Final Report for GS15-146
Project Information
Sweet corn IPM trials were conducted investigating insecticide efficacy for picture-winged flies and crop destruction methods to prevent the completion of fly development in the soil after harvest. Pyrethroid treated plots reduced silk fly populations and resulted in low levels of silk fly ear damage, but did not protect against fall armyworm. Spinetoram mixed with a feeding stimulant provided fall armyworm protection and intermediate silk fly protection in the fall and equivalent protection in the spring. Crop destruction techniques did not affect adult fly emergence from soil in the fall, but residue incorporation reduced fly emergence in the spring.
Introduction
The purpose of this project is to investigate more environmentally and economically sustainable approaches for silk fly management in fresh market sweet corn fields. Florida is the second largest producer of fresh market sweet corn (USDA/NASS 2016), and much of that is grown in southern Florida where silk flies (Diptera: Ulidiidae) are severe pests. Sweet corn is treated mostly with pyrethroids to prevent unacceptable damage to harvestable ears by silk flies. Females oviposit in fresh silks, and the larvae consume the developing ear. There are few non-pyrethroid options due to label and efficacy limitations. Adult flies quickly re-infest treated fields (Goyal 2010) and insecticide residues rapidly degrade. Fields are sometimes treated more than three times per week to prevent females from ovipositing in the silks (Anonymous 2009). Due to heavy pyrethroid reliance, silk flies are under heavy selection pressure for developing resistance. Growers have commented that flies are increasingly more difficult to control. Some of the flies present soon after treatment may have simply recovered from initial intoxication. Laboratory bioassays published in 2004 raised concerns over the potential for resistance development (Nuessly and Hentz 2004). This project will perform much needed resistance monitoring of pyrethroid treated flies in the field.
Poor canopy coverage and penetration when using contact insecticides can also lead to control failure. Silk flies congregate near the top of the canopy in the evening hours (Seal et al. 1995), but can be found throughout the crop canopy during the day. Most sweet corn acreage is treated by air, but ground equipment is used in areas where this is not possible. This project will examine if canopy penetration can be improved by modifying application volume or pressure or by using adjuvants.
There is need for additional chemical and cultural control tactics. Several newer reduced risk insecticides that have fly efficacy are available, but must be ingested by the flies to be effective. Some of these are currently incorporated into bait formulations for tephritid fruit fly control (Mangan et al. 2006). These products have not been extensively tested as a control option for silk flies. Mixing these materials with the feeding stimulant sucrose has been adopted by small fruit growers for control of Drosophila suzukii (Diptera: Drosophilidae) in the Northeast (Cowles et al. 2015). This project will examine if reduced-risk insecticides mixed with feeding stimulants can provide adequate silk fly control.
Finally, fields are often left standing for prolonged periods after harvest, allowing fly larvae to complete development. There have been no studies to determine the most efficient or timely means of removing crop residue to remove silk fly larvae that would contribute to the next generation of silk flies. Growers that do remove crop residue in a timely manner will often disc or mow fields. This project will document the effect of delayed crop destruction on silk fly production and which method of crop removal is the most efficient for preventing silk flies from completing their life cycle.
- Determine how insecticide application methods can be improved for increasing canopy penetration
- Determine if silk flies demonstrate reduced pyrethroid susceptibility in field settings
- Determine if the reduced risk insecticide spinetoram can be applied in a feeding stimulant to provide adequate fly control
- Determine most efficient and timely means of crop residue removal to reduce silk fly production
Cooperators
Research
For the fall experiment, three 20-row blocks of var. ‘Obsession’ sweet corn (Seminis Vegetable Seeds, St. Louis, MO) were planted on 76.2 cm beds at the Everglades Research and Education Center, Belle Glade, FL on 16 Sept using a John Deere Max Emerge 4-row vacuum planter. Seeds were spaced 20.3 cm apart. Whorl-feeding fall armyworm (FAW; Spodoptera frugiperda J. E. Smith, Lepidoptera: Noctuidae) were treated with Coragen (rynaxypyr, E. I du Pont de Nemours and Co., Wilmington, DE) and Rimon (novaluron, Crompton Manufacturing Co., Inc., Middlebury, CT) on 5 and 14 Oct, respectively. Unplanted 4.6 m alleys separated blocks, and 3.0 m alleys separated treatments within blocks. Each treatment plot was 45.7 m long. An RCB design was used with 3 replicates per treatment.
For the spring experiment, four 20-row blocks of ‘Obsession’ sweet corn were planted on 76.2 cm beds at the Everglades Research and Education Center, Belle Glade, FL on 18 February 2016 using a John Deere Max Emerge 4-row vacuum planter. Seeds were spaced 14.5 cm apart. Unplanted 4.6 m alleys separated blocks, and 3.0 m alleys separated treatments within blocks. Each treatment plot was 45.7 m long and arranged in an RCB design with three treatments.
