Final Report for GS14-131
Project Information
Squash bug is a major pest of squash and pumpkin in the U.S. Current control tactics involve insecticide applications that are injurious to pollinators and natural enemies. Based on a 3-year survey in Virginia and surrounding states, we found that >50% of squash bug egg masses were parasitized by the parasitic wasp, Gryon pennsylvanicum. We also evaluated the toxicity of various reduced risk insecticides to the parasitoid and squash bug and found that neither flupyridafurone, pyrifluquinazon, flonicamid, nor sulfoxaflor controlled squash bug nymphs as well as the pyrethroid, lambda-cyhalothrin, which was also the most toxic to the parasitoid adults.
Introduction
The squash bug, Anasa tristis DeGeer (Hemiptera: Coreidae), is an important pest of pumpkin (Cucurbita maxima) and squash (C. pepo) causing wilt in plants with its piercing-sucking mouthparts and by potentially vectoring Cucurbit Yellow Vine Decline (Bruton et al. 2003, Doughty et al. 2016). Many commercial growers of these crops typically apply broad-spectrum foliar insecticides, commonly pyrethroids (often tank-mixed with fungicides) ( Doughty et al. 2016). Cucurbits are pollination dependent and many of the registered insecticides recommended for use in commercial cucurbit production are known to be acutely toxic to bees (Agency 2015, Wyenandt et al. 2016). Beyond pollinators there are other organisms that may be impacted by pest management practices, for instance, natural enemies that could be keeping pests in check in cucurbit systems.
Egg parasitoids, in particular, are important natural enemies of heteropteran pests. Out of the native egg parasitoids of A. tristis, Gryon pennsylvanicum Ashmead (Hymenoptera: Scelionidae), has been found to have the highest fecundity and rate of reproduction than three other encyrtid wasp species that also attack squash bug (Nechols et al. 1989). Gryon pennsylvanicum also appears to be widespread in North America and was reported to occur in the mid-Atlantic U.S. as early as 1943 (Schell 1943). This scelionid wasp has a host range that is limited to the leaf-footed bugs (family Coreidae), and has been recently introduced as a classical biological control agent for the western conifer seed bug in Italy (Peverieri et al. 2013, Roversi et al. 2014). Plant hosts can play a potential role in the capacity of A. tristis to lay eggs (Bonjour et al. 1990), overall survival (Bonjour and Fargo 1989), and squash bug reproduction (Bonjour et al. 1993), without effect on the parasitization of G. pennsylvanicum (Vogt and Nechols 1993). Adult G. pennsylvanicum do not feed on the host eggs (Vogt and Nechols 1993), but rather feed on the exudate from cucurbit leaf trichomes (extra-floral nectaries) that serve as sources of basic sugars and protein (Olson et al. 1996). The feasibility of augmentative biological control has been explored, but Olson et al. (1996) found that it was not economically feasible compared to chemical control. Nonetheless, it is important to assess the natural control impact of this parasitoid. In Kentucky, Decker and Yeargan (2008) observed egg parasitism as high as 31% with G. pennsylvanicum as the predominant egg parasitoid. More recently, Cornelius et al. (2016) observed high rates of parasitism by this species in Maryland, particulalry later in the season. To our knowledge a survey of squash bug egg parasitism has not been conducted in Virginia. Herein, we report the results of a three year survey from 31 counties throughout Virginia and surrounding states to allow us to quantify the potential effects egg parasitism may have on squash bug population dynamics.
In addition we evaluated the efficacy of various reduced risk insecticides for control of squash bug and toxicity to the parasitoid.
References Cited:
Abney, M. R., and R. Davila. 2011. Evaluation of Foliar and Soil Applied Insecticides for Control of Squash Bug on Zucchini Squash, 2010. Arthropod Management Tests 36.
Agency, U. S. E. P. 2015. EPA's Proposal to Mitigate Exposure to Bees from Acutely Toxic Pesticide Products.
Authority, E. P. 2015. Addendum to the Evaluation and Review Report APP202145 - Mainman.
Beard, R. L. 1940. The Biology of Anasa tristis DeGeer with Particular Reference to the Tachinid Parasite, Trichopoda pennipes Fabr., pp. 593-685. In C. A. E. S. N. Haven [ed.].
Bommireddy, P. L., B. R. Leonard, K. D. Emfinger, and P. Price. 2007. Evaluation of Neonicotinoids and Flonicamid Against Cotton Aphids and Tarnished Plant Bugs in Cotton, 2006. Arthropod Management Tests.
Bonjour, E. L., and W. S. Fargo. 1989. Host Effects on the Survival and Development of Anasa tristis (Heteroptera: Coreidae). Environmental Entomology 18: 1083-1085.
