Final report for GS19-197
Project Information
In Georgia, pecan is one of the top agricultural commodities producing an average yield of 599.4 kg per hectare over 52,204 hectares in 2019 (NASS 2020). The farm gate value for pecan production in 2018 was $218,477,486 (Wolfe and Stubbs 2019). Pecan, like many other agricultural commodities, is susceptible to damage by an assemblage of pest whose life histories and management tactics vary (Wells et al. 2007). Three species of aphid feed on pecan in the southeast including yellow pecan aphid, Monelliopsis pecanis Bissell, blackmargined aphid, Monellia caryella (Fitch), and black pecan aphid, Melanocallis caryaefoliae (Davis), (Hemiptera: Aphididae) (Wood et al. 1987, Mizell III and Schiffhauer 1990, Wells and Conner 2007, Shapiro–Ilan et al. 2013). The yellow pecan aphid and blackmargined aphid are collectively referred to as the ‘yellow aphid’ complex. All three species of aphid feed on the leaves of the tree causing both direct and indirect damage. Feeding on the leaf removes valuable nutrients from the leaf which are valuable for vital functions. In addition, as pecan aphids feed they secrete honeydew as a byproduct. Honeydew coats the leaf and overtime promotes the growth of sooty mold which can hinder the photosynthetic capabilities of the plant (Tedders 1978, Cottrell et al. 2009, Paulsen et al. 2013).
While insecticides are the primary method of control for these pests, the threat of insecticide resistance and non-target effects is a growing issue for aphid management in many different systems (Kandil et al. 2017, Tang et al. 2017, Mingeot et al. 2020). Therefore, alternative methods are needed in order to address pecan aphid issues in the field. One method is the use of natural enemies that can keep aphid populations in check. An example of a natural enemy that can be implemented for aphid management is Aphelinus perpallidus, a primary parasitoid of pecan aphids (Tedders 1978, Bueno Jr and Stone 1983, Bueno Jr and Stone 1985, Bueno Jr and Stone 1987). Despite previous research on the effects of intraguild predation and lab-based insecticide exposure studies, there is still much information that needs to be acquired including the effects of seasonality and vertical stratification (a.k.a canopy height)(Tedders 1978, Bueno Jr and Stone 1983, Bueno Jr and Stone 1985, Bueno Jr and Stone 1987).
The objectives of this research was to assess environmental factors on pecan aphids and A. perpallidus. In our first objective, we assessed the effects of seasonality on pecan aphids and A. perpallidus in four commercial orchards with different pest management regimes. In our second objective, we assessed the abundance of pecan aphids and A. perpallidus at different canopy heights in pecan trees at an experimental orchard. A third objective, which was a lab-based study devoted to assessing pecan aphid and A. perpallidus interactions, was canceled due to the covid-19 pandemic.
We conclude from this research that while management regimes can change between orchards, aphid phenology often remains similar as we saw few consistent differences between orchards throughout the two years of study on objective 1. Aphids followed similar seasonal patterns as demonstrated in previous studies in experimental orchards despite being present in lower population numbers. A. perpallidus populations typically followed that of their hosts. In objective 2, we found that when significant differences were detected between canopy heights, aphid and A. perpallidus were more abundant in the lower half of the pecan trees.
What these conclusions show is that growers may be able to rely on natural enemies and other factors to manage aphid populations in their orchards. In objective 1, we found that growers were applying insecticides for aphids despite having numbers below threshold. This opens the possibility for growers to reduce or eliminate spraying as a tool for aphid management saving growers time and money. In objective 2, we showed that when pecan aphids and A. perpallidus were present in higher numbers, they were typically more abundant in the lower parts of the tree. This is beneficial information for growers as they can be confident that their insecticide applications are appropriately covering areas of the tree with the highest amount of aphids. A more detailed explanations of the results and discussion can be found below.
Literature Cited
- Bueno Jr, R., and J. Stone. 1983. Phenology of a Parasite of the Blackmargined Aphid in West Texas [Aphelinus Perpallidus, Monellia Caryella]. Southwest. Entomol.
- Bueno Jr, R., and J. D. Stone. 1985. Aphelinus Perpallidus Parasitism of Monellia Caryella Populations in Far West Texas. J. Entomol. Sci. 20: 325-330.
- Bueno Jr, R., and J. Stone. 1987. Reproductive Response of Aphelinus perpallidus (Hymenoptera: Aphelinidae) to Age of Its Parent and Density of Its Host, Monellia Caryella (Homoptera: Aphidae). Environ. Entomol. 16: 877-880.
- Bueno Jr, R., and H. Van Cleve. 1997. The Effect of Temperature and Host Density on the Reproduction of Aphelinus Perpallidus. Southwest. Entomol.
- Cottrell, T. E., B. W. Wood, and X. Ni. 2009. Chlorotic Feeding Injury by the Black Pecan Aphid (Hemiptera: Aphididae) to Pecan Foliage Promotes Aphid Settling and Nymphal Development. Environ. Entomol. 38: 411-416
- Kandil, M.A.; Abdallah, I.S.; Abou-Yousef, H.M.; Abdallah, N.A.; Fouad, E.A. 2017. Mechanism of Resistance to Pirimicarb in the Cowpea Aphid Aphis craccivora. Crop Prot. 94: 173-177.
- Mingeot, D.; Hautier, L.; Jansen, J.P. 2020. Structuration of Multilocus Genotypes Associated with Insecticide Resistance of the Peach Potato Aphid, Myzus persicae (Sulzer), in Potato Fields in Southern Belgium. Pest Manag. Sci.
- Mizell III, R. F., and D. E. Schiffhauer. 1990. Effects of Pesticides on Pecan Aphid Predators Chrysoperla rufilabris (Neuroptera: Chrysopidae), Hippodamia convergens, Cycloneda sanguinea (L.), Olla v-nigrum (Coleoptera: Coccinellidae), and Aphelinus perpallidus (Hymenoptera: Encyrtidae). J Econ Entomol 83: 1806-1812.
- NASS, U. 2020. United States Department of Agriculture National Agricultural Statistics Service. 2017. Quick stats database.
- Paulsen, C., T. Cottrell, and J. Ruberson. 2013. Distribution of the Black Pecan Aphid, Melanocallis caryaefoliae, On the Upper and Lower Surface of Pecan Foliage. Entomol. Exp. Appl. 146: 252-260.
- Shapiro–Ilan, D. I., T. E. Cottrell, M. A. Jackson, and B. W. Wood. 2013. Control of Key Pecan Insect Pests Using Biorational Pesticides. J Econ Entomol 106: 257-266.
- Tang, Q.-L.; Ma, K.-S.; Hou, Y.-M.; Gao, X.-W. 2017. Monitoring Insecticide Resistance and Diagnostics of Resistance Mechanisms in the Green Peach Aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae) in China. Pestic. Biochem. Phys., 143, 39-47.
- Tedders, W. L. 1978. Important Biological and Morphological Characteristics of the Foliar-feeding Aphids of Pecan, vol. 1578-1587, Department of Agriculture, Science and Education Administration, 1978.
- Watterson, G. P., and J. D. Stone. 1982. Parasites of Blackmargined Aphids and their Effect on Aphid Populations in Far-West Texas. Environ. Entomol. 11: 667-669.
- Wells, L. 2017. Pecan: America's Native Nut Tree, University of Alabama Press.
- Wells, L., and P. Conner. 2007. Southeastern Pecan Growers' Handbook.
Our objectives include:
1. Identifying the parasitoid species attacking pecan aphids in Georgia and quantifying their parasitism rates. We have updated this objective to include a seasonal phenology of all threes aphid species and A. perpallidus (the primary aphid parasitoid in Georgia).
2. Assessing parasitism rates on pecan aphids in the laboratory (Canceled)
3. Examining the relationship between canopy height and parasitism rates. We also included the effects of canopy height on aphid and parasitoid numbers.
