Final report for GS21-236
Project Information
Pecan orchards are often attacked by many arthropod pests, and sap-sucking aphids concern growers due to their potential to impair tree productivity. Aphid management practices in pecan orchards are currently reliant on the use of synthetic insecticides, which are known to negatively impact the environment, non-target organisms (e.g., natural enemies and pollinators) as well as humans. Hence, the development of more sustainable aphid management in pecan systems requires further research. Improving biological control using predatory natural enemies is a plausible option because pecan orchards are known to harbor diverse predators of aphids (e.g. ladybeetles, lacewings, and minute pirate bugs); however, the specific roles these biocontrol agents play in pecan fields has received relatively little attention. To advance the augmentative and/or conservational biological control programs, it is crucial to understand the trophic interactions among these common natural enemies. Thus, our objectives were to 1) examine the positive services (i.e. predation) and disservices (i.e. intraguild predation) of predatory arthropods in pecan systems through molecular gut content analysis, and 2) determine if services depend on different locations within pecan trees (i.e. in the upper and lower canopies) and vary during the season (early and late season). This study will contribute to boosting ecological-based pest management in southern US pecan orchards.
Our main goal with this project is to understand the specific roles that predatory natural enemies play within the southern US pecan agroecosystems and contribute to a strong ecological basis for advancing sustainable management through:
- Identifying the community of pecan-resident predators that contribute to the biological control of each aphid species (e. black pecan aphids, yellow pecan aphids, and blackmargined aphids).
- Identifying potential intraguild predation events among predators of aphids.
- Understanding how tree strata (upper versus lower canopy) or seasonal differences (early versus late season) play a role in the interactions among predators and with their prey.
Additionally, we will use the information acquired in 1, 2, and 3 to pursue our fourth goal with this project, which is:
- To produce a visual, durable, extension field guide to natural enemies and their role as biocontrol agents of pecan aphids for Georgia pecan growers.
Cooperators
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
Research
Site characteristics and experimental design
The research was conducted in a pecan experimental orchard located at the United States Department of Agriculture (USDA) Southeastern Fruit and Tree Nut Research Laboratory (32.665550, -83.729287), Byron, GA. The orchard consisted of approximately 110 mature pecan trees var Pawnee. The trees were approximately 40 years old. Six trees were randomly selected within the orchard and each tree was considered a replicate. Tree height was measured at the beginning of the experiment with the aid of an optical Rangefinder (Model Insight 400 XL - Opti-Logic, Tullahoma, TN) and a Telescopic Crawler Boom Lift (Model 660SJC - JLG Industries, McConnellsburg, PA) that reached up to approximately 20 meters of height. The boom lift platform was raised and leveled with the highest branches of the canopy and the rangefinder was pointed to the ground to get the measurement. Trees were, on average, 17.15 meters tall.
Sample collection and Predator Identification
Two samples were taken from three distinct positions within the pecan canopy vertical strata, specifically the upper, middle, and lower sections. The two samples were taken from opposite sides of the trees (one on the north side and one on the south side of the canopy). Height was reached with the aid of a telescopic crawler boom lift (JLG 660SJC). Insects were collected using a reverse blower (i.e., a suction sampler) that ran for 30 seconds and moved gently sideways around pecan branches that were within the reach of the operator standing on a hydraulic lift basket. Immediately after collection, the samples were placed in gallon-size zip lock bags and stored inside ice coolers to stop or delay biological processes (more specifically genetic material -DNA- degradation) and other ecological interaction events (e.g., predation) from occurring among the collected individuals. Samples were brought to the laboratory and stored in a -20 freezer. All predators within the samples were counted, identified to the family level, and transferred to individual vials containing 95% ethanol and stored in the -20 freezer until DNA extraction to avoid contamination and degradation. This process was performed in the 2021 and 2022 seasons.
Molecular gut content analysis: DNA isolation and screening for prey targets
We cleaned each individual predator using 10% bleach, PCR-grade water, and 95% ethanol. Then, ethanol was dried from each predator and transferred into a sterile micro-centrifuge tube before DNA extraction to reduce external DNA contamination. DNA within natural enemy samples was extracted using Qiagen DNeasy® Blood and Tissue 96-well kits (including a negative control containing all buffers and solutions, but no insect tissue) following manufacturer protocol (Qiagen, Chatworth, CA, USA). Extracted DNA was stored at -20 °C. We have begun this process following extensive identification of the predators and prey in the system, and we have purchased all the extraction kits needed and supplies with the grant funds. Following extraction, each predator DNA sample will be screened using polymerase chain reactions (PCR) for detection and estimation of feeding on aphids, alternative prey targets (i.e. flies, caterpillars), and intraguild predation. The diagnostic DNA approach will provide new information about pecan predators feeding on common pests and use of alternative food resources, non-pest prey species. PCR kits (Qiagen Multiplex Kit) were already purchased for the project using funds, and work will be completed over the next three months.
Data Analysis
The results from the molecular analysis will initially be used for a descriptive work of the roles of naturally occurring predators in aphid control as well as their interactions with other natural enemies. That is, we will identify the presence of prey in the predators’ gut as well as the frequency they eat it. Finally, we will analyze if there are effects of tree strata as well as seasonality in the services provided by these natural enemies. Specifically, ordination will be used to examine changes in predator community structure, and DNA detection frequencies combined with the abundance of prey will be used to examine the food web properties of the predatory community.
