Final report for GS18-193
Project Information
Many farms use leguminous cover crops as a nutrient management strategy to reduce their need for nitrogen fertilizer. When they are effective, leguminous cover crops are a valuable tool for sustainable nutrient management. However, the symbiotic partnership between legumes and nitrogen fixing rhizobia is vulnerable to several abiotic and biotic stressors that reduce nitrogen fixation efficiency in real world contexts. Sometimes, despite inoculation with rhizobial strains, this symbiosis fails to form. Such failure was observed in a 14-acre winter cover crop trial in the Rio Grande Valley (RGV) of Texas when three legume species produced no signs of nodulation or nitrogen fixation. This study examined the role of nitrogen, phosphorus, moisture, micronutrients and native microbial communities in the nodulation of Vigna unguiculata and assessed arbuscular mycorrhizal fungi as an intervention to improve nodulation. Results from two controlled studies confirm moisture and native microbial communities as major factors in nodulation success. Micronutrients showed mixed impacts on nodulation depending on plant stress conditions. Nitrogen and phosphorus deficiencies, however, were not likely causes, nor was mycorrhizal inoculation an effective intervention to improve nodulation. Inoculation method also had a major impact on nodulation rates. Continued research on improved inoculation practices and other ways to maximize nitrogen fixation efficiency will be required to increase successful on-farm implementation. The lessons learned in this project were shared widely with the south Texas agricultural community through 3 field days, 4 conference presentations, an educational video and online nodulation troubleshooting tool, and one peer-reviewed publication.
Objective 1: Determine the likely cause(s) of legume nodulation failure in the cover crop trials at Hilltop Gardens in Lyford, TX.
Objective 2: Assess the potential of inoculation with arbuscular mycorrhizal fungi to enhance nodulation and N fixation by buffering the legume-Rhizobium symbiosis from these three stressors: water stress, phosphorus availability, and micronutrient deficiencies.
Objective 3: If preliminary greenhouse data shows increased nodulation and N fixation, incorporate inoculation with mycorrhizae into winter 2018-2019 cover crop trials at Hilltop Gardens to determine the utility of this intervention under field conditions.
Objective 4: Disseminate project findings so that producers in this area and elsewhere can make better informed decisions about cover cropping as part of their farm management strategies.
Cooperators
- (Educator and Researcher)
- (Educator and Researcher)
Research
Dominant Factor Assessment
Five separate experiments were conducted concurrently in controlled greenhouse conditions (Edinburg, TX) to examine the association of abiotic (moisture, micronutrients, phosphorus, nitrogen) and biotic (native microbial communities) conditions with nodulation in cowpea (Vigna unguiculata). In each experiment, the potential interaction of AMF as a participant in successful nodulation of cowpea was also examined.
For all experiments, Iron and Clay cowpea seeds (Vigna unguiculata; Johnny’s Seeds, Winslow, ME) were soaked for 10 minutes in 55 oC water, then pregerminated for 3 days in petri dishes in the greenhouse at 30 oC. Pregerminated seeds were then transplanted into 15 cm diameter plastic pots with 1500 g of a 1:1 mixture of perlite and soil obtained from the field where nodulation failure occurred (Hilltop Gardens, Lyford, TX). Native soil was a Willacy fine sandy loam with a pH of 8.1. Nutrient extractions were conducted by Texas Plant and Soil Lab (Edinburg, TX). Nitrogen and phosphorus values are the average of 25 samples analyzed by Mehlich III extraction. Micronutrient values are from a single soil sample using the hot water method for boron extraction and DTPA for cobalt, copper, manganese, molybdenum, and zinc. Soil pH was measured using a multimedia pH meter (Bluelab, Tauranga, New Zealand) and soil texture was determined using the USDA NRCS Web Soil Survey and confirmed by hydrometer.