Cooperators
- (Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- (Researcher)
Research
Objective 1: Seasonal Phenology of Aphids in Commercial Orchards
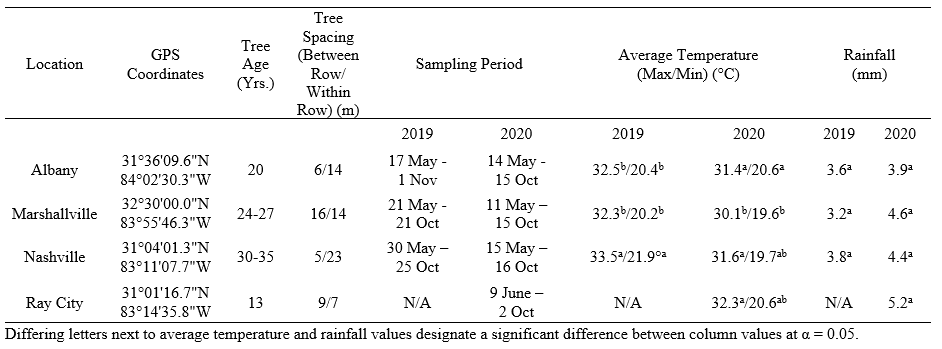
Table 1. GPS coordinates, tree age and spacing, climate data, and the corresponding sampling periods for each site sampled in Georgia, USA.
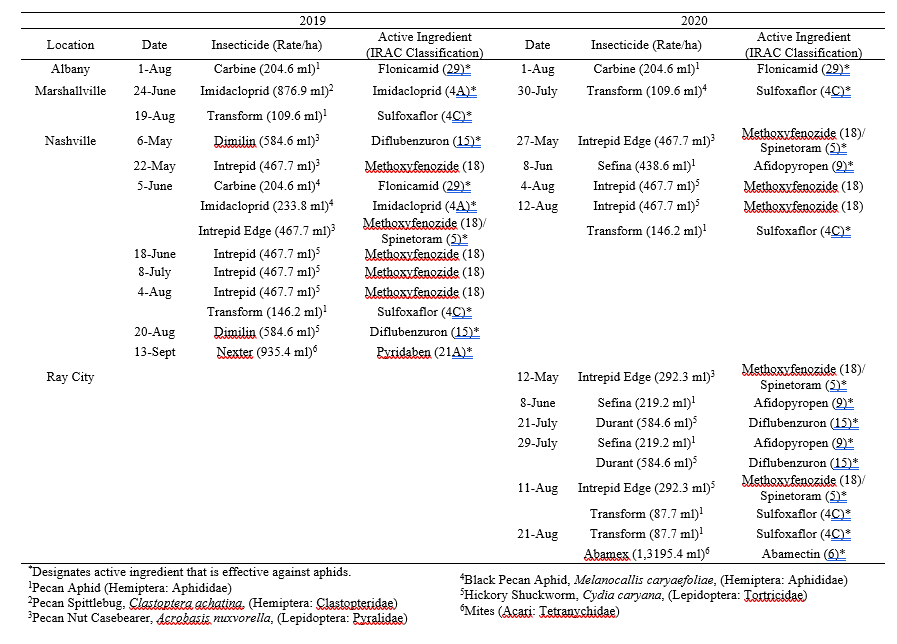
Table 2. Dates of 2019 and 2020 insecticide applications per site based on each grower’s personal management program.
All sampling sites were done in partnership with four large scale pecan producers in major pecan growing regions in southern Georgia, USA (Table 1). During the 2019 and 2020 growing season, two 0.4 ha sampling areas (20.1 x 201.2 m each) were measured at each location prior to sampling. Within each sampling area, five mature ‘Sumner’ variety pecan trees were selected at random. From each tree, five leaves were collected from the lower canopy (~1-2 m from the ground) using a pole pruner and stored in labeled 3.79 L Ziploc® bags. To standardize the leaf samples, only the middle three pairs of leaflets from each compound leaf were collected and examined. During transit, leaf samples were stored in a cooler containing an ice block in order to mitigate aphid movement in the bag. Leaf samples were stored in a refrigerator and examined within 48 hours of collection. The interior of all bags was examined to account for aphids/mummies that moved or fell off leaves during pre-examination.
Sampling was done every other week throughout the sampling period and sampling was ceased once growers began to harvest pecans (Table 1). Leaves were taken back to the lab to quantify the number of live aphids and parasitized aphids that were mummified (from here on referred to as mummies). Both emerged parasitoid mummies (i.e., mummies from which adult wasps successfully emerged from) and non-emerged parasitoid mummies (i.e., mummies on which no successful wasp emergence has occurred) were counted. In 2020, non-emerged parasitoid mummies were placed individually in plastic capsules (Size 0, 7.62 cm, Healthy Life Supply; Mound House, NV) and stored in an environmental chamber (25°C, 60% RH, 16:8 L: D, Percival© E36L2; Perry, IA). Parasitoids that emerged from the mummies were identified as either primary or hyperparasitoids using a reference collection identified by a parasitic hymenopteran taxonomist, James Woolley (Texas A&M University), and Hymenoptera of the World by Goulet and Huber (1993). This information was used to quantify the proportion of primary and hyperparasitoid emergence at each site. In addition to leaf sampling, one yellow sticky card (7.6 x 12.7cm, Olson Products Inc.; Medina, OH) was placed for one week in the lower canopy of five randomly selected trees in each sampling area (10 cards total) from July to September in 2019 and from May to October in 2020 to assess adult A. perpallidus populations.
For all analyses, adults and nymphs of yellow pecan aphid and blackmargined aphids were pooled together as the yellow aphid complex. Adults and nymphs of black pecan aphids were pooled together. Aphid mummy analysis was divided into emerged parasitoid mummies and non-emerged parasitoid mummies. Differences in seasonal mean aphid, mummy and adult parasitoid numbers across the sampling periods for each site and between sites were analyzed with a One-way ANOVA. Subsequently, Tukey’s HSD was used for post hoc analysis to separate means among sampling dates and between sites at α = 0.05. Spearman’s correlation was used to analyze the correlation between aphids and both mummies and adult parasitoids at α = 0.05. Difference among insecticide application at each site were analyzed using a Pearson’s chi-squared test at α = 0.05. All analyses were conducted in JMP® Pro 14.1.0 (SAS Version 14.1.0, Cary, NC).
Objective 2: Examining the parasitism rates of all three aphid species by pecan aphid parasitoids in the laboratory.
Canceled due to Covid-19 limiting lab experiments, have met with graduate committee in order to come up with and execute alternative projects.
Objective 3: Examining the relationships between canopy height and the rate of successful parasitism.
