Final report for GS17-171
Project Information
Florida is a major producer of zucchini squash (Cucurbita pepo L., Cucurbitaceae) and produced 17% of the US squash in 2017, valued at about $39 million dollars. Key insect pests including the sweetpotato whitefly (Bemisia tabaci Genn., B biotype, Hemiptera: Aleyrodidae), the melon and the cowpea aphid (Aphis gossypii Glover and Aphis craccivora C.L.Koch, Hemiptera: Aphididae), attack zucchini squash and transmit viral diseases that together cause yield losses up to 80%. Aphids transmit viruses of economic importance and reports of whitefly-transmitted viruses in Florida squash have increased in the last few years. Pesticides are generally used for insect and insect-transmitted disease management but the development of insecticide resistance and their adverse effects on non-target organisms are major concerns. This project evaluated a combination of non-pesticidal approaches including cultural practices, augmentation and conservation biological control to manage key pests in organic squash with the goal of reducing the amount of chemical inputs in the cropping system, limiting the adverse effects of insecticides on non-target organisms, and maintaining environmental quality. The effects of the African marigold (Tagetes erecta L., Asteraceae) and the sweet alyssum (Lobularia maritima (L.) Desv, Brassicaceae) used as companion plants, together with the predatory mite, Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) against key insect pests and its effects on viral incidence in squash production were assessed. The outcomes included the development of integrated pest and disease management strategies utilizing companion plants and the predatory mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) for organic squash and cucurbit growers in Florida.
The overall goal of this study is to evaluate the potential of three companion plant species, in absence or presence of A. swirskii, for biological control of key insect pest populations and its effect on viral incidence in organic zucchini squash. The objectives include 1) monitor the establishment of insect vectors and plant viruses in squash, 2) evaluate the effect of companion plants and A. swirskii introduced in zucchini squash crops, and 3) identify temporal and spatial distribution patterns of key pest species, A. swirskii and other natural enemies in a squash field using geostatistical techniques.
Cooperators
Research
This project consisted of two phases. One research phase with two field-seasons were conducted in fall 2017 and spring 2018 at the University of Florida’s Plant Science Research and Education Unit (PSREU) in Citra, FL. The second phase was a four-month on-farm demonstration that was carried out during the fall of 2018 in an organic farm in Hawthorne, FL.
Objective 1. For 2017 experiments, the zucchini squash ‘Cash Flow’ (Siegers Seed Co., Plant City, FL) was used as cash crop. The African marigold ‘Crackerjack’ (Stokes Seeds, Buffalo, NY) and ‘Tall White’ alyssum (Urban Farmer, Westfield, IN) were used as companion plants. Plants were sown in double rows at 35-cm intervals and fertigated. Samples collected during the experiments were processed at the University of Florida’s Small Fruit and Vegetable IPM Laboratory, Gainesville, FL.
Four replicates of seven treatments were arranged in a randomized complete block design for a total of twenty-eight 5.5-m wide × 4.3-m long plots. Each plot comprising three double row 0.9-m raised beds represented one treatment with squash grown in the outer beds and companion plants grown in the middle bed. Treatments were defined by the type of companion plant used and the presence or absence of A. swirskii, as follows: 1) marigold in absence of A. swirskii; 2) alyssum in absence of A. swirskii; 3) marigold in presence of A. swirskii; 4) alyssum in presence of A. swirskii; 5) no companion plant, only the release of A. swirskii for insect suppression; 6) no companion plant, only the use of M-Pede® for insect control; and 7) no pest management technique (control). For treatments including A. swirskii, 500-ml shaker bottles were purchased (Koppert Biological Systems, Inc., Howell, MI) and released onto the squash plants (100 – 150 mites/m2) three weeks after sowing the squash as a complementary strategy for insect suppression.
Aphids were sampled weekly from randomly selected squash and companion plants using the leaf-turn method (Nyoike & Liburd 2010) consisting on gently turning over a leaf and counting the number of aphids. Winged aphids were also monitored weekly using one clear pan trap with 6% detergent solution placed in the middle of each plot.
Two yellow sticky traps were placed per plot weekly and left in the field for 48 hours to monitor adult whiteflies and thrips. Immature whiteflies were monitored by collecting leaves from randomly selected squash and companion plants. Leaf discs were taken from each leaf and the number of immature whiteflies per disc was recorded.
A single young leaf showing viral symptoms was excised per plot and assayed by conducting DAS-ELISA and PCR to check for infection with aphid- (Potyvirus group) and whitefly-transmitted viruses (CuLCrV). Total marketable yield was recorded by harvesting and weighting all fruits per plot until crop termination.