At transplant, 1 ml of Bradyrhizobium sp. (Vigna) rhizobium inoculant solution (2 g inoculant/500 mL water; Verdesian Guard-N N-Dure ®, Cary, NC) was applied to the seed radicle. This inoculant is sold for use with peanut, cowpea, lespedeza, and mung bean. 1 mL of mycorrhizal inoculant solution (1 g inoculant/500 mL water; Wildroot Organic, Austin, TX) was also applied at transplant to the cowpeas assigned to mycorrhizae (MycM+) treatments. In all experiments, cowpeas were grown for 75 days in greenhouse conditions. Daily temperature ranged between 28 oC and 36 oC on average, and relative humidity between 52 and 86%. Soil pH measurements were taken initially upon planting (mean - 8.0 ± 0.1) and monthly during the experiment to check for pH changes from nutrient solutions, but none were detected.
Pots were watered based on daily moisture measurements using a moisture meter (ProCheck 5TE, Pullman WA). Except where otherwise indicated below, the pots were watered with 150 mL of tap water (or the designated nutrient solution) whenever their soil moisture fell below a lower threshold of 5%. This amount of water raised the soil moisture to an upper target of 15%. Tap water was used instead of deionized water in order to better simulate field conditions since both rainwater and local irrigation water sources carry trace minerals. However, in the absence of specific soil tests for nutrients of interest, exact treatment impacts cannot be determined. Levels listed in Table 2 should be considered lower thresholds. These lower thresholds (µg element/g dry soil) were calculated using the concentration for each nutrient solution, the total volume of solution applied over the course of each experiment, and the dry mass of the soil.
Table 1. Solution mixtures used for each nutrient treatment level in the three nutrient experiments.
|
Experiment |
Treatment |
Solution Mixture |
Cumulative amount added (L) |
|
Nitrogen |
Control |
5 mM CaCl2 |
3.070 |
|
Low |
5 mM CaCl2 |
3.160 |
|
|
High |
5 mM CaN2O6 |
3.343 |
|
|
Phosphorus |
Control |
2 mM KCl |
3.325 |
|
Low |
0.1 mM KH2PO4 + 1.9 mM KCl |
3.538 |
|
|
High |
2 mM KH2PO4 |
3.745 |
|
|
Micronutrients |
Control |
tap water |
3.445 |
|
High |
25 µM H3BO3 + 1.7 µM CoCl2 + 0.5 µM CuSO4 + 2 µM MnCl2 + 0.5 µM Na2MoO4 + 2 µM ZnSO4 |
3.763 |
Table 2. Macro- and micro-nutrients and their cumulative amounts added over the course of the three nutrient experiments. Cumulative amounts are expressed per kg of soil in each pot (µg nutrient/kg soil = ppm) in each treatment, with measured native soil concentrations present in field soil given for comparison.
|
|
Cumulative amount added by experiment & treatment (ppm) |
Native soil concentration (ppm) |
|||||||
|
|
Nitrogen |
Phosphorus |
Micronutrients |
||||||
|
Nutrient |
Control |
Low |
High |
Control |
Low |
High |
Control |
High |
|
|
N |
0 |
0 |
312 |
0 |
0 |
0 |
0.00 |
0.00 |
19 * |
|
Ca |
410 |
422 |
447 |
0 |
0 |
0 |
0.00 |
0.00 |
453 * |
|
P |
0 |
0 |
0 |
0 |
7 |
155 |
0.00 |
0.00 |
59 * |
|
K |
0 |
0 |
0 |
173 |
184 |
195 |
0.00 |
0.00 |
2088 * |
|
B |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.68 |
0.79 ** |
|
Cu |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.08 |
0.34 *** |
|
Mo |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.12 |
0.01 *** |
|
Mn |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.28 |
5.27 *** |
|
Zn |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.33 |
1.34 *** |
|
Co |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
0.25 |
0.04 *** |
|
* mean of 25 samples analyzed by Mehlich III extraction. |
|||||||||
|
** single soil sample using the hot water method. |
|||||||||
|
*** single soil sample using DTPA. |
|||||||||
Moisture
Using a 3x2 factorial design, this experiment compared all combinations of three levels of moisture – high, mid, and cycle – and two levels of mycorrhizal inoculation – with (M+) or without (M-). Moisture levels were designated as high (soil moisture between 15-25%), mid-range (soil moisture between 5-15%) or saturation/drought cycle (between wilting point and field capacity). In the high moisture treatment, pots received 200 mL of tap water when they reached a lower threshold of 15% soil moisture which raised them to field saturation (around 25%). For the saturation/drought cycle, plants received 500 mL of tap water after 3 days below a threshold of 2.5% soil moisture. They were watered in two 250 mL increments to minimize leaching and runoff. The three-day wait was set based on the average time cowpeas took to wilt after reaching 2.5% soil moisture in a pre-trial assessment.