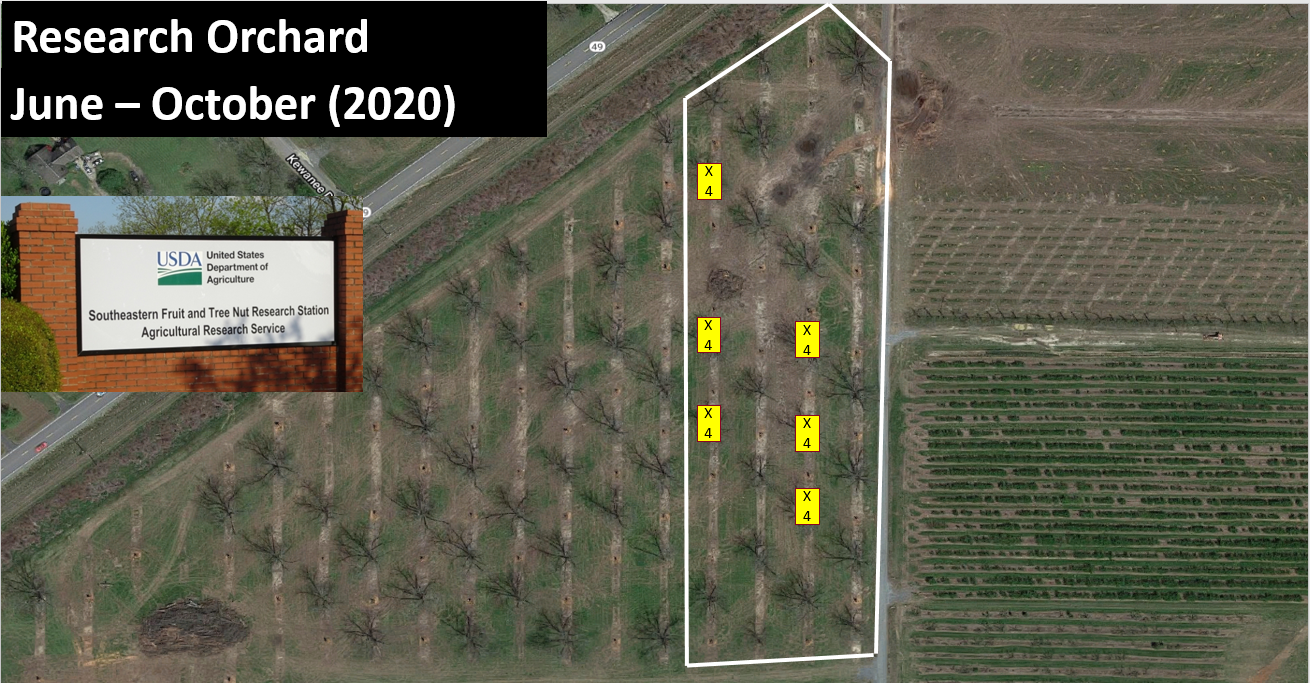
This study was conducted from June – October 2020 and 2021 in Peach County, Georgia, at the United States Department of Agriculture (USDA) Southeastern Fruit and Tree Nut Research Laboratory on mature ‘Stuart’ and ‘Sly’ pecan trees. All trees were ~15.2 meters or greater in height. A lift was used to perform all measuring and sampling. Prior to sampling, the tree canopies were measured and marked at 6 meters, 9 meters, 12 meters, and 15 meters in six trees in the orchard. Due to low numbers during June, sampling carbaryl + pyrethroid (Baythroid XL©, 119.8 g a.i./liter Bayer Cropscience) at 9.35 ml/h was applied on 20 July and 3 August to flare aphid numbers. Five whole pecan leaves were taken from each height in each tree once a month in order to quantify aphids and parasitoid pupa. In addition, a yellow sticky card was placed at each height in each tree in order to quantify the adult parastioids at each height.
For both years at each site, leaves and cards were taken back to the lab to quantify the number of live aphids, parasitoid pupa (both hatched and unhatched), and adult parasitoids. Since whole leaves were taken, the number of leaflets on each leaf was quantified. Effects of height on aphids and aphid parastioids were analyzed with a One-way Analysis of Variance. Tukey’s HSD was used for post hoc analysis to separate means among sampling dates and between sites at α = 0.05. Spearman correlation was used to analyze the correlation between aphids and both pupal and adult parasitoids at α = 0.05. All analyses were conducted in JMP® Pro 14.1.0 (SAS Version 14.1.0, Cary, NC).
Objective 1: Examine parasitoid species attacking pecan aphids in major pecan-growing regions of Georgia
Comparison of Overall Parasitoid and Aphid Populations Across Sites
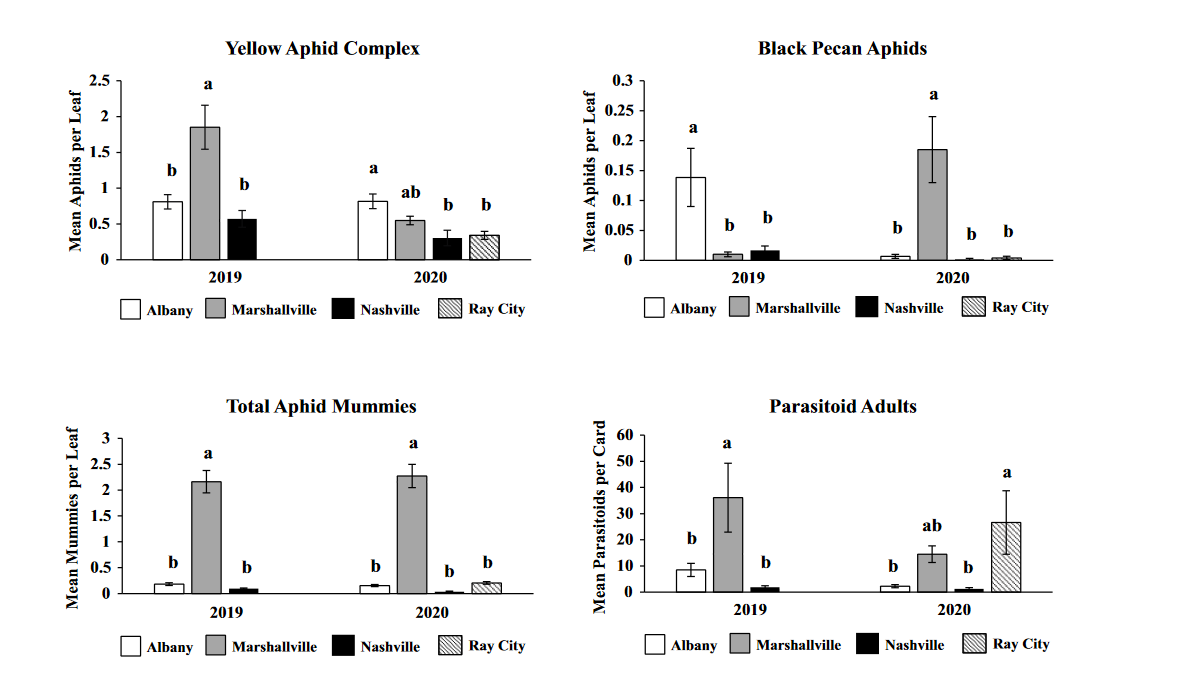
Comparison of aphids and parasitoids at each site in both years revealed variation among sites across both years of the study with little consistency from year to year. The only consistent trend among the sites was that Marshallville had the highest number of parasitoids during both years of the study. One interesting aspect of this analysis is that it suggests that intensive spraying may not be necessary to achieve low aphid density as aphid abundance often didn't differ significantly between sites that sprayed more frequently versus those that did.
Mummy Rearing Assessment

The largest number of unemerged mummies was found at the Marshallville site followed by Albany and Ray City. The majority of mummies reared did not emerge with emergence rates being very low. Hyperparasitism was found at Marshallville and Ray City which were sites with some of the highest parasitoid numbers. This may indicated that hyperparasitism maybe associated with primary parasitoid population density. The sole primary parasitoid that emerged during this was Aphelinus perpallidus Gahan. Molecular genetics performed in a seperate study, reveal that the hyperparasitoid assembledge for A. perpallidus consists of 5 hypers: Apaphes vulgaris, 2 species of Charipinae, 1 species of pachyneuron, and 1 species of Signophoridae.