Bonjour, E. L., W. S. Fargo, and P. E. Rensner. 1990. Ovipositional Preference of Squash Bugs (Heteroptera: Coreidae) Among Cucurbits in Oklahoma. Journal of Economic Entomology 83: 943-947.
Bonjour, E. L., W. S. Fargo, A. A. Al-Obaidi, and M. E. Payton. 1993. Host Effects on Repreoduction and Adult Longevity of Squash Bugs (Heteroptera: Coreidae). Environmental Entomology 22: 1344-1348.
Bruton, B. D., F. Mitchell, J. Fletcher, S. D. Pair, A. Wayadande, U. Melcher, J. Brady, B. Bextine, and T. W. Popham. 2003. Serratia marcescens, a Phloem-Colonizing, Squash Bug -Transmitted Bacterium: Causal Agent of Cucurbit Yellow Vine Disease. Plant Disease 87: 937-944.
Casida, J.E.; Durkin, K.A. 2013. Neuroactive insecticides: targets, selectivity, resistance, and secondary Effects. Annual Review of Entomology. 58: 99–117.
Chapman, A., T. Kuhar, P. Schultz, T. Leslie, S. Fleischer, G. Dively & J. Whalen. 2009. Integrating chemical and biological control of European corn borer in bell pepper. J. Econ. Entomol. 102: 287 295.
Cornelius, M. L., M. L. Buffington, E. J. Talamas, and M. W. Gates. 2016. Impact of the Egg Parasitoid, Gryon pennsylvanicum (Hymenoptera: Scelionidae), on Sentinel and Wild Egg Masses of the Squash Bug (Hemiptera: Coreidae) in Maryland. Environmental Entomology 45: 367-375.
Decker, K. B., and K. V. Yeargan. 2008. Seasonal Phenology and Natural Enemies of the Squash Bug (Hemiptera: Coreidae) in Kentucky. Environmental Entomology 37: 670-678.
Desneux N, A. Decourtye, and J. M. Delpuech 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52: 81–106.
Doughty, H. B., J. M. Wilson, P. B. Schultz, and T. P. Kuhar. 2016. Squash Bug (Hemiptera: Coreidae): Biology and Management in Cucurbitaceous Crops. Journal of Integrated Pest Management 7: 1.
Eiben, J., C. Mackey, W. Roberts, and J. V. Edelson. 2004. Foliar Applied Insecticides for Controlling Squash Bug, 2003. Arthropod Management Tests 29.
EPA, O. o. P. P. 2012. Ecological Risk Summary for the Section 3 New Chemical Registration of Pyrifluquinazon for Indoor Greenhouse Use. In O. o. P. P. EPA [ed.].
Fargo, W. S., P. E. Rensner, E. L. Bonjour, and T. L. Wagner. 1988. Population Dynamics in the Squash Bug (Heteroptera: Coreidae)-Squash Plant (Cucurbitales: Cucurbitaceae) System in Oklahoma. Journal of Economic Entomology 81: 1073-1079.
Foster, S. P., G. Devine, and A. L. Devonshire. 2007. Insecticide resistance, pp. 261–285 in H. F. van Emden and R. Harrington, Aphids as Crop Pests. CABI, U.K.
Ghidiu, G. M. & T. P. Kuhar. 2012. Chapter 16: Pepper insects and their control, pp. 216 226. In V. M. Russo [ed.], Peppers: Botany, Production and Uses. CAB International, Oxfordshire, United Kingdom., 280 pp.
IRAC, I. M. W. G. 2016. IRAC Mode of Action Classification Scheme, pp. 1-26.
Johansen, C. A. 1977. Pesticides and pollinators. Annu. Rev. Entomol. 22: 177-192.
Kilpatrick, A. L., A. M. Hagerty, S. G. Turnipseed, M. J. Sullivan, and W. C. J. Bridges. 2005. Activity of Selected Neonicotinoids and Dicrotophos on Nontarget Arthropods in Cotton: Implications in Insect Management. Journal of Economic Entomology 98: 814-820.
Kuhar, T. P., J. Speese, R. J. Cordero and V. M. Barlow. 2005. Evaluation of insecticides in pumpkins, 2004. Arthrop. Manag. Tests 30: E70.
Kuhar, T. P., C. R. Philips, K. Kamminga, and A. Wallingford. 2011. Pyrethroid resistance in green peach aphid in Southwestern Virginia (USA) and field efficacy of insecticides in peppers. Resist. Pest News. 21 (Fall): 8-11. Online. http://whalonlab.msu.edu/newsletter/index.html.
Kuhar, T. P., H. Doughty, K. Kamminga, A. Wallingford, C. Philips & J. Aigner. 2012. Evaluation of insecticides for the control of brown marmorated stink bugs in bell peppers in Virginia 2011 Experiment 1. Arthropod Managt. Tests 38: E37.