Micronutrients
Using a 2x2 factorial design, this experiment compared two levels of micronutrients – micronutrients added (Mi+) and field level (Mi-) – and two levels of mycorrhizal inoculation – M+ or M-. We compared impact of addition of copper (0.5 µM CuSO4), cobalt (1.7 µM CoCl2) boron (25 µMH3BO3), molybdenum (0.5 µM Na2MoO4), manganese (2 µM MnCl2), and zinc (2 µM ZnSO4) on root nodulation to a control with field level micronutrients (Table 2). Micronutrient concentrations were based on a modified Hoagland’s solution (Taiz et al. 2015).
Phosphorus
Using a 3x2 factorial design, this experiment compared three levels of P – field, low and high – and two levels of mycorrhizal inoculation – M+ or M-. The levels of P tested included field level P, low P (0.1 mM KH2PO4, 1.9 mM KCl), and high P (2 mM KH2PO4). Since P (target nutrient) was supplied as KH2PO4, potassium levels (non-target) were also raised. To avoid confounding the impacts of P and K, low and field level treatments were supplemented with potassium chloride (KCl) to match the K levels applied to the high P treatment (Table 2).
Nitrogen
Using a 3x2 factorial design, this experiment compared three levels of N – low, field, and high – and two levels of mycorrhizal inoculation – M+ or M-. We compared the impact on nodulation of field level N (Table 2) to N levels both higher (5 mM CaN2O6) and lower (1/2 field level). Higher N treatments had calcium nitrate added in solution while lower N was achieved through a 50/50 mix of field soil and sand. Since the high N treatment also received 5 mM Ca (non-target) in addition to 10 mM N (target), low and field level N treatments were supplemented with calcium chloride (5 mM CaCl2) to match the calcium levels applied to the high N treatment (Table 2). These adjustments were made to avoid confounding the impacts of Ca and N on nodulation.
Soil Sterilization
A sterilization experiment was included to isolate the effects of soil microbes impacting nodulation. For these treatments, soil media was steam sterilized in an autoclave at 121 oC for 30 minutes before planting. Using a 2 X 2 X 2 factorial design, this experiment compared all combinations of following three factors – sterilized (S+) or unsterilized (S-) soil, with (M+) or without (M-) mycorrhizal inoculation, with (R+) or without (R-) rhizobial inoculation. Steam sterilization can affect soil pH as well as nutrient content and availability. Therefore, separate tests were conducted to determine the baseline pH and nutrient levels for the sterilized soil.
Data collection
During the final week before termination (days 68-74), light-saturated photosynthesis measurements (Asat) were taken for three replicates from each treatment using a Portable Photosynthesis System (model LI-6400XT, LiCOR, Lincoln, NE, USA). Asat was recorded at 2000 umol m-2 s-1 PAR after the assimilation value had stabilized and the stomatal conductance value exceeded a threshold of 0.05 mol m‑2 s‑1. After 75 days, five replicates from each treatment were randomly chosen. Roots were cleaned and examined for nodules which were counted, weighed, and checked for internal color as an indicator of N fixation activity. Pink, red or brown nodules were counted as active while green, grey, tan, and any other color were considered inactive (Farquharson et al. 2012). Plants were then dried for at least 72 hours at 70oC and the dry biomass of root, stem, and leaf tissue for each plant were recorded.