Seasonal phenology of aphids, parasitized aphids and adult parasitoids at each location
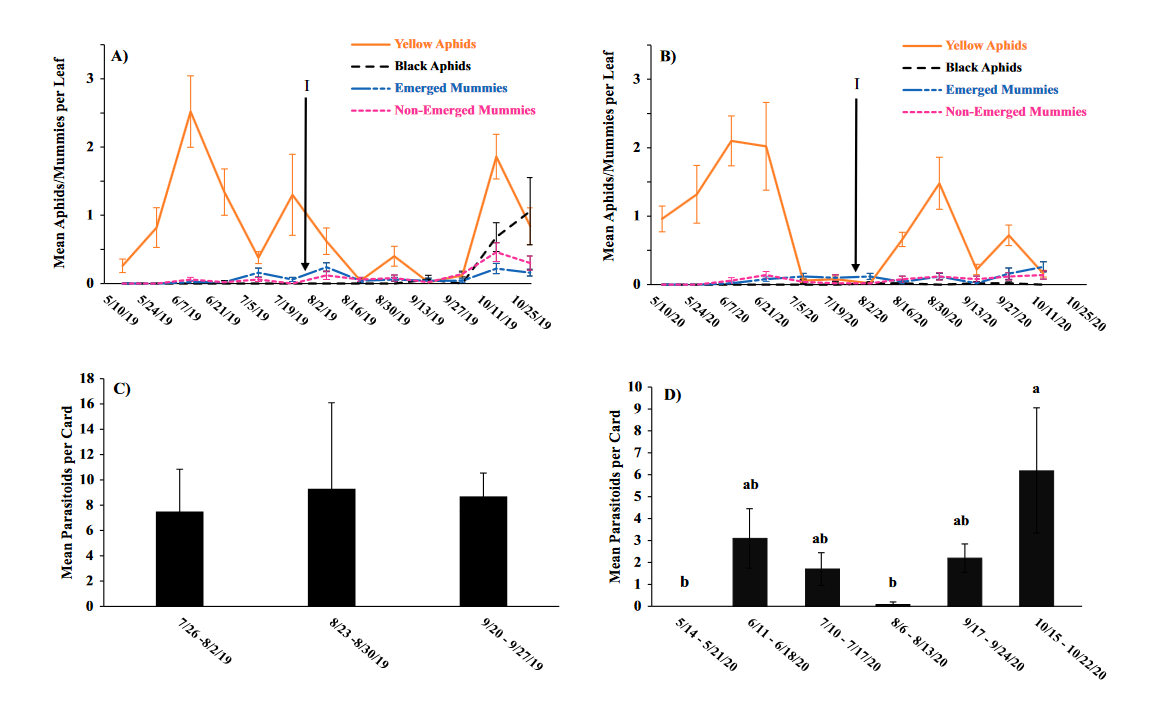
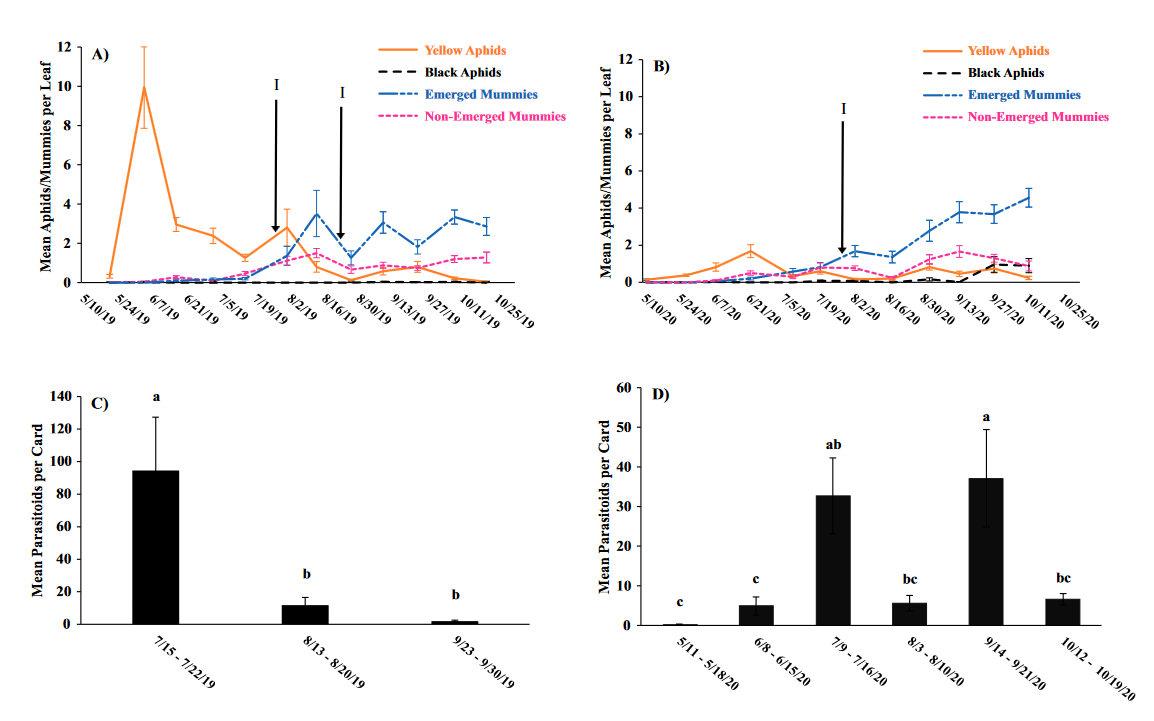
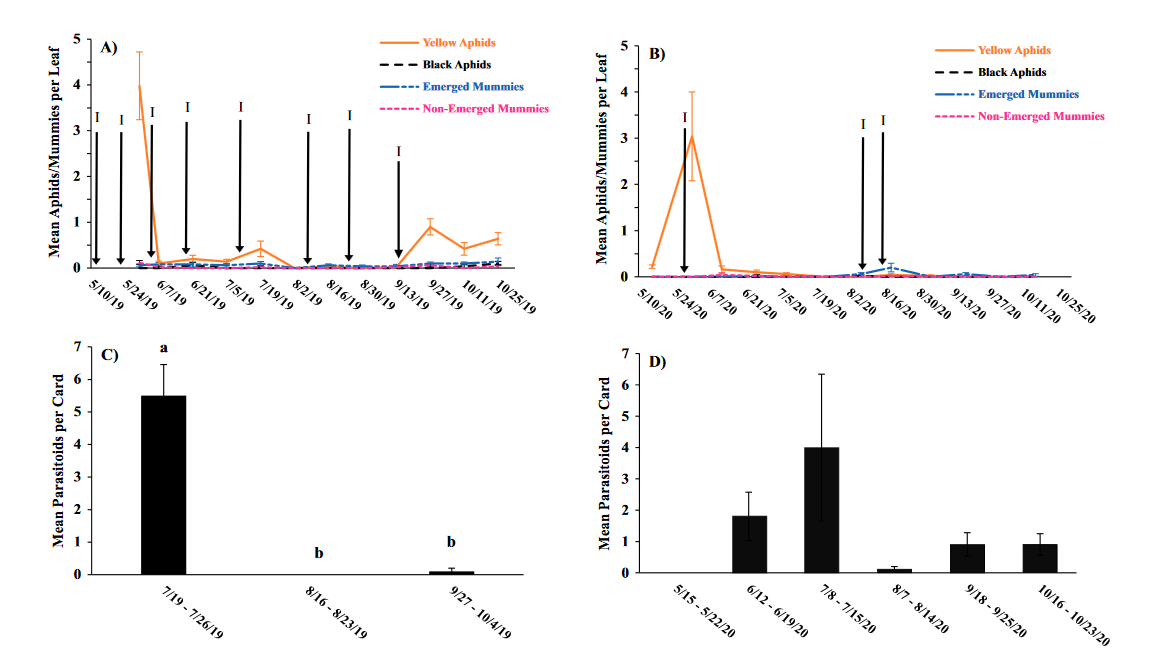
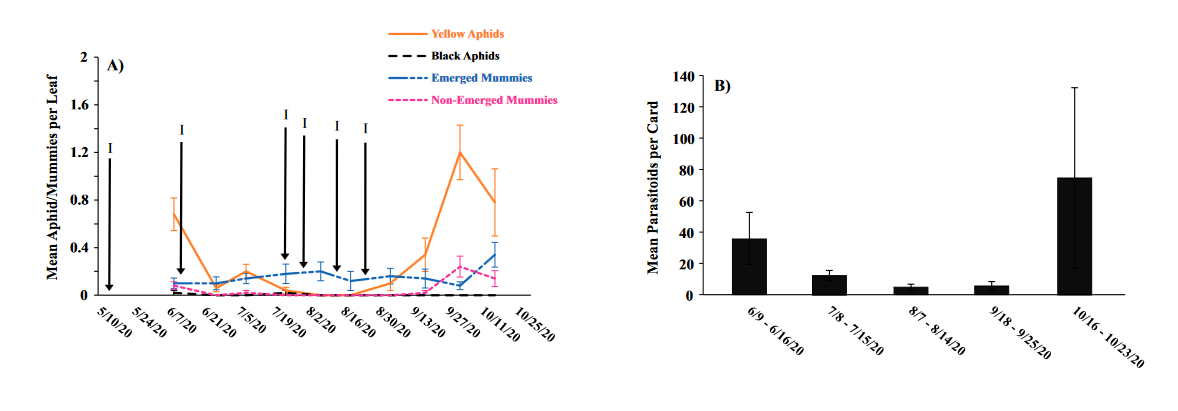
Throughout all sites yellow aphid complex numbers typically followed a similar pattern of rising and crashing throughout the season with peaks usually occurring in May and June followed by another peak in late September and early October. Black pecan aphids were rarely collected throughout the growing season usually being found in September and October. The population trends in our data are similar to previous studies on aphid phenology and life history (Tedders 1978, Dutcher et al. 2012). Interestingly, despite insecticide application at the sites in our study, aphid numbers still peaked at similar times as peaks at the unsprayed experimental orchards of other studies (Tedders 1978, Dutcher et al. 2012). However, the growth of these peaks does not appear to be as great. The yellow aphid complex collected during our study seem to achieve their highest abundance between May and June and September and October. This lines up with the recommendations of the UGA spray guide which discourages spraying for the yellow aphid complex in the early season (May and June) and when growers ceased treating and turn their focus to harvest (September and October) (Table 2). Thus, these peaks fall outside what is often a normal yellow aphid complex treatment window for most Georgia pecan growers. However, treatment for black pecan aphid appears to differ from the recommendations of the UGA spray guide, at least at some sites. For example, growers at the Marshallville and Nashville orchard treated for black pecan aphid despite black pecan aphid numbers being low throughout. Thus, there is potential for growers to reduce costs by using monitoring information to determine if black aphid numbers are at threshold values recommended in the spray guide.