Objective 1, Fall trial
Preliminary experiments with spray cards to assess spray penetration into the corn canopy were conducted in the Fall 2015 sweet corn crop. One week after silking, eight of the plots were sprayed with two different water volumes (103 or 207 liters/ha) using a high clearance sprayer (model 254 X-11, Hagie Manufacturing Co., Clarion, IA). The spreader Kinetic® (Helena Chemical Company, Collierville, TN) was mixed with water and applied to four of the plots. Plots were arranged in a split plot design with the presence or absence of the spreader as the main plot factor and the two water rates as subplot factors. Each water rate was replicated twice in the main plots. In each plot, 4 yellow spray cards (Water Sensitive Paper, Gempler’s, Janesville, WI) were attached to individual sweet corn tassels via paperclips, 4 were secured to the stem two leaves below the tassel on the same plant, and 4 were secured to the sides of the plant’s primary ears for a total of 12 cards per plot. Once the residues had dried, cards were removed from the field and scanned at 2400 dpi with a digital flatbed scanner (HP Scanjet 5590, HP Inc., Palo Alto, CA). Images were analyzed with DepositScan software (USDA-ARS Application Technology Research Unit, Wooster, OH) to determine percentage spray coverage.
Objective 1, Spring trial
Insecticide spray penetration in the sweet corn canopy was again evaluated in the Spring trial using two water rates and two pressures. Spray coverage was visualized using Kromekote spray cards measuring 5.1 x 7.6 cm (38.8cm2). Spray cards were placed horizontally on the flag leaf axil and the axil of the leaf above the primary ear on mature sweet corn 3 weeks after silking. Cards were wrapped around three stem sections to minimize the effect of card orientation to the sprayer and wind drift: the lowest portion of the tassel (upper stem), between the second and third leaf from the flag leaf (middle stem) and just above the ear (lower stem). No plant had more than one card. Four cards were placed at each location per replicate. A high clearance sprayer was used to apply treatments in previously untreated plots. Treatments were arranged in a split-split plot design with water rate (187 and 374 liters/ha) as the main plot factor, pressure (103 and 207 kPa) and card location on the plant were subplot factors. Treatment plots were replicated 3 times. The insecticide Hero (zeta-cypermethrin + bifenthrin; FMC Corporation, Philadelphia, PA) was applied with the sprays at its high label rate of 752.7 ml/ha. A blue marker dye (Mystic SPI Blue, Winfield Solutions, St. Paul, MN) was also added to the spray tank to visualize spray droplets on the spray cards. Upon drying, cards were removed from the field, scanned with the digital flatbed scanner, and analyzed with DepositScan as above.
After spray residues had dried on the plants, plants adjacent to those with spray cards were removed from the field. Leaf and stem sections with a surface area of approximately 38.8 cm2 were cut from the plants and placed in 150 ml snap cap vials. Three samples were collected for each card location per plot. Between 10-15 E. eluta flies (mixed sex) were introduced into each vial and held for observation after 24 h, at which time the number of flies that were apparently behaving normally (able to right themselves when shaken, move normally, exhibiting typical wing-waving behavior) were counted and categorized as alive flies.
Objective 2, Fall experiment
Insecticide treatments targeting ulidiid flies were initiated at first silk on 26 Oct. Pyrethroids were applied at their maximum label rates for silk flies or armyworms by a high clearance sprayer with a 12.2 m boom fitted with TeeJet 8003 nozzles and calibrated to deliver 280.6 liters/ha at 206.8 kPa. Sequential pyrethroid applications consisted of Baythroid XL (beta-cyfluthrin, Bayer Crop Science, Research Triangle Park, NC), Warrior II (lambda-cyhalothrin, Dow AgroSciences, Indianapolis IN), and Mustang (zeta-cypermethrin, FMC Corporation, Philadelphia PA) applied at their high label rates. Ten treatments were applied before harvest on 19 Nov. Before tassel push in the treated plot, four strips of plastic sheeting (Clear Poly Sheeting – 4 mil, Uline, Pleasant Prairie, WI), each measuring 1 m x 30.5 m, were laid on the ground between sweet corn rows in one of the blocks that was to be treated with a pyrethroid rotation for silk flies. The plastic sheeting was laid down in an attempt to collect silk flies that might be affected by an insecticide application and fall off of the plants. The sheeting completely covered the soil in the furrow between two adjacent rows of corn and was held in place by metal stakes. One furrow separated each of the four plastic sheets. Plants adjacent to the plastic sheeting were observed for healthy silk flies and morbid flies. Because the four sheets covered a combined 120 row-m, and the plants adjacent to the sheets were examined, a total of 240 row-m were examined for silk flies after each pyrethroid application.
Objective 2, Spring trial
The same insecticide treatments as in the fall experiment were applied in the Spring and at the same interval. Insecticide treatments targeting ulidiid flies were initiated at first silk on 15 April; treatments were applied 9 times before harvest on 6 May. The day after insecticide application, silk fly populations and species composition were assessed by visual observation of the flies present on plants in rows 6 and 14 of the pyrethroid and untreated check plots for a total of 274 row-m per treatment. Flies present on the stem or leaves between the tassel and the second leaf below the flag leaf on the tassel were considered to be in the upper canopy. Flies between the second leaf and the leaf above the ear were considered to be present in the middle canopy, and flies present at the ear and below were considered to be in the lower canopy. Three observations were made in the mid-morning hours between 10:00 and 11:30 AM (EDT). Two observations were made between 8:00 AM and 9:30 AM (EDT). Two observations were made between 11:30AM and 1:30 PM (EDT). Two observations were made in the evening after 5:30 PM (EDT).
Objective 3, Fall trial.