Kuhar, T. P., and H. Doughty. 2016. Evaluation of Foliar and Soil Insecticides for the Control of Foliar Insects in Summer Squash in Virginia, 2015. Arthropod Management Tests 41: tsw023.
Margolies, D. C., J. R. Nechols, and E. A. Vogt. 1998. Rapid Adaptation of Squash Bug, Anasa tristis, Populations to a Resistant Cucurbit Cultivar. Entomologia Experimentalis et Applicata 89: 65-70.
McLeod, P., J. Diaz, S. Eaton, and L. Martin. 2003. Evaluation of insecticides for control of squash bug on summer squash, 2002. Arthropod Manag. Tests (2003) 28 (1): DOI: http://dx.doi.org/10.1093/amt/28.1.E72
Morita M, Ueda T, Yoneda T, Koyanagi T, Haga T. 2007. Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest Manag Sci. 2007 Oct;63(10):969-73.
Natwick, E. T., and M. I. Lopez. 2016. Insecticide Evaluation for Aphid Control in Alfalfa, 2015. Arthropod Management Tests 41: tsw025.
Nauen, R. Peter Jeschke, Robert Velten, Michael E Beck, Ulrich Ebbinghaus-Kintscher, Wolfgang Thielert, Katharina Wölfel, Matthias Haas, Klaus Kunz, and Georg Raupach. 2015. Flupyradifurone: a brief profile of a new butenolide insecticide. Pest Manag Sci. 2015 Jun; 71(6): 850–862.
Neal, J. J. 1993. Xylem Transport Interruption by Anasa trsitis Feeding Causes Cucurbita pepo to Wilt. Entomologia Experimentalis et Applicata 69: 195-200.
Nechols, J. R., J. L. Tracy, and E. A. Vogt. 1989. Comparative Ecological Studies of Indigenous Egg Parasitoids (Hymenoptera: Scelionidae; Encyrtidae) of the Squash Bug, Anasa tristis (Hemiptera: Coreidae). Journal of the Kansas Entomological Society 62: 177-188.
Olson, D. L., J. R. Nechols, and B. W. Schurle. 1996. Comparative Evaluation of Population Effect and Economic Potential of Biological Suppression Tactics Versus Chemical Control for Squash Bug (Heteroptera: Coreidae) Management on Pumpkins. Journal of economic entomology 89: 631-631.
Pair, S. D., B. D. Bruton, F. Mitchell, J. Fletcher, A. Wayadande, and U. Melcher. 2004. Overwintering Squash Bugs Harbor and Transmit the Causal Agent of Cucurbit Yellow Vine Disease. Journal of Economic Entomology 97: 74-78.
Palumbo, J. C. 2006. Evaluation of Flonicamid and Acetamiprid for Aphid Control in Head Lettuce, Spring 2005. Arthropod Management Tests.
Palumbo, J. C. 2014. Evaluation of Pyrifluquinazon for Control of Sweetpotato Whitefly in Fall Cantaloupes, 2012. Arthropod Management Tests 38.
Palumbo, J. C. 2016. Control of Sweetpotato Whitefly Adults with Foliar Insecticide Alternatives in Spring Cantaloupes, 2014. Arthropod Management Tests 40: E38.
Peverieri, G. S., P. Furlan, D. Benassai, S. Caradonna, W. B. Strong, and P. F. Roversi. 2013. Host Egg Age of Leptoglossus occidentalis (Heteroptera, Coreidae) and Parasitism by Gryon pennsylvanicum (Hymenoptera, Platygastridae). Journal of Economic Entomology 106: 633-640.
Roversi, P. F., G. Sabbatini Peverieri, M. Maltese, P. Furlan, W. B. Strong, and V. Caleca. 2014. Pre-release risk assessment of the egg-parasitoid Gryon pennsylvanicum for classical biological control of Leptoglossus occidentalis. Journal of Applied Entomology 138: 27-35.
Sappington, K., and M. Ruhman. 2016. 2016 Addendum for the Proposed Section 3 Registration of Transform WG and Closer SC (Sufoxaflor) For Use on Various Crops, Turf, and Ornamentals.
Schell, S. C. 1943. The Biology of Hadronotus ajax Girault (Hymenoptera - Scelionidae), a Parasite in the Eggs of Squash-bug (Anasa tristis DeGeer). Annals of the Entomological Society of America 36: 625-635.
Slosser E, Pinchak WE, Rummel DR. 1989. A review of known and potential factors affecting the population dynamics of the cotton aphid. Southwestern Entomologist 14: 302-313.