Data analysis
For the moisture, micronutrient, phosphorus, and nitrogen experiments, 2-way analyses of variance were conducted to compare the main effects of each factor and mycorrhizal inoculation and the interaction effect between that factor and mycorrhizae on nodule number, biomass, and activity and plant indicators including Asat, root, stem, leaf and total biomass, root to shoot ratio and nodule to plant biomass ratio. Nodulation intensity was calculated as the ratio of dry nodule biomass to dry root biomass, which is affected by both nodule number and mean nodule size. Multiple comparisons were performed using the Holm-Sidak method. When assumptions of normality and equal variance were violated, a Kruskal-Wallis 1-way ANOVA on ranks was employed, followed by Dunn’s method for multiple comparisons. For the sterilization experiment, three-way ANOVAs were conducted to compare the main effects of sterilization, mycorrhizal inoculation, and rhizobial inoculation and the interaction effects among the three (SYSTAT™, San Jose, CA).
Micronutrient Experiment
In the second experiment of the series, developed based on the results of the first but following a modified protocol, Iron and Clay cowpea seeds (Vigna unguiculata; Johnny’s Seeds, Winslow, ME) were surface sterilized through immersion in 2% hypochlorite solution for five minutes, followed by five rinses with sterile water. Two seeds were then planted into each 15 cm plastic pots with 1500 g of a 1:1 mixture of perlite and soil obtained from the field where nodulation failure occurred. Nutrient solutions and estimated treatment impact compared to field soil levels are in Table 4.
Table 4. Micronutrient Experiment Nutrient Solutions and Treatment Impacts
|
Nutrients |
Nutrient Concentration |
Treatment impact (µg/g dry soil) |
|
Zinc |
2µM ZnSO4 |
0.21 |
|
Copper |
0.5 µM CuSO4 |
0.05 |
|
Cobalt |
1.7 µM CoCl2 |
0.16 |
|
Molybdenum |
0.5 µM Na2MoO4 |
0.08 |
|
Boron |
25µM H3BO3 |
0.43 |
|
Manganese |
2µM MnCl2 |
0.18 |
At planting, a 1 ml solution of rhizobium inoculant solution (2 g inoculant/500 mL water; Verdesian Guard-N®, Cary, NC) was applied to the seed. Pots were thinned to one plant each after 7 days. Cowpeas were randomly assigned to one of 8 treatments that were watered with a nutrient solution of B, Co, Cu, Mn, Mo, or Zn individually, all 6 micronutrients together, or tap water (control). The plants were grown for 45 days in Percival Environmental Growth Chambers (Perry, Iowa) with 15 hours of light (PAR – 440 µmol/m2/s) every 24 hours. Light period temperatures were 27 oC, dark period temperatures were 24 oC, and relative humidity ranged between 45 and 70%. Pots were watered with 150 mL of the designated nutrient solution every three days for a total of 16 waterings (2.4 L solution/plant). Each treatment had 9 replicates for a total of 72 individuals.
Data were collected 45 days after seeding from 9 replicates for each treatment. Pre-termination measurements included spectral signatures (ASD Handheld 2, Malvern Panalytical, Longmont, CO) and chlorophyll content (SPAD 502 Chlorophyll Meter, Spectrum, Aurora, IL). After termination, roots were cleaned and examined for nodules which were counted, weighed, and checked for internal color as an indicator of N fixation activity. Plants were then dried for at least 72 hours at 70oC and the dry biomass of roots, stems, and leaves for each plant were recorded. One-way analyses of variance were used to examine differences among treatments (SYSTAT™, San Jose, CA).
Dominant Factor Assessment Results
No significant interactions or effects of mycorrhizae on nodule biomass or nodulation intensity were detected at the 0.05 level of significance for any of the plant or nodule indicators in any of the experiments. Therefore, all results presented henceforth group together the M+ and M- results for each treatment.