Two insecticide regimes were compared to an untreated check. The first consisted of the pyrethroid rotation described above. The second treatment was Radiant (spinetoram, Dow Agro Sciences, Indianapolis IN) applied at its highest label rate for fall armyworm and mixed with the high rate of the feeding stimulant Nu-Lure (hydrolyzed corn gluten meal, Miller Chemical and Fertilizer Corporation, Hanover, PA). Ten treatment applications were made before harvest on 19 Nov. All treatments were applied using a high clearance sprayer with a 12.2 m boom fitted with TeeJet 8003 nozzles and calibrated to deliver 280.6 liters/ha at 206.8 kPa. At harvest, 90 ears from each plot were husked and evaluated for ulidiid ear damage, maggot infestation, and internal FAW damage. Fly damage rating was graded on a 0 to 5 scale: 0 = no maggots in the ear, 1 = silk damage only, 2 = tip damage, 3 = maggots feeding in the top ¼ of the ear, 4 = maggots feeding in the top ½ of the ear, and 5 = maggots feeding throughout the entire ear. Maggot infestation (i.e., load) was evaluated on a 0 to 3 scale: 0 = no maggots, 1 = <5 maggots, 2 = 5-20 maggots, and 3 = >20 maggots per ear. The percentage of ears with internal damage caused by Fall armyworm was determined for ears in each plot.
Objective 3, Spring trial.
The same insecticide treatments as in the Fall experiment were applied in the Spring and at the same interval, but with the addition of a spinetoram-only treatment in three plots applied at 438.5 ml/ha. Insecticide treatments targeting ulidiid flies were initiated at first silk on 15 April; treatments were applied 9 times before harvest on 6 May.
Objective 4, Fall trial.
After harvest, the untreated check plots (containing the greatest larval silk fly population) were mowed, disked once (disk1x), disked twice (disk2x), or left standing immediately after the harvest samples were taken from the field. Only ears that had been removed to sample silk fly and fall armyworm damage were removed from the plots prior to mowing and disking the plants. Fly larvae that completed larval development in corn ears drop into the soil for pupation. An emergence cage covering 0.58 m2 was erected over the soil to intercept new flies as they exited their pupae in the soil. Cage support frames were driven into the soil and the bottom edges of the cage mesh were covered with soil to prevent fly entry from outside the cages. Silk flies were removed from the cages three times per week and identified to species beginning 10 days after harvest and continuing until no more silk flies emerged from the soil. Treatments were replicated three times.
Objective 4, Spring trial.
The treatments in the Spring trial were changed slightly from the Fall trial based on results from the earlier experiment. In addition to treatments of disking twice and leaving the plants standing, the last treatment was plowing rather than disking once. The mowing treatment was not included in the Spring trial. In each treatment section, two white-mesh cages covering 0.58 m2 and a single brown-mesh cage covering 3.34 m2 were installed. Treatments were replicated three times. Adult flies emerging from the soil were removed from the cages twice weekly and identified to species beginning 6 d after harvest. Cages were monitored for 41 d. On two separate occasions, heavy rain combined with strong sustained winds and higher wind gusts blew many cages over. Cages were reinstalled over the plots in the same locations from which they had been dislodged by the storms.
Data Analysis. Species composition data of flies present in pyrethroid and untreated plots in the spring experiment after an insecticide application were analyzed using t-tests. Fly population density data are presented as number of flies per 30 m because several area crop consultants recommend insecticide applications if fly populations exceed 1 per 30.5 m. The time of day effect on fly position was analyzed using Proc Mixed in SAS with observation date a random variable (SAS Institute 2008). The fall spray card coverage data were log transformed and the spring spray card coverage data and the percentage of alive flies in treatment vials after 24 h were square root transformed and analyzed using Proc GLIMMIX. Insecticide efficacy data from both experiments (objective 3) were analyzed using ANOVA or Welch’s ANOVA when sample variance was heterogeneous using SAS JMP 11 (SAS Institute 2013). The number of flies emerging per m2 from the cages in the fall and spring trials (objective 4) was log transformed and analyzed using repeated measures in Proc GLIMMIX in SAS. Data from 24 May was not included, because 20 of the 27 cages had been blown over. Tukey-Kramer HSD was used for means separations of all analyses.
Objective 1.
In the Fall experiment, water sensitive cards exposed to the 103 liter/ha water rate received less overall spray coverage (13.5 ± 3.0 %) than the 207 liter/ha rate (30.1 ± 4.9 %; (F = 8.59, df = 1, 82, P = 0.004). Spray coverage was significantly affected by location of the spray card on the plant (F = 35.74, df = 2, 82, P <0.001). Cards on the ears received the least coverage (3.0 ± 1.2 %), stem-located cards received intermediate coverage (18.5 ± 4.0 %) and tassel-located received the greatest mean coverage (42.2 ± 5.9). Presence of the spreader adjuvant was not significant (no adjuvant = 27.7 ± 4.7%, adjuvant = 15.6 ± 3.4%; F = 0.77, df = 1, 82, P = 0.384). All interaction terms between adjuvant, location, and water volume were not significant (P > 0.05).
In the Spring trial, spray pressure and water rate did not have a significant effect on coverage (high pressure = 10.9 ± 0.8 %, low pressure = 8.6 ± 0.7 %; F = 3.27; df = 1, 7.46; P = 0.111). Water rate also did not have a significant effect on coverage (high water rate = 10.6 ± 0.8 %, low water rate = 9.0 ± 0.7 %; F = 5.04; df = 1, 7.46; P = 0.057). As in the Fall experiment, spray card location resulted in significant coverage differences (F = 181.70; df = 4, 169.1; P<0.001). Leaf coverage was greater than stem coverage for all locations (Table 1). The upper leaf received the greatest coverage, followed by the lower leaf. Among the stem sections, the greatest coverage was received on the upper stem, followed by the middle stem and the least coverage was on the lower stem. There was a significant rate x location interaction (F = 3.65; df = 4, 169.1; P = 0.007). Coverage was greatest with a high water rate (347 liters/ha) on the upper leaf and upper stem than with 187 liters/ha. Water rate did not affect card coverage at the middle stem, lower stem, and lower leaf. No other interaction terms were significant (P >0.05).