Objective 2. Natural enemies were monitored weekly using the leaf-turn method mentioned above. Natural enemies present in pan traps and sticky traps were recorded as well. Leaves collected for sampling immature whiteflies were also searched for A. swirskii. Numbers of A. swirskii eggs per leaf were recorded. Nymphs and adult males and females per leaf were recorded together as motiles.
Statistical analysis (objectives 1 and 2). Repeated measures analysis was performed for all response variables. The number of insect pests per plot collected by all sampling methods, were fitted using the PROC GLIMMIX procedure. Squash yields per plot were compared among treatments by using the PROC MIXED procedure. Comparisons of means among treatments at each week were obtained by requesting LSMEANS from each procedure using SAS 9.4. Data from squash plants and companion plants were analyzed separately.
Objective 3. Because sweet alyssum was not observed to serve as host for aphids or whiteflies and because of its attractiveness to predatory bug and predatory fly species, sweet alyssum planted as companion plant together with releases of A. swirskii were used as the pest management technique during the on-farm demonstration in 2018.
Two 8.2-m wide × 37-m long plots each with seven 37-m long raised beds were set up for the experiments. The treatment plot consisted of 25% of the area occupied by sweet alyssum and A. swirskii released in 23% of the squash plants (mixed cultivars) in the middle part of the plot and the control plot included only squash planted (mixed cultivars), no sweet alyssum nor A. swirskii released or any other pest management tactic. Twenty-five percent of the treatment plot area was planted with sweet alyssum. In the remaining 75% of the area in the treatment, the three cultivars of summer squash (‘Cash Flow’, ‘Gold Rush’, and ‘Zephyr’) were planted, one cultivar per row. One A. swirskii 10-ml sachet (Ulti-Mite Swirskii®, Koppert Biological Systems, Howell, MI) per plant was placed on 105 squash plants located in the middle of the treatment plot, three weeks after transplanting the squash. The following week, A. swirskii was released again due to fire antes moving the bran out of the Ulti-Mite sachets. Approximately 5-ml of bran was sprinkled per plant using a bottle shaker formulation.
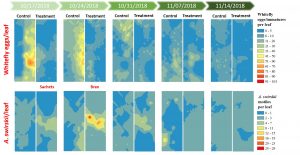
Seventy-seven sampling points were georeferenced per plot. Aphids, whiteflies eggs, and A. swirskii were monitored only in the plants within one meter of the georeferenced sampling points by collecting squash leaves weekly during a five-week period. Viral incidence was monitored using the same methodology described above. Ordinary kriging analysis was performed to track and compare the movement and abundance of whitefly eggs, aphids, and A. swirskii motiles between control and treatment plots. Distribution maps were generated using the Geostatistical Wizard in ArcGIS 10.6.1.
Aphids. Higher aphid pressure was observed during the spring compared to the fall for both apterae and winged aphids. Most of the collected aphids were identified as melon aphids (A. gossypii). There were no significant differences among treatments for densities of apterae aphids recorded by in situ counts during the spring season. Low numbers of apterae aphids were recorded in all treatments. Hardly any apterae aphids were found in the companion plants during the spring season. Similarly, there were no significant differences for densities of winged aphids collected by pan traps in the spring.
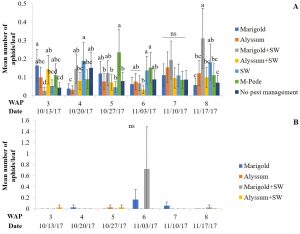
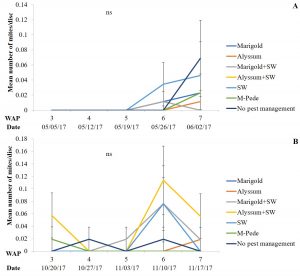
The number of aphids sampled differed over time during the fall season. Significant differences among treatments were identified for densities of apterae aphids (F30,258 = 4.17, P < 0.0001). The highest number of apterae aphids were found eight WAP in the squash where A. swirskii was released and planted next to marigolds and marigolds plus A. swirskii, whereas the lowest number of aphids was observed in the squash where A. swirskii was released and alyssum was used as companion plant (Fig. 1A). The highest number of apterae aphids were found in the marigolds from the treatment with marigolds plus A. swirskii six WAP in the fall season (Fig. 1B). There were no significant differences among treatments for densities of winged aphids collected by pan traps in fall. Nonetheless, traps around the squash treated with M-Pede showed the low numbers of winged aphids.