Moisture
Significant effects of moisture level were found for nodule biomass (F(2,24) = 4.941, p = 0.016). Mean nodule biomass was 0.80 g ± 0.42 for high moisture, 0.56 g ± 0.43 for mid, and 0.31 g ± 0.16 for cycle. Multiple comparisons showed a significant difference between high and cycle (p=0.013), but not between mid and either high or cycle treatments. Nodulation intensities for the three moisture levels were 0.38 ± 0.13 for high moisture, 0.42 ± 0.15 for mid moisture, and 0.28 ± 0.06 for cycle with no significant differences among them (F(2,24) = 3.354, p= 0.052).
Micronutrient
The addition of micronutrients significantly increased nodule biomass from 0.56 ± 0.43 to 1.02 g ±0.35 (F(1,16) = 7.671, p = 0.013). Nodulation intensity was 0.55 ± 0.20 for Mi+ and 0.42 ± 0.15 for the control (F(1,16) = 4.408, p = 0.051).
Phosphorus
The effects of P level on nodule biomass (F(2,24) = 0.719, p = 0.497) and nodulation intensity (F(2,24) = 0.765 , p = 0.476) were not statistically significant. Nodule biomass was 0.51 g ± 0.33 for field P, 0.63 g ± 0.44 for low P, and 0.75 g ± 0.56 for high P. Nodulation intensity was 0.45 ± 0.16 for field P, 0.48 ± 0.20 for low P, and 0.57 ±0.32 for high P.
Nitrogen
Nodule biomass was 0.31 g ± 0.11 for below field N, 0.39 g ± 0.26 for field N, and 0.0009 g ± 0.003 for high N. High N additions inhibited nodulation in all but one replicate. Due to the large number of zeros, nodule biomass data failed normality and equal variance. A Kruskal-Wallis one-way ANOVA on ranks was used instead and found significant differences in nodule biomass among the three N levels (H(2) = 21.425, p < 0.001). Nodule biomass for the high N treatments was significantly less than field N (Q = 4.053, p < 0.05), and low N (Q = 3.751, p < 0.05), but that field N and low N did not vary significantly from each other (Q = 0.295, p > 0.05).
The same pattern was true for nodulation intensity (H(2) = 22.314, p < 0.001) with a significantly lower nodulation intensity for high N compared to field (Q = 3.474, p < 0.05) and below field N (Q = 4.330, p < 0.05), but no difference between field and below field (Q = 0.836, p > 0.05. Nodulation intensity was 0.42 ± 0.11 for below field N, 0.34 ± 0.11 for field N, and 0 ± 0 for high N.
Sterilization
Soil sterilization had a significant impact on both nodule biomass (F(7,32)= 48.146, p <0.001) and nodulation intensity (F(7,32)= 65.493, p < 0.001). Nodule biomass was 0.70 g ± 0.42 for unsterilized soil and 0.08 g ± 0.07 for sterilized. Similarly, plants in sterilized soil showed much lower nodulation intensities (0.10 ± 0.05) compared to plants in unsterilized soil (0.47 g ± 0.21). This pattern was confirmed by the other measured variables as well, with sterilized plants performing poorly in every measured category.
Micronutrient Experiment Results
There were no significant differences among the micronutrient treatments for any of the measured variables, including nodule biomass (F(7,64) = 1.686, p = 0.128) and nodulation intensity (H(7) = 4.988, p = 0.661). We also found no significant differences in foliar N concentration among the treatments (F(7,63) = 1.223, p = 0.303).
Discussion
Eliminated Factors – Nitrogen, Phosphorus, Mycorrhizae
Based on the results of these experiments, two possible explanations for the nodulation failure and one proposed intervention can be eliminated from consideration in this context – phosphorus, nitrogen, and mycorrhizae.
P levels of 59.2 ppm like those present in this field are usually adequate for N fixation (O’Hara 2012). P was included as a potential determinant in the coarse assay experiment due to concerns about P accessibility in alkaline soils with high levels of calcium (von Wandruszka 2006). However, P additions did not improve nodulation, thereby discounting phosphorous deficiency as an explanation.