As with the spray cards, the spray residues significantly affected E. eluta survivorship depending on which section of the plant the flies had been exposed to (F = 44.26; df = 4,158; P<0.001). Fewer E. eluta survived exposure to leaf residues than stem residues (Table 2). Among stem locations, fewer E. eluta survived exposure to upper-stem residues, followed by mid-stem residues and then low-stem residues. With the spray cards, slightly greater coverage was achieved with the 374 liters/ha, but more flies survived when caged on these residues (28.0 ± 3.1 %) than flies exposed to residues deposited by a 187 liters/ha water rate (15.8 ± 2.5 %; F = 7.99; df = 1,58; P = 0.005). Although the location x water rate interaction was not significant (P > 0.05), this effect was pronounced with stem residues, probably because stems received far less coverage than leaves.
Table 1. Mean + SEM percentage spray droplet coverage on spray cards placed at different locations on sweet corn plants in Spring 2016. Sprays were applied with two different water rates and pressures.
|
Location |
Water application rate |
|
|
|
187 liters/ha |
374 liters/ha |
|
Upper leaf |
23.40 ± 1.07b |
31.05 ± 1.56a |
|
Low leaf |
9.33 ± 0.98c |
10.53 ± 1.14c |
|
Upper stem |
5.79 ± 0.99d |
8.79 ± 1.11c |
|
Mid stem |
4.08 ± 0.98ed |
3.14 ± 1.14e |
|
Low stem |
0.45 ± 0.98f |
1.19 ± 1.15f |
Significantly different means are followed by different letters within a column (Tukey-Kramer HSD, P < 0.05).
Table 2. Mean + SEM percentage of alive E. eluta flies after 24 hours caged on plant sections treated with a high label rate of Hero1 insecticide in Spring 2016.
|
Location |
187 liters/ha |
374 liters/ha |
|
Upper leaf |
2.46 ± 1.19 d |
2.99 ± 1.60 d |
|
Upper stem |
5.08 ± 1.14 c |
18.69 ± 3.02 c |
|
Mid stem |
18.96 ± 5.28 b |
37.23 ± 4.47 b |
|
Low stem |
46.97 ± 6.87 a |
73.25 ± 4.69 a |
|
Low leaf |
1.22 ± 0.82 d |
6.48 ± 2.2 d |
|
|
|
|
|
Pressure |
|
|
|
207 kPa |
11.83 ± 2.81 b |
29.35 ± 4.69 a |
|
103 kPa |
19.78 ± 4.16 ab |
26.69 ± 4.20 ab |
Significantly different means are followed by different letters within a column (Tukey-Kramer HSD, P < 0.05).
1zeta-cypermethrin + bifenthrin; FMC Corporation, Philadelphia, PA.
Objective 2. During the fall experiment, the dominant species observed in the pyrethroid plots at the beginning of the trial was C. massyla, but by the end of the trial, E. stigmatias was the most prevalent species. Silk fly populations in the untreated check plots were as great as 3 flies per row-m. A sum total of 456 healthy silk flies were observed on the plants from 240 row-m 4 h following 8 pyrethroid applications (Fig. 1). Early in the trial, the steepest population reductions followed the first Baythroid and Mustang applications. More flies were observed on plants following the two later Warrior applications, possibly due to greater populations in the field following the earlier Baythroid application. During this time, more E. stigmatias were entering the field. However, it is unclear if the population fluctuations were due to differential physiological effects of insecticide application or from silk fly movement in and out of the treated block.
A total of 67 dead or twitching flies were removed from plants and plastic sheets after pyrethroid treatments (red bars in Fig 1). Of these flies, only a single C. massyla and a single E. stigmatias fully recovered after Mustang and Warrior, respectively.
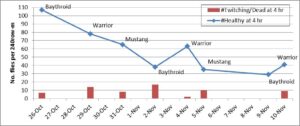
Figure 1. Total number of live healthy flies (blue line) and insecticide-affected flies (red bars) 4 h after each insecticide application. Insecticide-affected flies were taken to the laboratory for further observation. Of the insecticide-affected flies, a single C. massyla recovered from a Mustang application and a single E. stigmatias recovered from a Warrior application.
In the Spring trial, silk fly populations were routinely monitored by visual inspection. Five days before treatments were applied, pre-treatment population density was 0.2 per 30.5 row-m. Fly populations in the untreated check plot rapidly increased, averaging 70.3 per 30.5 row-m over the course of the experiment, but pyrethroid applications stabilized silk fly populations at a much reduced level compared to the untreated check plots. Average silk fly population density in the pyrethroid plots was 3.3 per 30.5 row-m. Populations in the pyrethroid treatment peaked at 7.3 per 30.5 row-m at which time the population in the check plots increased from 11.8 to 67.3 per 30.5 row-m (Figure 2).
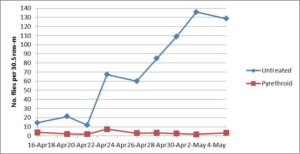
Figure 2. Silk fly populations in the untreated check plots and pyrethroid-treated plots the day after insecticide application in the Spring 2016 trial.