While it appears that aphid phenology in commercial orchards is not too different from previous studies in experimental orchards, it does appear that aphid abundance is lower in commercial orchards. Tedders (1978) found an average number of 100 aphids per 25 leaves during seasonal peaks with blackmargined aphid averaging around 900 per 20 leaves around October and December. Dutcher et al. (2012) found numbers closer to ours, but still found that during the peak aphid numbers could get as high as 40-100 aphids per shoot. This is much higher than our highest average peak of 10 yellow pecan aphids per leaf which was in June of 2019 in Marshallville, GA. Insecticide application pressure could be the likely explanation for this. The type of insecticide used may also be a cause of low aphid numbers, as the shift from broad-spectrum insecticides in the 1970’s such as organophosphates and pyrethroids (Ball 1977, Harris and Cutler 1977) to products which are designed to primarily target piercing-sucking insects (Mulder et al. 2018, Acebes-Doria and Halliday 2020) may help to conserve natural enemies such as H. axyridis and A. perpallidus that can put pressure on aphid populations.
While it appears that aphid phenology in commercial orchards is not too different from previous studies in experimental orchards, it does appear that aphid abundance is lower in commercial orchards. Tedders (1978) found an average number of 100 aphids per 25 leaves during seasonal peaks with blackmargined aphid averaging around 900 per 20 leaves around October and December. Dutcher et al. (2012) found numbers closer to ours, but still found that during the peak aphid numbers could get as high as 40-100 aphids per shoot. This is much higher than our highest average peak of 10 yellow pecan aphids per leaf which was in June of 2019 in Marshallville, GA. Insecticide application pressure could be the likely explanation for this. The type of insecticide used may also be a cause of low aphid numbers, as the shift from broad-spectrum insecticides in the 1970’s such as organophosphates and pyrethroids (Ball 1977, Harris and Cutler 1977) to products which are designed to primarily target piercing-sucking insects (Mulder et al. 2018, Acebes-Doria and Halliday 2020) may help to conserve natural enemies such as H. axyridis and A. perpallidus that can put pressure on aphid populations.
The adult parasitoid phenology typically exhibited a similar trend to the aphids in terms of peaks and crashes. This suggests that adult parasitoid numbers rise and fall with that of their host. This can be supported by the positive correlation we found between adult parastioids and aphids in Marshallville in 2019 and Ray City in 2020. Aphid mummies differed in their correlation with aphid numbers regardless of whether they were emerged or non-emerged. This can possibly be explained by the persistence of mummies on the leaf when other factors, such as insecticide application, rainfall, or natural population crashes eliminate the aphids in an area. Even after the parasitoid has hatched, the remaining mummy can be found on the leaf afterwards. Bueno and Stone (1985) saw a similar trend when blackmargined aphids were sprayed. They attributed this to having an ample number of aphids available to sustain A. perpallidus populations even after treatment. In addition, we observed several positive correlations between black pecan aphid and both hatch and unhatched mummies at several sites. This is interesting as black pecan aphid mummies were not found in this study and are noted to be rarely parasitized by A. perpallidus (Tedders 1978). Several previous studies have assessed aphid mummies and parasitism rates in correlation with pecan aphid numbers, but assessment of adult numbers is lacking (Bueno Jr and Stone 1983, Bueno Jr and Stone 1985). Our study is one of the first studies to look at the population density of adult A. perpallidus in the field.
Overall, this study was one of first to perform a multi-site assessment of pecan aphid and A. perpallidus populations in commercial pecan orchards. The findings on aphid seasonal activity confirmed that of previous studies by Tedders (1978) and Dutcher et al. (2012). While the study did not find any drastic differences in aphid phenology compared to experimental orchards it did reveal that aphid numbers are lower in commercial orchards than in experimental orchards. Additional years of study may be necessary to see if this is a due to management pressure or simply a temporary lull in populations. This study was also among the first to plot a seasonal phenology for adult A. perpallidus. Future studies could help expand upon this and help reveal long-term population trends. The literature available on this topic is currently quite limited and many of the points analyzed in this study could be further examined to reveal more information. Further regular and continued assessment is important to help growers develop pecan aphid management plans.
References Cited
- Acebes-Doria, A. L., and W. G. Hudson. 2019. 2020 commercial pecan spray guide. Univ. Georgia Coop. Ext. Pub. 841. Tifton, GA.
- Acebes-Doria, A. L., and P. L. Halliday. 2020. Insecticide efficacy against pecan aphids and pecan leaf scorch mites, 2018. Arthropod Manag. Tests. 45: 1-2.
- Ball, J. C. 1977. Aphid control on pecans, 1976. Insecticide and Acaricide Tests. 44: 1-2
- Bueno Jr, R., and J. D. Stone. 1983. Phenology of a parasite of the blackmargined aphid in west Texas [Aphelinus perpallidus, Monellia caryella]. Southwest. Entomol. 8: 73-79.
- Bueno Jr, R., and J. D. Stone. 1985. Aphelinus perpallidus parasitism of Monellia caryella populations in far west Texas. J. Entomol. Sci. 20: 325-330.
- Buitenhuis, R., G. Boivin, L. Vet, and J. Brodeur. 2004. Preference and performance of the hyperparasitoid Syrphophagus aphidivorus (Hymenoptera: Encyrtidae): fitness consequences of selecting hosts in live aphids or aphid mummies. Ecol. Entomol. 29: 648-656.
- Cottrell, T. E., B. W. Wood, and X. Ni. 2009. Chlorotic feeding injury by the black pecan aphid (Hemiptera: Aphididae) to pecan foliage promotes aphid settling and nymphal development. Environ. Entomol. 38: 411-416.
- Dutcher, J. D. 2005. Chemical control of aphids on pecan foliage. Arthropod Manag. Tests. 30: 1-2.
- Dutcher, J. D., H. Karar, and M. G. Abbas. 2010. Effectiveness of two insecticides for control of foliage feeding pecan aphids. Arthropod Manag. Tests. 35: 1-2.
- Dutcher, J. D., H. Karar, and G. Abbas. 2012. Seasonal abundance of aphids and aphidophagous insects in pecan. Insects 3: 1257-1270.
- Forrest, J. R. 2016. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 17: 49-54.