High N levels can inhibit nodule formation and nitrogenase activity (Walley et al. 2005; Serraj et al. 1999). Our results are congruent with these findings, but they do not support that N was a primary driver of the observed nodulation failure. We found little or no nodulation in plants where excessive rates of N were added, reinforcing the recommendation that leguminous cover crops are best employed where N may be deficient. In this agroecosystem, nodules did form at field level N (18.8 ppm, 42 kg/ha) and there was no significant difference between nodulation at field level N and below field level N. Reducing the N content by half did not increase the number or weight of nodules over the field level soil. Nonetheless, we did find that enhanced nodulation promotes greater leaf biomass which may in turn promote greater cover crop development and subsequent N fixation in a positive feedback. In farms where synthetic N inputs are unlikely to reach excessively high N levels, leguminous cover crops may assist in maximizing plant-available N while providing other benefits to soil health, such as weed control (Rugg 2017), and soil microbial biodiversity (McDaniel et al. 2014; Soti et al. 2016).
Other studies have suggested mycorrhizal inoculation as an intervention to improve nodulation (Chalk et al. 2006; Ortas 2003), but the results of this experiment did not confirm the utility of this practice as predicted. Mycorrhizal inoculation was not observed to significantly impact nodule biomass in any component of this trial. This may be related to the inoculant, which could have lacked viable infective propagules or been poorly suited for soil and climate conditions. Based on these results, locally adapted mycorrhizal inoculants are highly recommended for farmers and farm managers looking to employ this strategy on-farm. It can also be difficult to observe the impact of mycorrhiza in relatively small greenhouse pots. The benefits of mycorrhizae come from the hyphal network that expands nutrient mining beyond the range accessible to the plant’s root system on its own. In a small pot setting where the root system can mine the available soil volume effectively, the potential benefit of mycorrhizae is lessened (Poorter et al. 2012).
Confirmed Factors – Moisture
The experimental results suggest that increased moisture and increased frequency of watering improve nodulation outcomes. Unfortunately for dryland farmers, moisture limitation is particularly difficult to mitigate in the field. In a semi-arid region like the Rio Grande Valley, dry soils may consistently reduce the N fixation potential of leguminous cover crops, even when those soils remain moist enough for plants to survive. For farmers with irrigation access, it may not be cost-effective to water a crop that they do not intend to harvest. Cost-benefit calculations must be carefully considered, but legumes may provide a more consistent return on investment in regions that receive more regular rainfall.
When a legume enters a period of water stress, nodules are the first to lose their water supply since the process of N fixation is more sensitive to drought than plant growth (Serraj 1999). In this experiment, the minimum moisture attained is more influential on nodulation than the total amount of moisture received during the season. For example, plants under the saturation/drought cycle regime received approximately the same amount of total water over the course of the experiment as mid-range moisture plants (3515 mL and 3445 mL respectively), but we found that acute drought stress, similar to conditions in a dry-land agriculture, put the plants at a serious disadvantage compared to plants with metered moisture.
One proposed solution for dryland farmers is to choose legumes based on drought tolerance. However, these results suggest that even cowpea, one of the most heat and drought tolerant legume options, is vulnerable to the impact of moisture stress on nodulation and N fixation. Other studies on cowpea have shown that nodule water potential and nitrogenase activity show major declines after periods of drought, even before leaf water potential shows any changes (Pararajasingham et al. 1990). Even minor 15% declines in photosynthesis in a moisture stress situation can be accompanied by 90% drops in nitrogenase activity (Venkateswarlu 1989). More research on alternative solutions could help improve nodulation outcomes in semi-arid regions like the Rio Grande Valley. In addition to testing other drought tolerant legume species, further research could help determine the nitrogen tradeoffs between longer growing periods during drier seasons and shorter growing periods if planting is delayed until historically wetter times of year.