A total of 3,814 silk flies were observed from the untreated check plots; 66.0% were E. eluta, 29.6% were E. stigmatias, 2.9% were C. massyla, and 1.4% were unidentified Euxesta spp. (including E. annonae). Pyrethroid treatments affected the proportion of E. eluta and E. stigmatias observed in the plots (E. eluta proportion t = 8.91; df = 47.0; P <0.001; E. stigmatias: t = 6.20; df = 35.8; P < 0.001). Of the 172 silk flies observed from the pyrethroid plots, 22.1% were E. eluta, 65.7% were E. stigmatias, 8.1% were C. massyla, and 4.1% were unidentified Euxesta spp. (including E. annonae.
Time of day had a significant effect on the fly distribution in untreated check plots. The proportion of flies in the upper canopy was greatest in the evening and morning hours (F = 19.18; df = 3, 20; P = <0.001). Although the proportion of flies in the middle canopy was numerically great in the late morning hours, the difference with the other times of day was not significant (F = 3.66; df = 3, 5.32; P = 0.093). The proportion of flies in the lower canopy at the ear level or below was greatest at noon, and significantly less in the middle morning and evening hours (F = 7.30; df = 3, 5.44; P = 0.024; Figure 3).
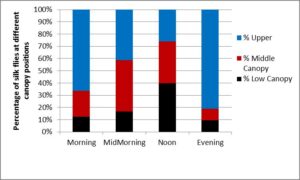
Figure 3. Percentage of silk flies observed in the sweet corn canopy at four different times of day. Observations were conducted between 16 April and 5 May 2016. Fly locations: upper canopy = the stem or leaves between the tassel and the second leaf below the flag leaf on the tassel; middle canopy = the second leaf and the leaf above the ear; and at the ear and below were considered to be in the lower canopy. Observation periods: morning = between 8:00 AM and 9:30 AM (EDT); mid-morning between 10:00 and 11:30 AM (EDT); noon = between 11:30AM and 1:30 PM (EDT); evening = after 5:30 PM (EDT).
Objective 3.
In the Fall experiment, pyrethroid treatments resulted in the lowest ulidiid fly damage to ears and the lowest maggot load in the ears (Table 3). Radiant + Nu-Lure fly damage ratings and maggot load were intermediate, but closer to the pyrethroid treatment than the check plots. The Radiant + Nu-Lure treatment had the lowest proportion of FAW damaged ears. Although there were fewer FAW damaged ears in the pyrethroid treatment than the check plots, the pyrethroid treatment was more similar to the untreated check than the Radiant + Nu-Lure treatment.
Table 3. Mean + SEM insect damage to sweet corn ears following 9 different insecticide applications in the Fall 2015 experiment.
|
Treatment |
Rate (liter/ha) |
Fly Damage Rating1 |
Maggot Load2 |
% Ears with internal FAW damage |
|
Check |
na |
4.3a |
2.2a |
51.5a |
|
Radiant + Nu-Lure Nu-Lure Radiant |
3.5 0.4 |
2.2b |
1.0b |
4.8c |
|
Pyrethroid Rotation Baythroid XL Warrior Mustang
|
0.2 0.1 0.3 |
1.4c |
0.6c |
35.3b |
|
ANOVA |
|
F = 97.20 df = 2,22 P <0.001 |
F = 98.26 df = 2,22 P <0.001 |
F = 46.20 df = 2,22 P <0.001 |
Significantly different means are followed by different letters within a column (Tukey-Kramer HSD, P < 0.05).
1Fly damage rating: 0 = no maggots in the ear, 1 = silk damage only, 2 = tip damage, 3 = maggots feeding in the top ¼ of the ear, 4 = maggots feeding in the top ½ of the ear, and 5 = maggots feeding throughout the entire ear.
2Maggot load: where 0 = no maggots, 1 = <5 maggots, 2 = 5-20 maggots, and 3 = >20 maggots per ear.
In the Spring trial, silk fly damage was less severe than in the fall, and all treatments reduced silk fly infestation and damage to the ears (Table 4). There was no separation between spinetoram with and without Nu-lure for any insect damage category. Even though worm pressure was less severe than during the fall experiment, ear protection from fall armyworm was best in the spinetoram treatments; pyrethroid protection was intermediate between spinetoram and the untreated check.
Table 4. Mean + SEM insect damage to sweet corn ears following 9 different insecticide applications in Spring 2016.
|
Treatment |
Rate (liter/ha)
|
Fly Damage Rating1 |
Maggot Load2 |
% Fly-free Ears |
% Ears with internal FAW damage |
|
Check |
na |
2.1 ± 0.2a |
1.1 ± 0.1a |
27.4 ± 4.5b |
15.2 ± 2.9a |
|
Radiant |
0.4 |
0.8 ± 0.1b |
0.4 ± 0.1b |
70.0 ± 2.5a |
1.5 ± 1.0b |
|
Radiant + Nu-Lure Nu-Lure Radiant |
3.5 0.4 |
0.8 ± 0.1b |
0.4 ± 0.1b |
72.2 ± 3.9a |
1.5 ± 0.5b |
|
Pyrethroid Rotation Baythroid XL Warrior Mustang
|
0.2 0.1 0.3 |
0.7 ± 0.1b |
0.3 ± 0.1b |
74.8 ± 3.4a |
8.9 ± 2.4ab |
|
ANOVA |
|
F = 16.70; df = 3, 17.5; P <0.001 |
F = 31.06; df = 3, 35; P <0.001 |
F = 35.98; df = 3, 35; P <0.001 |
F = 9.05; df = 3, 15.31; P = 0.001 |
Significantly different means are followed by different letters within a column (Tukey-Kramer HSD, P < 0.05).