Smith, T. M. and G. W. Stratton. 1986. Effects of synthetic pyrethroid insecticides on nontarget organisms. Residue Reviews: Reviews of Environmental Contamination and Toxicology: 93-120.
Stansly, P. A., and B. C. Kostyk. 2016. Control of Sweetpotato Whitefly With Foliar Insecticides on Staked Tomatoes, Fall 2013. Arthropod Management Tests 40: E61.
Vogt, E. A., and J. R. Nechols. 1993. The Influence of Host Deprivation and Host Source on the Reproductive Biology and Longevity of the Squash Bug Egg Parasitoid Gryon pennsylvanicum (Ashmead) (Hymenoptera: Scelionidae). Biological Control 3: 148-154.
Wyenandt, A., T. P. Kuhar, G. C. Hamilton, M. J. VanGessel, E. Sanchez, and D. Dugan. 2016. 2016 Mid-Atlantic Commercial Vegetable Production Recomendations. Virginia Cooperative Extension.
Objective 1: To survey the egg parasitoids of squash bugs throughout Virginia.
- Collections of squash bug egg masses
- The specimens collected will be taken to the Virginia Tech Vegetable Entomology Lab and reared in environmental chambers for identification to species where possible. Records of location, host plant, parasitism levels, and squash bug hatch rate will be made.
Objective 2: To assess the effects of narrow-spectrum insecticides on the eggs and nymphs of squash bug and its egg parasitoid.
Egg masses collected will be utilized in screening assays to by location. Assays will screen for effects of specific narrow-spectrum insecticides against the controls and a commonly used broad-spectrum insecticide. The controls will provide baseline data on the parasitism levels and squash bug hatch rates for each location. The assay will be structured as follows:
Bioassay replicated 5 to 6 times. For each rep per treatment, 1o egg masses will be dipped in treatments.
Treatments : water control, Lambda-Cyhalothrin, flupyridafurone, Pyrifluquinazon, Flonicamid, and Sulfoxaflor
All treatment concentrations will be at maximum label rate
Assays were replicated as masses became available
Cooperators
Research
Obj. 1. Parasitoid survey:
Throughout the summer months from 2013 to 2015, we collected squash bug egg masses from squash and pumpkins located throughout Virginia and a few nearby states. In 2013, we collected 71 egg masses from Montgomery County and 10 egg masses from Washington County, VA. In 2014 and 2015, we increased the robustness and geographic reach of the survey, by sampling >25 counties in Virginia, Tennessee, South Carolina, Maryland, and Kentucky. Egg masses were found in untreated fields of squash or pumpkins on research farms, organic farms, and community gardens. We also searched for egg masses on several conventional commercial squash fields, and seldom found any in sufficient densities. Presumably, the frequent use of insecticides eliminated squash bug densities in those fields. Surveys subsequently focused on insecticide-free cucurbits. Across the survey, there were 31 egg masses recovered from Virginia’s coastal region. The piedmont of Virginia collections accounted for 153 egg masses and the Appalachian mountain region comprised of 301 samples.
Squash bug egg masses were removed from leaves in the field by cutting around the egg mass and placing it individually into 60 x 15 mm Petri dishes, which were kept at room temperature until all nymphs and adult parasitoids had emerged. Once all nymphs and parasitoids had perished, samples were processed and parasitism and squash bug hatch rates were recorded by location.
Obj. 2. Insecticide Bioassays
Our study aimed to utilize A. tristis egg masses that were collected during a concurrent survey of G. pennsylvanicum throughout the Commonwealth of Virginia (Chapter 2)). Collections were made when A. tristis eggs were prevalent and freshly laid on squash or pumpkins. These conditions were most frequently met on farms where chemical control methods were infrequent or actively avoided. The collection period spanned the 2014 and 2015 summer months and included 25 counties; the egg masses utilized in the present experiments were from those collection efforts.
Squash bug nymph toxicity bioassays.
All bioassays were set up when a sufficient number of individuals were available. Insecticide treatments were commercial formulations of products provided by the manufacturers (Table 1). During the 2015 and 2016 field seasons, A. tristis egg masses were placed in a mesh cage at room temperature. Once nymphs emerged, they were place on young potted plants of either ‘Lioness’ yellow summer squash or ‘Tigress’ zucchini grown in the greenhouse. When sufficient numbers of 2nd - 4th instars were available in this colony, they were used immediately in the bioassay. All insecticides were mixed at label rates (Table 1). Fresh cut discs (8-10 cm diameter) of summer squash fruit were dipped in insecticide solution and five nymphs were placed with the disc into 150 x 15 mm Petri dishes. Dishes were kept at room temperature (TEMP range) and mortality was assessed at 24 and 48 hours after treatment. Control mortality was corrected with Abbott’s formula.