- Gómez-Marco, F., A. Urbaneja, J. A. Jaques, P. Rugman-Jones, R. Stouthamer, and A. Tena. 2015. Untangling the aphid-parasitoid food web in citrus: Can hyperparasitoids disrupt biological control? Biol. Control 81: 111-121.
- Goulet, H., and J. T. Huber. 1993. Hymenoptera of the world: an identification guide to families. Agriculture Canada, Ottawa, Canada.
- Harris, M. K., and B. L. Cutler. 1978. Pecan, pecan weevil and hickory shuckworm management, 1977. Insecticide and Acaricide Tests 3: 59-60.
- Kistner, E. J. 2017. Climate change impacts on the potential distribution and abundance of the brown marmorated stink bug (Hemiptera: Pentatomidae) with special reference to North America and Europe. Environ. Entomol. 46: 1212-1224.
- Kunkel, B. A., and T. E. Cottrell. 2007. Oviposition response of green lacewings (Neuroptera: Chrysopidae) to aphids (Hemiptera: Aphididae) and potential attractants on pecan. Environ. Entomol. 36: 577-583.
- Lefort, M. C., S. Wratten, A. Cusumano, Y. D. Varennes, and S. Boyer. 2017. Disentangling higher trophic level interactions in the cabbage aphid food web using high-throughput DNA sequencing. MBMG. 1: e13709.
- Mizell III, R. F., and D. E. Schiffhauer. 1990. Effects of pesticides on pecan aphid predators Chrysoperla rufilabris (Neuroptera: Chrysopidae), Hippodamia convergens, Cycloneda sanguinea (L.), Olla v-nigrum (Coleoptera: Coccinellidae), and Aphelinus perpallidus (Hymenoptera: Encyrtidae). J. Econ. Entomol. 83: 1806-1812.
- Mizell, R. F. 2007. Impact of Harmonia axyridis (Coleoptera: Coccinellidae) on native arthropod predators in pecan and crape myrtle. Fla. Entomol. 90: 524-536.
- Mulder, P. G., S. K. Seuhs, M. E. Payton. 2020. Insecticide efficacy for controlling pecan aphids, 2018. Arthropod Manag. Tests. 45:1-2.
- Paulsen, C., T. Cottrell, and J. Ruberson. 2013. Distribution of the black pecan aphid, Melanocallis caryaefoliae, on the upper and lower surface of pecan foliage. Entomol. Exp. Appl. 146: 252-260.
- Shapiro–Ilan, D. I., T. E. Cottrell, M. A. Jackson, and B. W. Wood. 2013. Control of key pecan insect pests using biorational pesticides. J. Econ. Entomol. 106: 257-266.
- Tedders, W. 1978. Important biological and morphological characteristics of the foliar-feeding aphids of pecan. USDA Technical Bulletin. 1579. Hyattsville, MD.
- (USDA-NASS) U.S. Department of Agriculture National Agricultural Statistics Service. 2020. Pecan production (January 2020). USDA, NASS. Washington, D.C.
- Wells, L., P. Conner, B. Goff, M. Nesbitt, B. Wood, P. Bertrand, T. Brenneman, J. Brock, M. Hotchkiss, C. Reilly, K. Stevenson, J. Dutcher, W. Hudson, H. Ellis, J. Payne, L. Tedders, K. Harrison, and P. Sumner. 2007. Southeastern Pecan Growers Handbook. Univ. Georgia Coop. Ext. Pub. 1327. Tifton, GA.
- Wolfe, K., and K. Stubbs. 2019. Georgia Farm Gate Value Report 2018. Univ. Georgia Coop. Ext. Pub. 17. Tifton, GA.
- Wood, B. W., W. L. Tedders, and J. D. Dutcher. 1987. Energy drain by three pecan aphid species (Homoptera: Aphididae) and their influence on in-shell pecan production. Environ. Entomol. 16: 1045-1056.
Objective 3: Examining the relationships between canopy height and the rate of successful parasitism.
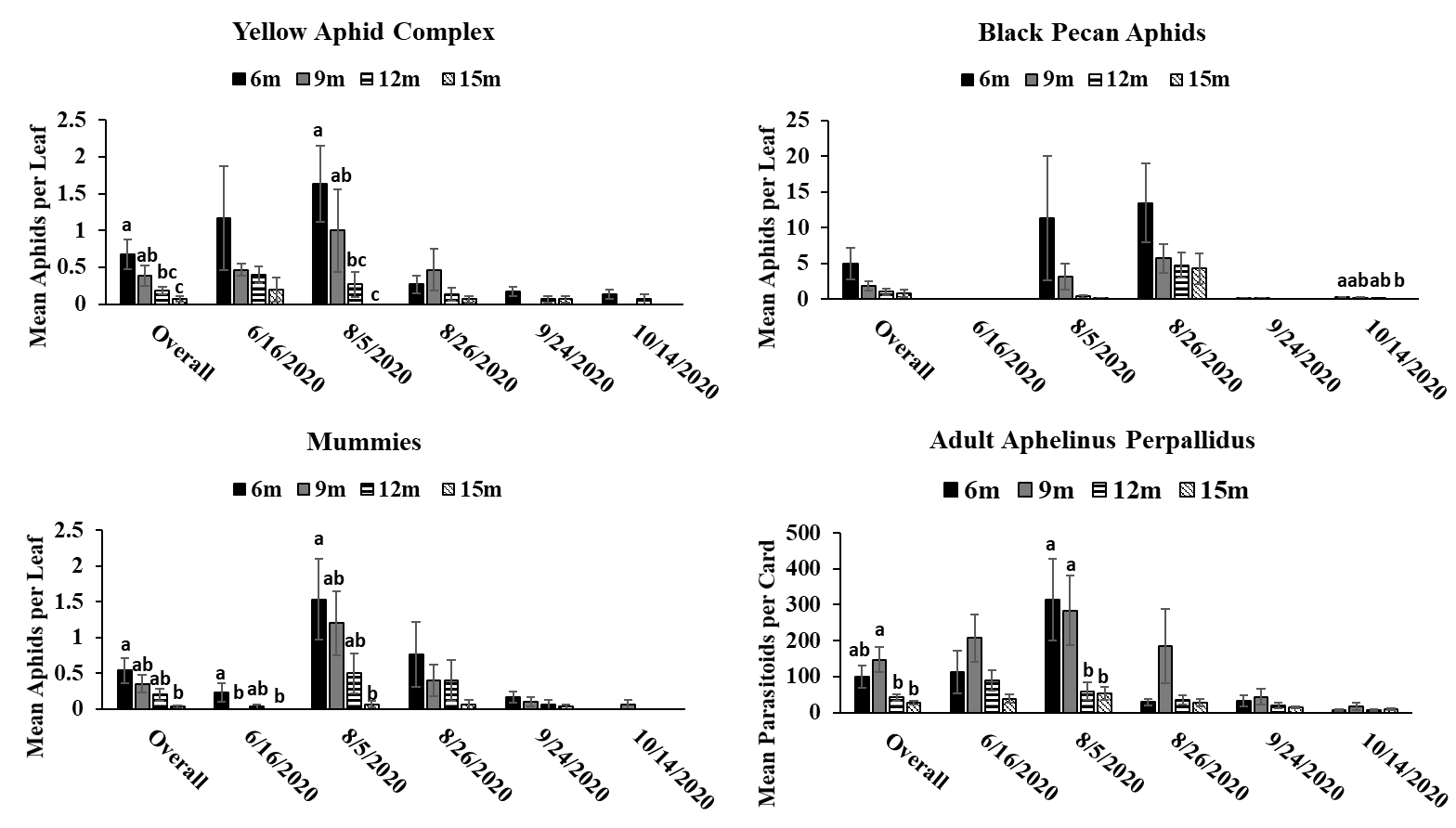
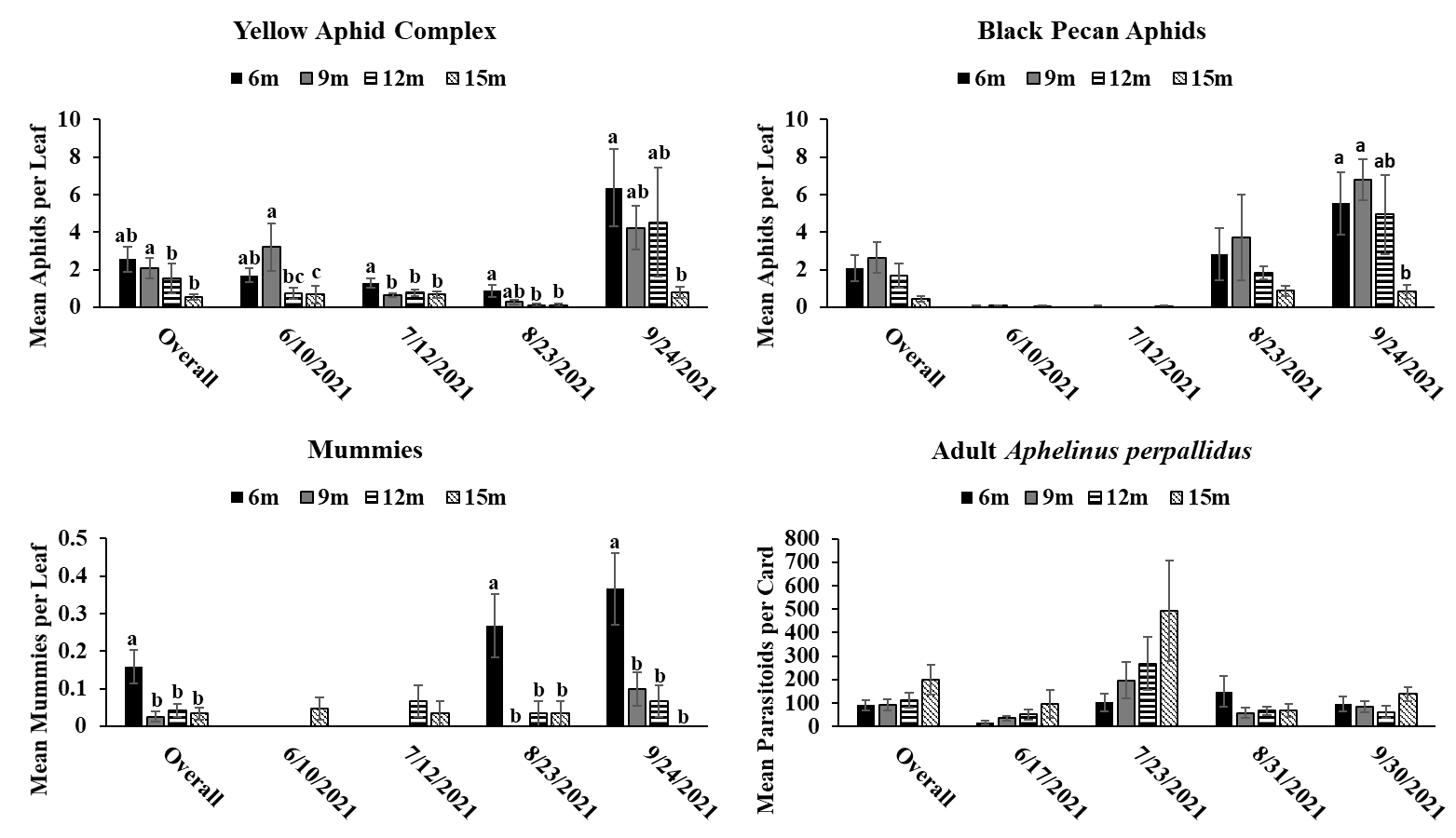
The results of this study provide evidence that pecan aphid abundance differs based on canopy height. While significant differences in pecan aphid abundance were not detected during every month of the study, when aphid numbers were significantly different, it was apparent that pecan aphids were more abundant in the lower parts of the canopy in both the experimental orchard and commercial orchard. These results are similar with previous studies showing that aphids prefer the lower canopies of woody plants (McClure 1982, Dahlsten et al. 1999, Dixon 2005, Platková et al. 2020). This higher abundance in the lower canopy may be due to a more suitable climate and better-quality food in the lower part of the canopy compared to the upper canopy (Dixon 2005, Platková et al. 2020). Previous studies have shown that leaf quality is poorer in the upper canopies of other tree