforts but also emphasize the need for further investigation into its performance in complex field conditions. The variability observed in biochar's effectiveness suggests that its utility as a remediation agent will depend heavily on site-specific factors. Furthermore, the use of XRF as a practical method for Pb quantification holds promise, particularly in resource-limited environments, although calibration is critical to ensure the accuracy of results.
Education & outreach activities and participation summary
Participation summary:
1: Conference presentations
In 2024, I presented a poster and rapid-fire talk at the ASA, CSSA, SSSA International Annual Meeting in San Antonio, TX. This opportunity allowed me to share my research on biochar and converse with other graduate students and soil science professionals from around the world. I had many insightful conversations about biochar and urban soils at the conference.
In 2025, I presented a poster on my XRF work at two student conferences held on the Penn State campus: the Gamma Sigma Delta Research Expo, and the Penn State Interdisciplinary Environmental Research Symposium (PIERS). I was able to chat with students and professors from different departments about my project, many of whom were interested in using XRF on liquid samples in their own research.
Both of my posters are available under the Information Products section of this report.
2: Penn State’s Ag Progress Days Exposition
In both 2024 and 2025, my lab hosted a booth at Ag Progress Days, a free event that features over 400 exhibitors and over 42,000 attendees each year. We were able to share our work with people from a wide array of backgrounds, including industry professionals, academics, farmers, curious gardeners, and children--roughly 100 visitors each year. In both 2024 and 2025, we held demonstrations of portable XRF for soil testing. In 2025 we focused more on my biochar research: I wrote a fact sheet on biochar to hand out alongside small bags of biochar provided free of charge by Metzler Biochar. The fact sheet is available under the Information Products section of this report.
3: YouTube Video
In Fall 2025, we produced a video with the Dutton Institute in Penn State’s College of Earth and Mineral Sciences to teach viewers about biochar and its potential uses in soil remediation. The 8-minute video details how biochar is made, its uses, and my research project. By including background information on biochar, we hoped to make the video accessible to non-academic viewers as well as the scientific community.
We first uploaded the video to the Dutton Institute’s YouTube in early January 2026 and it amassed 202 views before we re-uploaded a second version, which now has 50 views. We hope to also post the video on Penn State’s College of Agriculture channel.
Project Outcomes
My work on biochar will hopefully both draw attention to biochar as a potentially beneficial soil amendment, and also serve to educate researchers and the public on its variability. Biochar has been shown to adsorb heavy metals such as lead, but we need to further investigate how this reaction plays out in the complicated system of the soil environment. At Penn State's Ag Progress Days, I was able to share information about biochar with about 100 farmers. I have also worked with my connections at Metzler Biochar and Phospholutions in Central Pennsylvania to share ideas about innovations in biochar.
My findings on the use of XRF for analyzing liquid samples are very promising. We hope to publish articles on our method in technical journals, which should reach researchers at laboratories around the U.S. Encouraging labs to investigate cheaper, faster alternatives for soil tests will help bring down overall costs for farmers and homeowners. As a service lab, we have been able to offer XRF testing to other researchers at Penn State who are interested in measuring metals in liquid samples. XRF on liquids has also become a regular topic in our tours of the lab given to undergraduate students taking SOILS 101 at Penn State.
Finally, our video on biochar for soil remediation has already garnered 252 views, hopefully making information on the topic more accessible for the general public.
During this project, I learned about the importance of making science accessible, especially to small-scale urban growers. My advisor and I were challenged with making outreach content that could be useful to people from all walks of life, rather than just conference presentations and research papers. Working on a YouTube video for the first time was exciting to me, as we had to determine how to present our work in an engaging and concise way.
In 2024, Sage Schmouder, an undergraduate student at Penn State, joined me as a research assistant. Since then, she has applied for and received two grants from Penn State for her work with biochar and contaminated soils. When I met Sage, I discovered that we are both natives of Johnstown, PA, and had separately decided to study soil contamination after having lived in an area contaminated by a history of industry. For both of us, this project was an opportunity to learn more about the problems faced by farmers and gardeners in the Rustbelt, and hopefully we will one day be able to contribute to solving some of the environmental issues we've grown up surrounded by.
During this project, I enjoyed making connections in both academic and non-academic laboratories and hope to become a researcher at a soil lab following my graduation. Environmental testing is critical for ensuring the safety, sustainability, and security of America's food supply and natural resources.
The most challenging aspect of the project for me was the experimental set-up and data analysis for our soil experiments. There were many potentially confounding variables to consider when working with soil, and yet these laboratory trials were highly simplified compared to field conditions! In retrospect, I would have liked to have been able to use many different biochars, soil types, variable conditions, and longer periods of time, but that was not possible in only two years.
I found the XRF method development to be the most exciting part of the project. I would like to see future work carried out involving different solution matrices and analytes--for instance, total Pb digestions from soil (like EPA Method 3050B) or digestions for other heavy metals.