Table 1. List of insecticides and concentrations used in laboratory toxicity bioassays on A. tristis and G. pennsylvanicum.
|
Active ingredient (AI) |
Product (Manufacturer) |
IRAC Insecticide Group |
Relative Toxicity to Bees |
|
flonicamid |
Beleaf (FMC) |
29 |
No Observable Adverse Effect1 |
|
flupyradifurone |
Sivanto (Bayer) |
4D |
Highly Toxic (oral)2 |
|
λ-cyhalothrin |
Warrior II (Syngenta) |
3A |
Acutely Toxic3 |
|
pyrifluquinazon |
Nichino America |
|
Practically Non-toxic4 |
|
sulfoxaflor |
Closer (Dow) |
4C |
Very Highly Toxic2 |
1 (Authority 2015) 2 (Sappington and Ruhman 2016) 3 (Agency 2015) 4 (EPA 2012)
Squash bug egg mass dip toxicity bioassays
In 2014, five bioassays, with 387 egg masses, while the 2015 field season permitted the completion of five separate bioassays, utilizing over 300 egg masses. Included in these bioassays were egg masses from 3 counties and 6 sites, all with known occurrence of, or visible parasitization. A. tristis egg mass dip assays were carried out with the aforementioned insecticide treatments (Table 1) with 10 egg masses per treatment, samples averaged 18 eggs per mass. Egg masses were left on leaf discs and dipped in insecticide treatment solutions. The assays were replicated as masses were encountered in the field for a total of nine replications over the two field seasons. Treatment concentrations were based on field tank spray concentrations from recommended label rates for pumpkin or squash commercial vegetable production (Table 1). Leaf discs were returned to 60 x 15 mm Petri dishes and A. tristis nymphs or parasitoids contained within were allowed to develop at room temperature. Counts of nymph and parasitoid emergences were made after all emerged insects died to reduce losses from live specimens escaping. Each egg mass was examined separately for partially emerged specimens as well. Data from the nine replicates were pooled and an ANOVA was used to analyze for significant treatment effects on nymph and adult parasitoid emergence.
Adult G. pennsylvanicum toxicity bioassays
In 2015, emerging G. pennsylvanicum adults were collected and provided with a 50:50 honey: water solution ad libitum at room temperature (~ 27ºC). The adults were then utilized in a contact assay. Treatments followed those outlined in Table 1 and 5.5 cm P8 filter paper discs were dipped in the insecticidal solutions and allowed to dry under a ventilation hood. Once dry, these discs were then used to line 15 ml conical tubes. Adult parasitoids were gently aspirated from the colony and placed in conical tubes evenly distributed in groups of seven or more across all six treatments. Adult mortality was assessed at 24, 48, and 72 hours after introduction to the tubes and control mortality was corrected using Abbott’s formula. Proportion mortality data of A. tristis nymphs and G. pennsylvanicum adults from direct exposure bioassays as well as numbers of A. tristis or G. pennsylvanicum adults emerging from field-collected and dipped eggs were analyzed using ANOVA in JMP 11. 0 (SAS, Cary, NC). Fisher’s Protected LSD test was used to compare means among different treatments.
Obj. 1.
In 2013, the parasitoid wasp G. pennsylvanicum was recovered from 85% of the 71 egg masses from Montgomery County Virginia, and 90 % of the 10 masses collected in Washington County Virginia. The average rate of parasitization per egg mass was 83%. Occasional egg masses from one Montgomery County site included the eupelmid wasp Anastatus reduvii Howard.
In 2014, we sampled 34 counties in Virginia, Tennessee, South Carolina, Maryland, and Kentucky and recovered G. pennsylvanicum from 29 of those counties. Egg masses were collected from the Eastern Shore to the mountains of Virginia and on average 45% of collected egg masses were parasitized. In 2015, 18 of the 25 sampled counties had the egg parasitoid G. pennsylvanicum with 50% of collected egg masses were parasitized. The parasitoid was widespread in squash bug eggs causing varying egg mortality across the region, with 100% parasitization recorded from multiple counties each year. The predominant parasitoid species was G. pennsylvanicum, previously reported from squash bug eggs in North Carolina, Kansas, and Kentucky, but at much lower parasitism levels (Schell 1943, Olson et al. 1996, Decker and Yeargan 2008). Dividing Virginia into mountain, piedmont and coastal areas revealed variation in sample numbers and parasitization rates. There were 31 egg masses recovered from Virginia’s coastal region, 30% of those were parasitized. The piedmont of Virginia collections accounted for 153 egg masses and 39% parasitization. The mountain region of Virginia samples was comprised of 301 samples with a 72% parasitization rate.