species (Dixon 2005, Platková et al. 2020). In addition, aphids are more vulnerable to wind, rain, and ultraviolet light in the upper parts of the canopy (Dixon 2005, Platková et al. 2020) . Temperature can also be a factor in aphids choosing to avoid the upper canopy as temperature in the upper canopy can differ by as much as 10 between the upper and lower canopies of some trees (Dixon 2005). The highest abundance of pecan aphids in the lower canopy was most apparent during peak times of year for each aphid species (yellow aphid complex: June-September; black pecan aphid: September-October) (Tedders 1978). This may indicate that pecan aphids congregate and build up populations in the lower canopy were conditions are better. Previous research on canopy distribution of pecan aphids has shown that canopy preference decreases as populations increase (Edelson and Estes 1987). We did not observe this in our study due to lack of steady population increase in our aphid populations preventing our aphids from reaching population saturation. Future studies during more heavy pecan aphid population may be useful in order to see if the distribution of pecan aphids in the canopy changes as populations increase.
Aphelinus perpallidus numbers only differed significantly according to canopy height in the experimental orchard during August of 2020 where they were more abundant in the lower portions of the canopy. This trend was also observed during the overall 2020 season. However, in the commercial orchard in 2021, A. perpallidus were found in higher abundance in the upper canopy compared to the lower canopy. This contrasts with previous studies on parasitoids in forests where specialists often were found a specific part of the canopy in contrast to more generalist parasitoids which were more evenly distributed throughout the canopy (Šigut et al. 2018). This may have been due to low aphid numbers throughout the season, which may have forced A. perpallidus to distribute more throughout the canopy in order to find hosts. Such long distance dispersal have previously been reported in parasitoids due to host scarcity so it is possible A. perpallidus may be displaying this behavior here (Cameron et al. 1981). Our multivariate analysis found a positive correlation between the number of yellow pecan aphids and A. perpallidus, especially in the lower canopy. Interestingly, the positive correlation of this relationship often decreased in significance as canopy height increased indicating that increases in canopy height may weaken some of ecological relationships.
Mummies were low throughout the season which may have coincided with low aphid numbers. Mummies typically were found in the lower canopy in both the experimental orchard and commercial orchard often following the population trends of yellow pecan aphids. This make sense as A. perpallidus often prefer yellow pecan aphids as host to black pecan aphids (Tedders 1978, Paulsen et al. 2013). Interestingly, we saw a positive correlation between mummies and black pecan aphids in 2021. This may have been due to decrease in yellow aphid populations via parasitism allowing black pecan aphid populations to increase. However, we did not see this trend in 2020 so further study may need to be done to see if A. perpallidus parasitism of yellow pecan aphid positively benefits black pecan aphid.
While A. perpallidus were detected more often in the lower canopy, it is still impressive to see that both prey and parasitoid can still colonize trees up to 15 meters despite potential dispersal limits due to size and mobility. This can be useful as it means parasitoids can possibly be relied upon to manage pest populations in the upper canopy. This is useful as previous research has shown that insecticide coverage decreases significantly a pecan canopy height increases (Bock et al. 2015). A study looking at spray coverage at different heights in mature pecan trees, has shown that spray coverage decreases significantly as canopy height increases (Bock et. al, 2015). This indicates that parasitoids can play a key role in pest management in areas where insecticidal control may fail.