Zero samples were infected with aphid-transmitted viruses (Potyvirus group) during the spring season. Low incidence was recorded in the fall season with up to three samples infected in the treatment where no companion plant was used but A. swirskii was released (Table 1).
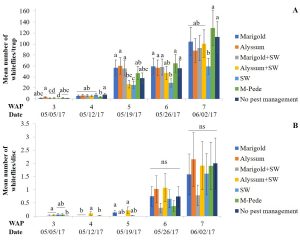
Whiteflies. Densities of whitefly adults were significantly different among treatments (F24,72 = 1.89, P = 0.02) in the spring. Starting at week five there was an increase in the numbers of adult whiteflies with the highest numbers seven WAP in the treatment including M-Pede applications and the treatment with no pest management. Yellow sticky traps from the treatment with A. swirskii released (no companion plants) showed the lowest numbers of adult whiteflies throughout the season (Fig. 2A). Significant differences among treatments were identified for densities of whitefly immatures (F14,72 = 18.2, P < 0.0001) in spring. Immature numbers increased six and seven WAP with the highest numbers in squash with alyssum as companion plant and alyssum together with A. swirskii release in the squash. The lowest numbers of immature whiteflies were recorded in the squash planted together with marigolds and A. swirskii release (Fig. 2B).
There were no significant differences among treatments for densities of whitefly adults in fall. High numbers of whitefly adults were recorded throughout the sampling period and was difficult to identify treatment differences. Densities of whitefly immatures were significantly different among treatments (F24,90 = 1.86, P = 0.02) in fall. The numbers of whitefly immatures are somewhat consistent with the numbers of adult whiteflies collected by the yellow sticky traps. High numbers of immatures were found on the squash in weeks three and four for most treatments. The squash treated with M-Pede and the treatment with A. swirskii release and no companion plant showed significantly lower numbers of immatures. No immature whiteflies were found in the companion plants during both the spring and fall seasons.
PCR was conducted to screen for CuLCrV only during the fall season because no symptoms were observed in the squash plants during the spring. The virus was detected in samples from all treatments at similar incidence across treatments. Likewise, CuLCrV was detected in 63% (38 out of 56) of the samples assayed in fall. CuLCrV incidence was not influenced by treatments (Table 1).
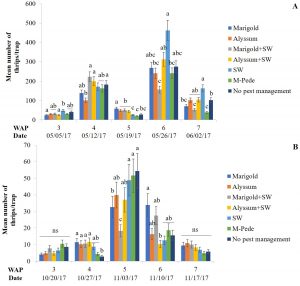
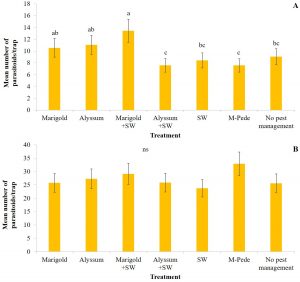
Thrips. Florida flower thrips (Frankliniella bispinosa) was the dominant species during both seasons. There were significant differences among treatments for densities of F. bispinosa in spring (F24,84 = 2.81, P = 0.0002) and fall (F24,72 = 1.86, P = 0.02). In the spring, the number of thrips was highest six WAP in the treatment where A. swirskii was released but no companion plant was used, whereas it was lowest in the treatment with release of the predatory mite, A. swirskii and marigold as companion plant (Fig.3A). In the fall, the treatment with no pest management showed the highest numbers of thrips five WAP followed by the treatment where M-Pede was applied (Fig. 3B).
High numbers of thrips were found in marigold flowers in both seasons whereas alyssum (alone or together with A. swirskii release) inflorescences showed low thrips numbers. Significant differences among treatments were identified for numbers of thrips (F12,125 = 2.17, P = 0.01) in spring. Low numbers of thrips were recorded across treatments; however, more numbers of thrips were recorded in treatments including marigolds when A. swirskii was released in the squash planted next to marigolds during week five. Low numbers of thrips were found in treatments where alyssum was present. There were no significant differences among treatments for thrips numbers in fall. However, the same tendencies mentioned above were observed with high numbers in marigold flowers and low numbers in alyssum inflorescences.
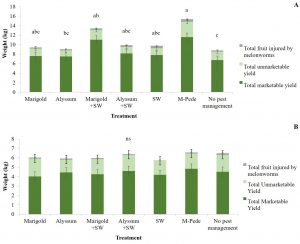
Yield. There were significant differences in marketable (F6,24 = 5.04, P = 0.001) and unmarketable yield (F6,22 = 3.10, P = 0.02) among treatments during the spring. Both marketable and unmarketable yield showed highest yield in plots where M-Pede was applied followed by the treatment with marigold as companion plants and release of A. swirskii. The lowest yield was recorded in the control were no pest management was implemented (Fig. 4A). No significant differences in yield were found among treatments in fall. Nonetheless, the marketable yield was approximately 50% less in fall compared to the spring probably due to CuLCrV infecting the squash during the fall (Fig. 4B).