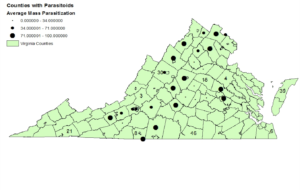 Figure 1: Map of Virginia and its counties showing the average mass parasitization by size of the circle in the respective county.
Figure 1: Map of Virginia and its counties showing the average mass parasitization by size of the circle in the respective county.
Obj. 2.
Squash bug nymph toxicity bioassays.
There were no significant differences in mortality across treatments at 24 hr after treatment. At 48 hr, there was a significant treatment effect with λ-cyhalothrin having significantly higher rate of mortality than the other insecticide treatments (F=4.472; df=4,14; p<0.0249; Fig. 2). There were no significant effect of treatment on the number of emerging squash bug nymphs from the dipped eggs (F=1.7142; df=4,566; p<0.1294; Fig. 3. There was a significant effect of treatment on the number of G. pennsylvanicum adults emerging from dipped egg masses with λ-cyhalothrin having fewer emerging parasitoids than the other treatments (F=4.5810; df=5, 566; p<0.001; Fig. 4). There was also a significant effect of treatment on % mortality of G. pennsylvanicum adults exposed to treated filter paper (F=24.5841; df= 4, 9; p<0.0017); sulfoxaflor and λ-cyhalothrin were found to have a higher rate of mortality when compared to the other treatments at 72 hours after treatment (Fig. 5). The percent mortality at 24 and 48 hours after treatment were found to have no significant differences (F=3.871; df= 4, 9; p<0.0871) and (F=0.7726; df= 4, 9; p<0.5870) respectively, see figure 5.
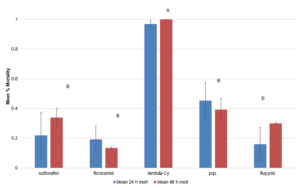 Figure 2. Mean ± SEM proportion mortality of squash bug nymphs from three separate toxicity bioassays where nymphs were held on treated (insecticide-dipped) squash discs . Columns within the same assessment time with a letter in common n are not significantly different. Pqz = pyrifluquinazon; flupyrid. = flupyridafurone
Figure 2. Mean ± SEM proportion mortality of squash bug nymphs from three separate toxicity bioassays where nymphs were held on treated (insecticide-dipped) squash discs . Columns within the same assessment time with a letter in common n are not significantly different. Pqz = pyrifluquinazon; flupyrid. = flupyridafurone
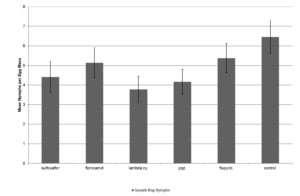 Figure 3. Mean ± SEM number of emerging A. tristis nymphs per egg mass dipped in various insecticide treatments over nine separate bioassays over the 2014 and 2015 field seasons
Figure 3. Mean ± SEM number of emerging A. tristis nymphs per egg mass dipped in various insecticide treatments over nine separate bioassays over the 2014 and 2015 field seasons 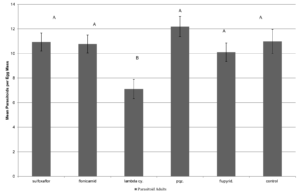 Figure 4. Mean ± SEM number of emerging Gryon pennsylvanicum per egg mass dipped in various insecticide treatments over nine separate bioassays over the 2014 and 2015 field seasons. Columns within the same assessment time with a letter in common n are not significantly different.
Figure 4. Mean ± SEM number of emerging Gryon pennsylvanicum per egg mass dipped in various insecticide treatments over nine separate bioassays over the 2014 and 2015 field seasons. Columns within the same assessment time with a letter in common n are not significantly different. 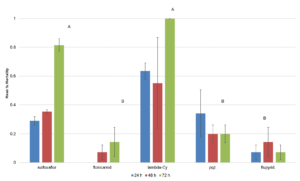 Figure 5. Mean ± SEM mortality of adult Gryon pennsylvanicum wasps after exposure to insecticide. Columns within the same assessment time with a letter in common n are not significantly different.
Figure 5. Mean ± SEM mortality of adult Gryon pennsylvanicum wasps after exposure to insecticide. Columns within the same assessment time with a letter in common n are not significantly different.
Educational & Outreach Activities
Participation Summary:
Publications:
Wilson, J. 2016. The Effects of Insecticides on Squash Bug, its Egg Parasitoids and Pollinators in Virginia Cucurbit Production. Ph.D. Dissertation, Virginia Tech.