Confused Factors – Micronutrients, Native Soil Communities
Micronutrient addition increased nodulation by 79% over the control in the initial coarse assay, but the same was not observed in the follow-up micronutrient experiment. This discrepancy could have been caused by differences in stress (Figure 2) faced by the two sets of cowpeas due to the role of micronutrients in plant stress mediation (Hajiboland 2012; Rubio et al. 2007). It was also difficult to determine micronutrient levels that were biologically significant without risking toxicity. This experiment could be repeated with higher micronutrient concentrations. Further research is required to pinpoint specific micronutrients that can facilitate improved nodulation under field conditions in alkaline subtropical soils. However, in both experiments, cowpeas consistently formed nodules even with no added micronutrients. Micronutrients, therefore, cannot be the sole explanation for the failure of nodules to form in the field, but instead may interact with additional factors.
The role of native soil communities also remains unclear. Microbial diversity, particularly of plant growth-promoting rhizobacteria, plays a key function in healthy soils (Lugtenberg and Kamilova 2009). We found that plants in sterilized soil (with no major changes in soil nutrient levels) had significantly reduced nodule biomass and were less healthy by every measure than plants in unsterilized native soil. This was true both for cowpeas that received rhizobial and mycorrhizal inoculation after sterilization and those that were uninoculated. Far from causing the nodulation failure, the complex microbial communities of native soils seem to have facilitated effective nodulation and other metrics of plant growth and vigor. A better understanding of the ecology of microbial communities and the impact of introductions such as rhizobial and mycorrhizal inoculants into agroecosystems is required to maximize the functionality of cover crops and overall soil health.
Ignored Factors – Rhizobial Inoculation
The factors investigated in our experiments, while supported by the scientific literature, may have overlooked a more fundamental problem – effective rhizobial inoculation. Nitrogen, phosphorus, moisture, micronutrients, and competitive soil microorganisms can all impact nodulation. However, the first requirement for nodule formation is that live rhizobia and viable legume seeds are present in the soil together. If rhizobia capable of infecting the legume species are neither present in the native soil nor introduced through an inoculant, the legume-rhizobia symbiosis cannot form and even the best habitat and climatic conditions cannot mitigate this fundamental problem. Although we can neither test the original rhizobial inoculant for viability (Lupwayi et al. 2000) nor measure the number of rhizobia that survived on each seed (Materon and Weaver 1985) post-facto, we suggest that inoculation problems may have been a major cause of the initial nodulation failure.
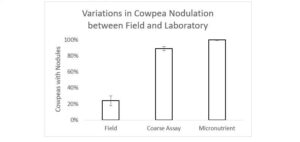
While we expected that nodule initiation would fail for many of our treatments, given the nodulation failure in the field, the only treatment in which nodulation was completely inhibited was the highest N treatment, for reasons discussed above. In a subsequent field trial at site of the original nodulation failure, 24% of cowpea plants formed at least one nodule. In the coarse assay and micronutrient experiments, 95% of plants formed at least one nodule (Figure 3). The scale of this improvement was unmatched by any of the other nodulation drivers under consideration.
In field scale inoculations, a peat-based inoculant is commonly applied to lightly wetted seeds, then planted via a seed spreader or drill. In our controlled study, a rhizobial inoculant slurry was pipetted directly onto pregerminated seed root radicles. Obviously, an inoculation method this precise would not be feasible in the field. That said, any alternative method for farm-scale inoculation that increases rhizobial adherence to seeds such as adhesive additives (gum arabic, methyl cellulose, or vegetable oil) would improve the possibility of successful infection and nodulation and thus nutrient management (Elegba 1984; Hoben et al. 1991). Improved inoculation methods are a prime candidate for further research to improve nodulation success in South Texas.
Educational & Outreach Activities
Participation Summary:
This work was presented in poster form at the Soil Science Society of America annual meeting in San Diego, CA (Jan. 2019), Subtropical Agriculture and Environments Conference in Weslaco, TX (Feb. 2019) and at the Texas Organic Farmers and Gardeners Conference in Corpus Christi, TX (Feb. 2019). It was also the basis of an oral presentation at the Texas Hispanic Farmers and Ranchers Conference in McAllen, TX (Dec. 2018).
The results were also included in four field day presentations, two of which were held at Hilltop Gardens in Lyford, TX (Nov. 15, 2018 and June 6, 2019), one field day at PPC farms in Mission, TX (July 11, 2019), and one at Terra Preta Farms in Edinburg, TX (Feb. 25, 2020).