1Fly damage rating: 0 = no maggots in the ear, 1 = silk damage only, 2 = tip damage, 3 = maggots feeding in the top ¼ of the ear, 4 = maggots feeding in the top ½ of the ear, and 5 = maggots feeding throughout the entire ear.
2Maggot load: where 0 = no maggots, 1 = <5 maggots, 2 = 5-20 maggots, and 3 = >20 maggots per ear.
Objective 4.
In the Fall trial, a total of 2,582 silk flies were removed from the 12 cages (Fig. 4). The vast majority of these (95.5%) were E. stigmatias, followed by C. massyla (2.9%). Euxesta eluta was extremely uncommon during the fall trial, and comprised only 1.0% of the emerging flies. There were no significant differences between the residue destruction treatments and the standing corn treatment on fly emergence from the cages (F = 1.58, df = 3, 120, P = 0.199). Although there was a significant date x treatment interaction (F = 2.34, df = 42, 120, P <0.001), no clear, consistent treatment pattern on fly emergence was observed. Fly emergence from the disked and mowed plots peaked on 7 December, and peak emergence from the standing corn plots occurred on 18 December. Extrapolating the number of silk flies captured from the emergence cages by the total area that the cages covered to a full acre would mean that one acre of infested sweet corn could potentially produce 1.5 million silk flies.

Figure 4. Fall trial mean adult silk fly emergence from soil within cages erected over the control plots where plants were left standing, mowed, disked once or disked twice.
In the spring trial, a total of 5,604 silk flies were removed from the cages. The species complex composition during the first two weeks (3,175 flies captured) was 60.2% E. stigmatias, 31.1% E. eluta, 4.1% C. massyla, 0.6% were E. annonae, and 4.1% unidentifiable. During the second half of the cage study (2,429 flies captured), the proportion of E. stigmatias decreased to 43.3%, while E. eluta increased to 45.8%, C. massyla increased to 7.2%, 1.2% were E. annonae, and 2.5% were unidentifiable. The species complex captured by the emergence cages differed from that observed in the field during the corn reproductive stages when out of 3,267 flies observed during the silking period, 64.2% were E. eluta, 29.8% were E. stigmatias and 1.7% were C. massyla.
Flies had already begun emerging out of the soil by the first sampling date which occurred 5 d after harvest, indicating that many larvae had already finished development and left the ear before harvest and their development in the soil was nearly complete by the time cages were installed. Fly emergence per m2 per day in the cages over standing corn initially peaked on 31 May, decreased, and then increased to a greater second peak on 17 June (Fig 5). A similar ‘double emergence peak’ was observed from the emergence cage experiment in December 2015.
Treatment, date, and the interaction between treatment and sampling date significantly affected silk fly counts in the emergence cages (F = 98.78; df = 2,270; P <0.001; F = 11.57; df = 11, 270; P < 0.001; F = 2.19;df = 22, 270; P = 0.002). For all sampling dates except 17 May, fewer flies emerged from disked plots than standing corn plots. Fly emergence from ploughed plots did not differ significantly from standing corn plots on 17 May, 27 May, and 7 June. Data from 7 June were included in the analysis even though 9 cages were blown down by a heavy windstorm the evening before; treatment capture did not significantly separate (P = 0.062). Fly emergence between disked and ploughed plots only differed significantly on 26 May (Fig 5). Extrapolating the number of silk flies captured from the standing corn plots by the total area that the cages covered to a full acre would mean that one acre of infested sweet corn in the spring would have produced more than 570,000 silk flies.
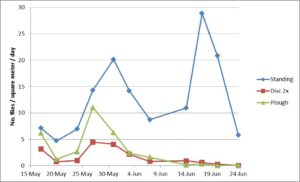
Figure 5. Spring trial mean adult silk fly emergence from soil within cages erected over the control plots where plants were left standing, ploughed, and disked.
Discussion
The spray cards indicated that coverage significantly decreases between the upper canopy, middle canopy and lower canopy. This decrease in coverage could influence spray efficacy for silk flies, and different ground application methods were tested for improving coverage. Spray coverage was slightly improved with increased water rate but not increased pressure. Nozzle selection has been assessed using similar methods in cotton; several were recommended for canopy penetration sprays targeting stink bugs (Sumner et al. 2007). In soybean, the denser the plant canopy, the fewer droplets penetrate the canopy. This results in decreased insecticide efficacy targeting Lepidoptera pests (Hutchins and Pitre 1984). Further areas of research include nozzle selection, incorporating drop nozzles, and comparing aerial application methods with each other for improved canopy penetration and insecticide efficacy.
Euxesta stigmatias is not as susceptible to some of the commonly used pyrethroids as E. eluta (Owens et al. 2016), and if flies are not located in the upper canopy, this decreased susceptibility might allow some to survive an insecticide application. Unexpectedly, E. eluta survivorship was greater when exposed to residues from the high water rate, possibly due to a decrease in active ingredient concentration. The observation data further indicate that E. stigmatias is not as impacted by pyrethroids as E. eluta. While the fly populations were significantly reduced in the pyrethroid plots, the species composition shifted from majority E. eluta to majority E. stigmatias.