Naturally occurring predators. Significant differences among treatments were identified for the numbers of minute pirate bugs collected with pan traps in spring. The highest numbers of Orius spp. were collected in the treatment with marigolds planted as companion plant and the release of A. swirskii in the squash followed by the treatments planted with marigolds and no predatory mites. The lowest numbers of Orius spp. were recorded in traps from treatments with alyssum planted as companion plant together with A. swirskii release, A. swirskii released without companion plants, and plots treated with M-Pede (Table 2). No minute pirate bugs were collected during the fall.
At least four long-legged fly species in the genera Condylostylus (Diptera: Dolichopopidae) were collected during the experiments. The numbers of these generalist predators were higher in the spring compared to the fall season and significant differences among treatments were identified during both seasons. Long-legged flies were higher in traps from treatments including companion plants and highest when alyssum was planted as companion plant. Lowest numbers were collected from treatments where only A. swirskii release was used (Table 2).
Naturally-occurring parasitoids. Forty-six morphospecies of parasitoid wasps from 12 families were collected during the experiments. For comparisons across treatments, the numbers from all parasitoid morphospecies were pooled together as total parasitoid wasps. There were significant differences among treatments for total parasitoid numbers in spring (F6,102 = 4.49, P =0.0004). More parasitoids were collected from treatments that included companion plants except for treatments including alyssum together with A. swirskii release. The latter showed the lowest values of total parasitoids followed by the treatment with M-Pede applications (Fig. 5A). No significant differences across treatments were found for total number of parasitoids collected in fall (Fig. 5B). In spring, Platygastridae was the most abundant family overall parasitoid families with highest numbers in treatments including alyssum together with A. swirskii release and treatment with M-Pede applications. Mymmarydae was the second most abundant parasitoid family. In fall, Platygastridae continue to show the highest numbers followed by Encyrtidae.
The introduced predator, Amblyseius swirskii. There were no significant differences among treatments for numbers of A. swirskii motiles in spring and fall. The predatory mites released three WAP were found in the leaf samples five WAP only in samples from the squash with A. swirskii release and no companion plants, and the squash planted next to marigolds and A. swirskii release (Fig. 4-1A). Despite being introduced deliberately in three out of seven treatments evaluated, A. swirskii was also found on leaf samples from other treatments. Six and seven WAP A. swirskii was recorded in squash samples from most treatments (Fig. 4-1A). In fall, numbers of A. swirskii motiles fluctuated over time, but the lowest numbers of predatory mites were recorded on squash without any pest management tactic (Fig. 6).
Spatio-temporal distribution patterns. Sweetpotato whitefly infestation levels were high since the beginning of the sampling period indicating that whiteflies established at early stages of the crop. Higher densities of whitefly eggs were recorded overtime in the control plot compared to the treatment plot. The number of whitefly eggs declined at the end of the season six and seven WAP and lower numbers of eggs per leaf were observed where A. swirskii motiles were present. Highest numbers of predatory mites were recorded three and four WAP in the treatment plot where they were deliberately released. Numbers peaked after the second release event four WAP and similar numbers of A. swirskii motiles were recorded in the control plot where they were not originally introduced. Reductions in the numbers of whitefly eggs were recorded during the weeks when A. swirskii showed higher numbers and the weeks following predatory mite release. Predatory mites were collected from both plots until the end of the sampling period. Lowest numbers of predatory mites were recorded in the squash six and seven WAP in both plots (Fig. 7).
Lower densities of aphids were recorded in the treatment plot where the alyssum was planted compared to the control plot including squash monoculture during the 2018 experiments. This reduction in aphid numbers could be related to the presence of sweet alyssum that is attractive to parasitoids and predators (i.e., braconid wasps, syrphid and dolichopodid flies, among others) that feed on aphid species.