Two online educational tools were developed based on this work. A short video about troubleshooting nodulation in legumes is available at https://www.youtube.com/watch?v=jNCNEbLlASA&feature=youtu.be and a digital nodulation troubleshooting tool can be found at https://www.utrgv.edu/agroecology/research/subtropical-soil-health/nodulation-guide/.
A journal article reporting the results of this work was published in Sept. 2019: Kasper, S.; Christoffersen, B.; Soti, P.; Racelis, A. Abiotic and Biotic Limitations to Nodulation by Leguminous Cover Crops in South Texas. Agriculture 2019, 9, 209. https://doi.org/10.3390/agriculture9100209
Project Outcomes
Widespread adoption of leguminous cover crops requires higher rates of nodulation success and more efficient implementation to realize benefits to soil health and fertility. When N fertilizer replacement value is considered alone (excluding long-term soil health benefits), the high cost of legume seeds can outweigh the low cost of synthetic N fertilizer (Mallory et al. 1998). Replacing synthetic N inputs with biological N from legumes has ecological benefits, such as reduced nitrous oxide emissions and lower nutrient runoff (Vitousek et al. 1997; Crews et al. 2004) but on-farm implementation depends on the cost-effectiveness of this strategy. If efficient N fixation cannot be assured, farmers may opt for non-legume cover cropping options with lower seed costs. Research such as this on factors optimizing nodulation and N fixation in leguminous cover crops is therefore needed to address these impediments to increased adoption.
This project has tried to promote more awareness in our local and regional agricultural community about the care and attention required to maximize the efficiency of nitrogen fixation by leguminous cover crops. It was important to us to translate our scientific results into practical information that would be useful and applicable to farmers as they try to successfully implement more sustainable soil and nutrient management strategies. The video, online nodulation troubleshooting tool, and the contents of the field day presentations were all tailored with these goals in mind. With continued research on appropriate legume varieties for this region and successful inoculation strategies, leguminous cover crops may become a more environmentally and economically viable practice in south Texas.
This project began with a concerning surprise: fourteen acres of leguminous cover crops that failed to nodulate and fix nitrogen. Forage pea (Pisum sativum), hairy vetch (Vicia villosa), and crimson clover (Trifolium incarnatum) were inoculated with
peat-based rhizobial inoculants at planting. Aboveground, the plants appeared healthy, but root
checks for nodulation during the season showed no signs of nodules for any of the legume
species. The legumes still provided some benefits like weed suppression and increased organic
matter but fell short on a primary goal of nitrogen fixation. This nodulation failure launched a year-long effort of conversations with farmers and
other experts, a literature review for published explanations, and several experiments to try to better understand what factors may have been responsible for this result and what, if any, interventions might be possible.
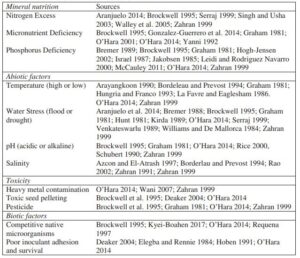
Through this project we learned about the long list of factors that can cause nodulation to fail in leguminous cover crops (Table 1) and narrowed that list down to several candidates most likely to cause problems in our region, most notable moisture constraints and inoculation issues.
Although this study does not lend itself one easy solution for improving legume performance in south Texas, it does clearly eliminate some of the factors from consideration in this context and provides a more focused foundation for future work in the efforts to develop efficient cover cropping systems in the Rio Grande Valley.
As a researcher with a background in sustainable farming, it was important to me that this research be driven by real-world
problems faced by farmers in the Rio Grande Valley. This project grew organically out of a true puzzle we noticed out in the field and was informed and guided at every step by the expertise of our farm partners in this research. This experience has helped develop my skills as a "boundary spanner," someone who can stand at the border between information producers (agricultural researchers) and information users (sustainable farmers) and facilitate communication between the two in a way that is credible and useful to both (Safford et al. 2017).