A greater proportion of the silk flies in the field were observed in the upper canopy in the early morning and early evening, approximately 2 hours after sunrise and before sundown. These observations confirm earlier reports of greater silk fly activity in the early morning and evening hours (Seal et al. 1995). Silk flies congregate on the tassel in the evening, and females on the tassel late in the day had fewer eggs in their ovaries (Seal et al. 1996), which may stimulate flies to seek food sources and therefore come into greater contact with an insecticide. Because flies remain in the upper canopy during the night, it is possible that an evening spray would be more effective than a morning spray.
The silk fly population and subsequent damage to ears was greatest in the Fall experiment than in the Spring experiment. There was also severe armyworm pressure during the vegetative and reproductive corn stages in the Fall trial compared with the Spring trial. Scouts have previously observed greater silk fly abundance in armyworm infested fields. Furthermore, C. massyla is reported to be attracted to noctuid damaged grasses (Allen and Foote 1992), and the armyworm infestation in the Fall experiment may have contributed to the greater silk fly population.
A spinosad bait used for tephritid management, GF-120® NF Naturalyte® (Dow Agro Sciences, Indianapolis, IN), was used in shade houses to control C. massyla, where it was hampering efforts to grow the giant reed Arundo donax as a host for its gall wasp biological control agent (Goolsby and Mangan 2010). If applied in an efficacious manner, spinetoram’ s unique mode of action could help reduce the possibility of silk flies developing resistance to pyrethroids by reducing pyrethroid selection pressure. The equivalent protection spinetoram provided compared with pyrethroids in the spring could be due to silk flies ingesting the active ingredient while foraging on other material or exploring the environment with their mouthparts. During the Fall trial, when silk fly populations were greater, spinetoram provided intermediate efficacy, but there were no plots in which Nu-Lure was not included in the spinetoram treatment. However, in both Fall and Spring, spinetoram treated plots had the least amount of armyworm damage. It is possible that if spinetoram were used to treat armyworms in reproductive stage corn, some selection pressure would be removed from the pyrethroids. More research is needed in order to understand how feeding stimulants might improve spinetoram efficacy and what rates would be more effective in a rotation with pyrethroids.
The effect of crop destruction on reducing the silk fly emergence from the soil was inconsistent. Seasonal weather variation may influence survivorship in the soil. For example, Caribbean fruit fly survivorship from composting infested fruit was lowest in compost piles that were warmest (Kendra et al. 2007). Heavy rains and waterlogged soil may have also contributed to reductions in fly emergence in the disked plots in the Spring. Between 17-21 May, the research station received 14.1 cm of rain. During the month of June, the research station received 18.1 cm of rain. In the Fall 2015 cage study when disking did not decrease fly emergence, only 6.4 cm of rain were recorded throughout the duration of the emergence cage study. Disked plots often contained standing water after heavy rain events that the standing corn plots did not. Increasing moisture content of the soil following harvest and disking is another possible management strategy. However, until more rigorously controlled experiments are performed, soil incorporation should not be considered as an effective means of preventing silk fly larvae from completing their life cycle. Therefore, the crop should be removed as quickly as possible to prevent adult females from continuing to utilize the field for oviposition. The species composition emerging from the soil differed from that observed in the field during the corn reproductive stages. The ecological interaction of the three species utilizing the same reproductive host needs to be further examined to determine if the three fly species differ in terms of damage potential and whether some level of larval competition within ears alters the final proportion of emerging fly species.
References
Allen, E. J. and B. A. Foote. 1992. Biology and immature stages of Chaetopsis massyla (Diptera: Otitidae), a secondary invader of herbaceous stems of wetland monocots. Proc. Entomol. Soc. Wash. 94: 320-328.
Anonymous. 2009. Sweet corn pest management strategic plan (PMSP). Belle Glade, Fl. (http://www.ipmcenters.org/pmsp/pdf/FLsweetcornPMSP.pdf).
Cowles, R. S., C. Rodriguez-Saona, R. Holdcraft, G. M. Loeb, J. E. Elsensohn, and S. P. Hesler. 2015. Sucrose improves insecticide activity against Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 108: 640-653.
Goolsby, J. A. and R. Mangan. 2010. Use of spinetoram bait (BF-120) for management of Chaetopsis massyla in shadehouse-grown Arundo donax. Southwest. Entomol. 35: 573 – 574.
Goyal, G. 2010. Morphology, biology and distribution of corn-infesting Ulidiidae. Ph. D. dissertation, University of Florida, Gainesville.
Hutchins, S. H. and H. N. Pitre. 1984. Effects of soybean row spacing on spray penetration and efficacy of insecticides applied with aerial and ground equipment. Environ. Entomol. 13: 948-953.
Kendra, P. E., M. K. Hennessey, W. S. Montgomery, E. M. Jones, and N. D. Epsky. 2007. Residential composting of infested fruit: a potential pathway for spreas of Anastrepha fruit flies (Diptera: Tephritidae). Fla. Entomol. 90: 314-320.
Mangan, R. L., D. S. Moreno, and G. D. Thompson. 2006. Bait dilution, spinosad concentration, and efficacy of GF-120 based fruit fly sprays. Crop Prot. 25: 125-133.
Nuessly, G. S. and M. G. Hentz. 2004. Contact and leaf residue activity of insecticides against the sweet corn pest Euxesta stigmatias (Diptera: Otitidae). J. Econ. Entomol. 97: 496-502.