Wilson, J. M., Kuhar, T.P. Benefits of an Insecticide Seed Treatment for Pumpkin Production in Virginia (2015) Virginia Cooperative Extension. Pub: # ENTO-174NP
Doughty, H.B., Wilson, J.M., Kuhar, T.P., Schultz, P.B. 2016. The Squash Bug (Anasa tristis (DeGeer)): Biology and Management in Cucurbitaceous Crops. Journal of Integrated Pest Management DOI: http://dx.doi.org/10.1093/jipm/pmv024 1 First published online: 19 January 2016
Wilson, J.M., Kuhar, T.P. Management of Squash Bug on Cucurbit Crop Proceedings of 2015 The Mid-Atlantic Fruit & Vegetable Convention Hershey, PA, pp. 219-220
Presentations:
Wilson, J., T. Anderson, and T. Kuhar. 2015. Tiny terrors: Egg parasitoids of Virginia squash bugs. Virginia Academy of Science 93rd Annual Meeting, Harrisonburg, VA, May 21-23, 2015
Kuhar, T. and J. Wilson. 2015. Squash bug update, 2015 Mid-Atlantic Fruit and Vegetable Convention, Hershey, PA, January 27-29, 2015
Federovitch,Casey L., Wilson, J. M., Kuhar, T.P. Will The Good Wasp Inside Survive?
Effect of Varied Insecticides on Squash Bug Egg Parasitoid, Gryon pennsylvanicum 2016 Undergraduate Research and Creative Scholarship Conference. Virginia Polytechnic Institute and State University. Feb. 2016 Undergraduate Research Symposium Student Poster
Wilson, J.M., Anderson, T.D., Kuhar, T.P. Bees, Wasps, and Insecticides; Maintaining a Healthy Mix in Cucurbit Production. Annual Meeting of the ESA Eastern Branch. Philadelphia, PA. Jan. 2016 Phd Poster Competition
Wilson, J.M., Anderson, T.D., Kuhar, T.P. The Impact of a Squash Bug Egg Parasitoid and its Sensitivity to Selective Insecticides Entomological Society of America Annual Meeting, Minneapolis, MN. Nov. 2015. Oral Presentation
Wilson, J.M. Improving Integrated Pest Management in Virginia Cucurbit Production Virginia Agricultural Council Farm Tour, Kentland Farm, Blacksburg, VA. Aug. 2015. Oral Presentation
Wilson, J.M., Kuhar, T.P. Management of Squash Bug on Cucurbit Crop Proceedings of 2015 The Mid-Atlantic Fruit & Vegetable Convention Hershey, PA, pp. 219-220
Wilson, J.M. Cucurbit Pest Management and Biological Control of Squash Bug. 2015 Virginia Tech College of Agriculture and Life Sciences Pesticide Applicator Recertification Course. Virginia Polytechnic Institute and State University, Blacksburg, VA Jan. 2015. Invited Lecturer
Wilson, J.M., Kuhar, T.P., Anderson, T.D. Squash Bug (Anasa tristis DeGeer) egg, nymph, and associated parasitoid fate; a reduced risk insecticide screening. Entomological Society of America Annual Meeting, Portland, OR Nov. 2014. Poster Presentation
Wilson, J.M. Cucurbit Pests and Beneficial Insects in Conventional Farming. Shenandoah Valley Produce Auction Vegetable Grower Meeting, Dayton, VA Jul. 2014. Oral Presentation
Wilson, J.M. Parasitism of Squash Bug Eggs in Organic Farming Systems. On Farm Twilight Vegetable Grower Meeting, Water Penny Farm. Sperryville, VA Jul. 2014. Oral Presentation
Wilson, J.M. Pests of Plants in the Cucumber Family and their Associated Natural Enemies. YMCA Twilight Grower Meeting. Blacksburg, VA Jul. 2014. Invited Oral Presentation
Wilson, J.M., Kuhar, T.P., Anderson, T.D. Egg Parasitism Levels of Squash Bug (Anasa tristis DeGeer) in Virginia. Annual Meeting of the ESA Eastern Branch. Williamsburg, VA. Mar. 2014. Poster Presentation
Wilson, J.M., Kuhar, T.P. Insect Pest Management for Pumpkins in Virginia. Virginia Pumpkin Growers Association Meeting. Hillsville, VA Jan. 2014. Invited Oral Presentation
Project Outcomes
Sampling throughout Virginia showed widespread occurrence of G. pennsylvanicum that was at times accompanied by very high rates of parasitism. Rates of 100% parasitization were sporadic and often associated with low sample sizes, but parasitization averaged 72% in samples (n= 301 egg masses) collected from the Appalachian ridge and valley region of Virginia. These rates of parasitism exceed what has been reported in Kentucky (Decker and Yeargan 2008), and recently in Maryland (Cornelius et al. 2016). However, Cornelius et al. (2016), did show that parasitism increased significantly later in the summer. Other than effective chemical control of squash bug, no other observable factors in this survey were found to limit the observance of parasitization by G. pennsylvanicum. However, lower rates were found in the Coastal Plain and Piedmont counties. Additional exploration in Virginia and the surrounding states may provide more information on those limiting factors.