The effects of elevation on natural enemies are poorly understood and analysis of a single family or even species should not be used as a blanket statement for all beneficial insects. A study on lady beetle (Coleoptera: Coccinellidae) populations at different heights revealed that lady beetles may respond negatively, neutrally, or positively to height depending on the species (Cottrell, 2017). A study on Ichneumonid wasps found that the community differed between the lower canopy and the upper canopy. Certain groups of Ichneumonid were captured in one location over the other while the other groups were captured equally in both locations (Di Giovanni et al. 2015). In addition, studies assessing parasitism rates of leaf-chewing pest found that parasitism rates increased from the first level (area closest to the ground floor) to the third level (uppermost area of a given tree) in smaller tree species and decreased from first level to the third level in taller tree species (Šigut et al. 2018). This phenomenon in smaller trees was believed to be due to spatial avoidance of predators by parasitoids. Since predators were more abundant in the first level, which was close to the forest floor, parasitoids may have moved higher in the tree to avoid predation. For the taller tree species, it was argued that parasitoids avoid the harsh abiotic conditions of the third level and thus are more prevalent in the lower, two levels. In addition, due to the first level being much farther from the forest floor there was less risk of predation (Šigut et al. 2018). This suggests that all natural enemies are not affected by elevation equally even on a species to species level. Future studies should look at the effects of elevation on numerous species of natural enemies in order to fully understand canopy height effects.
Trends between the commercial and experimental orchard were similar with pecan aphids and mummies being found in higher abundance in the lower parts of the tree. This information could be beneficial for growers as it means they can continue to scout the lower canopy and acquire valuable information on aphid numbers regardless of how tall their trees get. By only having to spend time on the lower part of the canopy regardless of rather a tree is 9 meters tall or 15 meters tall growers may be able to save time and manpower on scouting. In addition, this information can be useful for implementing pest management plans by informing growers on which management tactics (i.e. chemical control vs biological control) could be most effective.
Literature Cited
- Bock, C. H., M. W. Hotchkiss, T. E. Cottrell, and B. W. Wood. 2015. The Effect of Sample Height on Spray Coverage in Mature Pecan Trees. Plant Dis. 99: 916-925.
- Cameron, P., G. Walker, and D. Allan. 1981. Establishment and dispersal of the introduced parasite Aphidius eadyi (Hymenoptera: Aphidiidae) in the North Island of New Zealand, and its initial effect on pea aphid. New Zealand journal of zoology 8: 105-112.
- Cottrell, T. 2017. Trap Height Affects Capture of Lady Beetles (Coleoptera: Coccinellidae) in Pecan Orchards. Environ. Entomol. 46: 343-352.
- Dahlsten, D., R. Zuparko, A. Hajek, D. Rowney, and S. Dreistadt. 1999. Long-term sampling of Eucallipterus tiliae (Homoptera: Drepanosiphidae) and associated natural enemies in a northern California site. Environ. Entomol. 28: 845-850.
- Di Giovanni, F., P. Cerretti, F. Mason, E. Minari, and L. Marini. 2015. Vertical stratification of ichneumonid wasp communities: the effects of forest structure and life‐history traits. Insect Science 22: 688-699.
- Dixon, A. F. G. 2005. Insect herbivore-host dynamics: tree-dwelling aphids, Cambridge University Press.
- Edelson, J., and P. Estes. 1987. Seasonal abundance and distribution of predators and parasites associated with Monelliopsis pecanis Bissell and Monellia caryella (Fitch)(Homoptera: Aphidae). J. Entomol. Sci. 22: 336-347.
- McClure, M. S. 1982. Distribution and damage of two Pineus species (Homoptera: Adelgidae) on red pine in New England. Ann. Entomol. Soc. Am. 75: 150-157.
- Paulsen, C., T. Cottrell, and J. Ruberson. 2013. Distribution of the Black Pecan Aphid, Melanocallis caryaefoliae, On the Upper and Lower Surface of Pecan Foliage. Entomol. Exp. Appl. 146: 252-260.
- Platková, H., P. Pyszko, A. Coeur d´ Acier, E. Jousselin, and P. Drozd. 2020. Spatial distribution of aphids in the canopy of a temperate forest: where can they be found? Agricultural and Forest Entomology 22: 379-389.
- Šigut, M., H. Šigutová, J. Šipoš, P. Pyszko, N. Kotásková, and P. Drozd. 2018. Vertical canopy gradient shaping the stratification of leaf‐chewer–parasitoid interactions in a temperate forest. Ecology and evolution 8: 7297-7311.
- Tedders, W. L. 1978. Important Biological and Morphological Characteristics of the Foliar-feeding Aphids of Pecan, vol. 1578-1587, Department of Agriculture, Science and Education Administration, 1978.
- Wells, L., and P. Conner. 2007. Southeastern Pecan Growers' Handbook.
Educational & Outreach Activities
Participation Summary:
Due to the covid-19 pandemic, many conferences were canceled which limited the ability to perform outreach. However, we were able to do a couple things to fulfill this requirement. In 2020, at the virtual National Entomological Society of America (ESA) meeting, a virtual poster was presented that detailed the results collected during the 2020 field season for the elevation study (Objective 3). A poster will be presented at the 2021 Southeastern branch meeting that will detail the findings of the Seasonal Phenology study (Objective 1). In addition, we have submitted an article to The Pecan Grower magazine for publication in a future issue over the seasonal phenology (Objective 1) and have also submitted a research manuscript to the Journal of Environmental Entomology for the same objective that is currently in review.
Presentations
- Entomological Society of America National Meeting. Effects of Vertical Stratification on the Abundance of Pecan Aphid Parasitoids in Georgia Pecan Orchards. Slusher, E.K., T. Cottrell, A.L. Acebes. November 11-25 2020, Virtual
- Entomological Society of America Southeastern Branch Meeting. Multi-Site Seasonal Monitoring of Pecan Aphids and Their Parasitoids in Commercial Pecan Orchards. Slusher, E.K., W. Hudson, A.L. Acebes. March 29-31 2020, Virtual
Publications
- The Pecan Grower Magazine. Multi-Site Seasonal Monitoring of Pecan Aphids and Their Parasitoids in Commercial Pecan Orchards. Slusher, E.K., W. Hudson, A.L. Acebes.
- Journal of Environmental Entomology. Multi-Site Seasonal Monitoring of Pecan Aphids and Their Parasitoids in Commercial Pecan Orchards. Slusher, E.K., W. Hudson, A.L. Acebes.
Project Outcomes
Based off the findings of objective 1, it has become apparent that pecan aphid numbers are much lower than what they were in the past. Two of the orchards we studied during this research applied few insecticides throughout the year while two orchards sprayed more frequently. Despite this, orchards that sprayed less frequently still had very low aphid numbers. This may indicate that growers in Southeast Georgia may not need to apply insecticides at a high frequency in order to manage pecan aphids. This can have both economic and environmental benefits as growers may be able to mitigate insecticide application thus saving money while also reducing the amount of insecticide that makes its way into the surrounding environment. In objective 3, we found that parasitoids can still colonize the upper canopy of pecan trees. This indicates they may be able to provide some sort of control to aphids in the upper canopy. This can have economic and environmental benefits as well as growers can rely on natural enemies to provide biological control even in the upper canopy of pecan trees.
My advisor and I have gained a better understanding of the ecological relationships between pecan aphids and their parasitoids. Prior to the research done in objective 1, little research had been done on the seasonal phenology of pecan aphids and their parasitoids in commercial orchards in the Southeast. Prior studies done in the Southeast were primarily restricted to single experimental orchards. In addition, little to no analysis of the seasonal phenology of adult Aphelinus perpallidus had been done prior to this study. We have also gained a better understanding of how populations of pecan aphid and A. perpallidus vary at different commercial sites and also how diverse grower management tactics are. Talking with growers has allowed us to understand their concerns with regards to pecan management which allows us to adjust future studies accordingly. For example, as previously mentioned, Georgia growers are often more concerned with black pecan aphid than yellow pecan aphid, and thus future studies can be crafted to help growers manage black pecan aphid more effectively. In objective 3, we have learned more about the effects of elevation on pecan aphids and their parasitoids. The effects of elevation on pests and natural enemies in pecan orchards has been a poorly studied topic and is important when addressing pecan pests. Knowing where in the tree that certain insects have a preference for can help spot discrepancies and overlap in pest and predator/parasitoid populations.