Table 1. Percentage of samples showing positive results for infection with aphid- (Potyvirus groups) and whitefly-transmitted viruses (CuLCrV) during the fall season.
|
Treatment |
%Potyvirus group |
%CuLCrV |
|
Marigold |
1.79 |
8.93 |
|
Alyssum |
0.00 |
10.71 |
|
Marigold+SW |
1.79 |
7.14 |
|
Alyssum+SW |
3.57 |
12.50 |
|
SW |
5.36 |
8.93 |
|
M-Pede |
1.79 |
10.71 |
|
No pest management |
1.79 |
8.93 |
n =56 samples (2 per plot) used for ELISA assays and PCR
SW = Amblyseius swirskii
Table 2. Mean number (±SE) of predators collected per pan trap over a five-week period during the spring and fall seasons. Treatments with the same letter are not significantly different (P ≤ 0.05).
|
Treatment /Family |
Marigold |
Alyssum |
Marigold +SW |
Alyssum +SW |
SW only |
M-Pede |
No pest management |
Treatment effect |
||||||
|
Spring |
||||||||||||||
|
Anthocoridae (Minute pirate bugs) |
0.03 ± 8.79ab |
0.03 ± 7.88ab |
0.04 ± 10.59a |
0.01 ± 0.01c |
0.01 ± 0.01c |
0.01 ± 0.01c |
0.02 ± 6.11b |
F6,21 = 37.99, P < 0.0001 |
||||||
|
Araneae (Spiders) |
0.52 ± 0.17 |
0.01 ± 0.27 |
0.04 ± 5.12 |
0.42 ± 0.16 |
0.03 ± 4.3 |
0.04 ± 5.35 |
0.70 ± 0.21 |
F6,105 = 0.20, P = 0.97 |
||||||
|
Dolichopodidae (Long-legged flies) |
1.64 ± 0.43a |
3.87 ± 0.84abc |
2.81 ± 0.64abc |
2.25 ± 0.54abc |
0.92 ± 0.31abc |
2.24 ± 0.54a |
1.95 ± 0.49b |
F6,102 = 4.20, P = 0.0008 |
||||||
|
Fall |
||||||||||||||
|
Coccinellidae (Lady beetles) |
0.09 ± 21.23 |
0.1 ± 23.31 |
0.06 ± 14.78 |
0.13 ± 32.81 |
0.08 ± 20.18 |
0.09 ± 21.82 |
0.09 ± 20.55 |
F6,105 < 0.0001, P = 1 |
||||||
|
Dolichopodidae (Long-legged flies) |
0.04 ± 8.16a |
0.04 ± 8.97a |
0.01 ± 1.19c |
0.01 ± 0.94abcd |
0.01 ± 0.86abcd |
0.01 ± 0.74d |
0.10 ± 23.34b |
F6,90 = 9.6, P < 0.0001 |
||||||
Means within rows followed by the same letters are not significantly different (P ≤ 0.05).
SW = Amblyseius swirskii
Educational & Outreach Activities
Participation Summary:
The results of this project were presented during the 2018 Organic Summit (Gainesville, FL). Based on our findings, a workshop was conducted to train approximately 20 scientists and farmers on how to identify key major pests and natural enemies present in squash crop systems. Our findings were also presented during the 2019 ESA-SEB meeting (Mobile, AL).
Project Outcomes
This project developed a pest management program using complimentary techniques including the use of African marigold and alyssum as companion plants and the release of the predatory mite A. swirskii for management of insect key pests in squash. Our approach avoided additional set up or maintenance, and a single release of A. swirskii improved the sustainability of squash production. Amblyseius swirskii become successfully established on zucchini squash.
Geostatistical techniques allowed us to track the movement of A. swirskii within the squash plants and as a result we determined their effect on whitefly numbers. Amblyseius swirskii successfully suppressed whitefly populations.
Companion plants increased natural enemies around the squash. African marigold and alyssum are inexpensive, easy to establish in the field and their flowers generated income for the grower. Both were attractive to pollinators and ensured good and adequate fruit development in squash production. Furthermore, African marigolds and alyssums increased predatory and parasitoid species including hoverflies (Diptera: Syrphidae), long-legged flies (Diptera: Dolichopodidae), and Orius spp. (Hemiptera: Anthocoridae) that suppressed whitefly, thrips, and aphid populations.
The combination of techniques implemented can be adopted not only by organic squash growers but also by conventional growers and other cucurbit producers in Florida, and the rest of the southern U.S. including southern states (e.g., Georgia) that are attacked by similar insect pests and share similar disease pressure. The enhancement of natural enemies suppressed insect pests, reduced the number of insecticide applications, reduced the cost of production, and the exposure of pollinators and farmers to toxic chemicals.
N/A
- Marigolds can be used as companion plants together with swirskii for management of key insect pests when high aphid or thrips pressure is detected in the field because marigolds seemed to play a role as trap crop for thrips and aphids.
- Sweet alyssum can be used as companion plants together with A. swirskii for management of key insect pest when low aphid and thrips infestation levels and high whitefly pressure is detected in the field.