- pennsylvanicum has been considered for augmentative biological control of squash bug in the past (Olson et al. 1996), but was not cost efficient when compared to conventional squash bug management. Based on this three year survey, G. pennsylvanicum was determined to be a major natural enemy of squash bug eggs in Virginia. Conservation of this natural enemy should therefore be a priority for integrated pest management programs in cucurbits. In Virginia, organic and or reduced spray managed squash and pumpkin fields will likely benefit from the conservation of G. pennsylvanicum. Using an integrated approach to squash bug management as suggested by Doughty et al. (2016) may be the most sound and sustainable way to control squash bug.
Currently only pyrethroids and neonicotinoids are recommended for chemical control of squash bug. Although they are efficacious (Eiben et al. 2004, Abney and Davila 2011), pyrethroids are highly toxic to pollinators and natural enemies (Smith and Stratton 1986) and can result in outbreaks of secondary pests such as A. gossypii (Slosser et al. 1989). Neonicotinoids are also effective at controlling squash bug (McLeod et al. 2003, Eiben et al. 2004, Kuhar et al. 2005, Abney and Davila 2011, Kuhar and Doughty 2016), but are toxic to many non-target organisms and are generally not compatible with pollinator protection plans. In the present study, none of the reduced risk insecticides that were evaluated, flonicamid, flupyradifurone, pyrifluquinazon, or sulfoxaflor, provided effective control of squash bug nymphs in lab bioassays. However, in field experiments, flupyradifurone and sufoxaflor have resulted in significant reductions in squash bug densities compared to untreated plots (Kuhar and Doughty 2016). In another field experiment, Eiben et al. (2004) showed that flonicamid did not reduce the number of squash bugs on summer squash. To our knowledge, there is no previously reported efficacy of pyrifluquinazon on squash bug. Thus, the aforementioned insecticides may not be the sole answer for squash bug control, but they have been shown to be highly efficacious for control of homopteran pests such as whiteflies and aphids (Palumbo 2006, Bommireddy et al. 2007, Palumbo 2014, Kuhar and Doughty 2016, Natwick and Lopez 2016, Palumbo 2016, Stansly and Kostyk 2016). Thus, knowledge of their potential to at least suppress squash bug densities is important as is their impact on a key egg parasitoid of squash bug. In our bioassays, none of the reduced risk insecticides had a negative effect on the number of adult G. pennsylvanicum emerging from dipped squash bug eggs, and only sulfoxaflor caused significant mortality in adult parasitoid exposure assays. In comparison, λ-cyhalothrin reduced the number of G. pennsylvanicum emerging from dipped egg masses and was toxic to adults.
In general, we showed little to no ovicidal activity to squash bug from any of the insecticides that we tested. Ovicidal activity has been found in the neonicotinoid class of insecticides (4A) on eggs of the bollworm (Kilpatrick et al. 2005). We did not find the two compounds that share a similar mode-of-action (IRAC 2016), competitive modulators of the nicotinic acetylcholine receptor, the sufoxamine (4C) or the butenolide (4D), to have significant ovicidal effects. Current pest management recommendations call for control efforts to made after emerged nymphs can be found in the field, and our results further support this recommendation (Doughty et al. 2015). We have seen that the effectiveness of the native parasitoid G. pennsylvanicum can be locally great (Obj. 1), thus, an integrated approach in cucurbit production that minimizes early season foliar applications of broad-spectrum insecticides could support a conservation biological control in some settings.
Economic Analysis
N/A
Farmer Adoption
Through the open-access J. IPM publication on squash bug management and through Extension bulletins, as well as presentations at field days and workshops, we estimate that that over 400 growers were educated on the results of this project. We are confident that we brought significant awareness of this important egg parasitoid in the cucurbit agroecosystems. We have demonstrated through a 3-yr survey that the effectiveness of the native parasitoid G. pennsylvanicum can be locally great contributing significantly to reducing squash bug population levels, thus, an integrated approach in cucurbit production that minimizes early season foliar applications of broad-spectrum insecticides could support a conservation biological control. Unfortunately, we were not able to identify a reduced risk insecticide that was efficacious for controlling squash bug, and thus, use of a pyrethroid like lambda-cyhalothrin may be needed. However, we did demonstrate that if the parasitoid is developing within squash bug eggs, then they should not be impacted by these insecticide applications.
Areas needing additional study
Further evaluation of less toxic insecticides to natural enemies for use against squash bug.