Owens, D., G. S. Nuessly, D. R. Seal, T. A. Colquhoun. 2016. Variable pyrethroid susceptibility among the sweet corn infesting Ulidiidae (Diptera) in Florida and new baseline susceptibilities. J. Econ. Entomol. 109: 1283-1288.
SAS Institute. 2008. SAS/STAT 9.2 user’s guide. SAS Institute. Cary, NC.
SAS Institute. 2013. Using JMP 11. SAS Institute Inc. Cary, NC.
Seal, D. R., R. K. Jansson, and K. Bondari. 1995. Bionomics of Euxesta stigmatis (Diptera: Otitidae) on sweet corn. Environ. Entomol. 24: 917-922.
Seal, D. R., R. K. Jansson, and K. Bondari. 1996. Abundance and reproduction of Euxesta stigmatis (Diptera: Otitidae) on sweet corn in different environmental conditions. Fla. Entomol. 79: 413-422.
Sumner, P. E., P. M. Roberts, R. P. Edwards. 2007. Comparison of low-drift nozzles for canopy penetration in cotton. ASABE Annual International Meeting, Minneapolis MN.
United States Department of Agriculture [USDA]: National Agricultural Statistics Service (NASS). 2016. Vegetables 2015 Summary (February 2016). (http://usda.mannlib.cornell.edu/usda/current/VegeSumm/VegeSumm-02-04-2016.pdf).
Educational & Outreach Activities
Participation Summary:
Two research publications stemming from this project are currently in progress.
- Owens, D., G. S. Nuessly, N. Larsen, and N. Asbani. Investigations of the efficacy of Chemical and cultural control techniques for reducing silk fly populations in sweet corn. J. Econ Entomol. in preparation
- Owens, D., N. Larsen, and G. S. Nuessly. 2016. Evaluation of two insecticide regimens for control of sweet corn ear pests, 2015. AMT. in revision.
Silk fly research from this project was presented to stakeholders during two outreach events. In February 2016, a sweet corn pest management update was held at the EREC conference center and included presentations on weed and disease management, new sweet corn varieties, pesticide stewardship, insect pest management, and specifically silk fly pest management research. The event was sponsored by Syngenta and CEUs were provided to growers and crop consultants. Forty five researchers, growers, crop consultants, and extension agents were in attendance.
In May 2016, a sweet corn and bean field day was held at EREC to demonstrate to stakeholders the sweet corn experiments associated with this project and present preliminary harvest results. Twenty five researchers, growers, crop consultants, and extension agents were in attendance.
Final results of these experiments will be presented orally and in handouts to sweet corn growers at one of their weekly meetings held during the harvest season.
Project Outcomes
Some decreased pyrethroid susceptibility in E. stigmatias was indirectly observed during the spring trial. This reinforces the need to improve management practices. Spinetoram demonstrated some effect on silk flies, and appears to be a good product to use when armyworms need to be treated. New research is being planned to continue examining the effect of feeding stimulants on field efficacy of spinetoram and other insecticides labeled for use in sweet corn. The large plot sizes used during these experiments resulted in treatment separation not previously observed in small plot trials for these flies, providing a template for further insecticide trials. Recommendations were made to spray insecticides in the early morning and evening when more flies are located in the upper canopy where they are exposed to the greatest insecticide residues. After a sweet corn crop is harvested, it needs to be removed from the field as quickly as possible. Crop destruction resulted in inconsistent silk fly emergence from the soil. Flies must be denied access to sweet corn so that they cannot continue to oviposit and develop in harvested or unmanaged sweet corn fields.
Farmer Adoption
Two field days were held at the EREC to discuss both the Fall and Spring trials. Growers and crop consultants were very interested in the work; 70 stakeholders attended the field days. Recommendations were made to growers to target silk flies with insecticide applications in the early morning or early evening hours when silk flies are in the upper canopy. Some crop consultants are asking applicators if at all possible to make applications in the evenings to improve insecticide contact with silk flies as a result of the data collected in this experiment and in previous investigations. Species identification was stressed during these two field days, because pyrethroid applications may not be as effective on E. stigmatias. If spraying by ground, lower water rates may result in greater fly mortality, but this needs to be confirmed with additional testing. If armyworms need to be controlled, spinetoram also provides some fly protection. Crop residue needs to be destroyed as soon as possible after harvest to prevent female silk flies from continuing to oviposit in the crop. Fall data suggests that once eggs are deposited, sufficient ear residue is available for them to complete development in the soil after shallow incorporation.
Areas needing additional study
This research will stimulate new research areas that will improve silk fly management, and there are several possible investigative avenues that could be pursued building off of these experiments. The relationship between insecticide concentration in spray droplets and coverage needs to be further examined to be able to predict how efficacious an application will be given different crop canopy characteristics, application methods, and insecticide selection. Laboratory studies suggest that incorporation of feeding stimulants with spinetoram increases fly mortality, but the spring field study did not demonstrate any significant increase in protection. Three variables that could influence the efficacy of a feeding stimulant mixed with an insecticide include insecticide rates, application frequency, and droplet size. Additional feeding stimulants should be investigated. The current studies employed Nu-lure, a corn protein hydrolysate, but other protein matrices and sugars may be as or more effective. Additional insecticides should also be tested in combination with feeding stimulants to determine if efficacy can be improved, particularly with pyrethroids. The inconsistent impact that crop destruction had on silk fly emergence from the soil needs to be further explored with additional replication.