Objectives 1 and 2: Identification of naturally occurring predators, contributions to aphid biocontrol, and intraguild predation events.
Common predators of aphids in pecans
As part of objectives 1 and 2, we have successfully completed sampling and identified predators that likely contribute to aphid suppression in pecan orchards (Table 1). These predators are being screened for their molecular gut contents for the presence of aphids.
Table 1. Primary families of aphid predators (accounting for over 90% of the total predator community observed) collected from pecan trees during the 2021 and 2022 seasons.
|
Predator groupa |
Family |
Abundance |
|
Assassin Bugs |
Reduviidae |
169 |
|
Long-legged flies |
Dolichopodidae |
210 |
|
Ladybeetles |
Coccinellidae |
284 |
|
Green Lacewings |
Chrysopidae |
278 |
|
Minute Pirate Bugs |
Anthocoridae |
98 |
Upon the completion of the molecular analysis, we will be able to pinpoint and rank these common predators and their contributions to aphid control as well as the frequency that they engage in intraguild predation events.
Objective 3. Understanding how the temporal and spatial activity of commonly found predators play a role in the services they provide for aphid suppression in pecan systems.
Temporal activity of aphid predators in the pecan canopy
The temporal activity of predators varied among the groups collected. Some groups were found to be more active early in the season while others increased their population later in the season. Additionally, we found that some predators maintain relatively high populations throughout the entire season (e.g., green lacewings). This initial assessment of their temporal presence in the orchard suggests that different predators contribute to aphid control in different parts of the season. Minute pirate bugs (Orius sp.) and lacewings (Chrysopidae), seem to contribute to aphid biocontrol services at the beginning of the season, as their population is higher than other predators. On the other hand, assassin bugs (Reduviidae) had higher populations towards the end of the season (in September). Long-legged flies (Dolichopodidae) and Ladybeetles (Coccinellidae) were variable over the season and between years, potentially responding to other factors in the system.
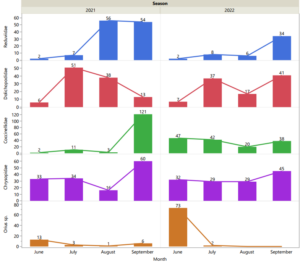
Distribution (spatial activity) of aphid predators in the pecan canopy
Even though all predators were retrieved from all three canopy locations assessed, their activity varied within the canopy strata. These findings suggest that predators may complement each other in their roles within the pecan canopy (Figure 2).

Upon completion of molecular analysis, this information will be used to understand their contributions to aphid management during the pecan season as well as in different parts of the canopy. We will merge their temporal and spatial activity with their contributions to aphid management providing an ecological framework for the future development of ecological-based pest management strategies in pecans. It is worth mentioning that during the molecular phase, we intend to extend these aphid-related objectives to other pests, using different primers that can detect the presence of important pecan pests (Lepidopterans) in these predators’ guts.
Educational & Outreach Activities
Participation Summary:
As part of our efforts to demonstrate how management decisions affect the sustainability of pest management in the pecan system, we have shared this data on a few occasions along with other components of the system that we have studied (e.g., the consequences of pruning pecans for insect management). We presented this data, in part, in a scientific meeting and at two of the major grower-based conferences for pecans in the nation, with the participation of a large group of growers and professionals (as listed below).
Scientific conferences
The PhD student who received this grant, Pedro Toledo, had the opportunity to showcase his work through this project at the main conference of his field of study (Entomological Society of America Annual Meeting). He was awarded first place in the PhD student competition for the presentation.
- ESA, ESC, and ESBC Joint Annual Meeting 2022 – Vancouver, Canada
Scientific presentation: Hedged-pruning enhances natural enemy recruitment in the pecan canopy: implications of canopy architecture and chemical pest control
Grower-based conferences
The Ph.D. student who received this grant, Pedro Toledo, also had the opportunity to speak at two important conferences that aim to provide pecan growers with the most up-to-date information on the system and discuss current issues related to the pecan industry. In both of these events, he showcased our findings related to the potential implications of pruning mature pecan trees for sustainable arthropod management in these agroecosystems, including partial results and developments of this grant project. Combined the presentations reached (at least) 60 growers and industry representatives.
- 2023 Southeastern Pecan Grower Association (SEPGA) Annual Conference.
115th Annual Convention & Trade Show – Gulf Shores, AL
Speech: Hedge pruning pecans: Potential implications of hedge pruning for pest management.
- 2023 Georgia Pecan Grower Association (GPGA) Annual Conference
58th Annual Educational Conference and Trade Show – Perry, GA
Speech: Hedge pruning pecans: Potential implications of hedge pruning for pest management.
Near-future perspectives for outreach in this project
Objective 4. Creating an informative outreach material that provides information on the common predators collected in this study, as was as when they are likely present in the field (temporal activity shown in Objective 3) and contributions to aphid control. After molecular data has been analyzed, we will be able to complete either a visual field guide or include this information in an app that has been used by growers (MyIPM pecans) where the information can be easily reached through their mobile phones. We intend to make this information easy to access and reach a great audience of growers who can make informative decisions. Additionally, at least two peer-reviewed scientific manuscripts will be published showcasing our findings during this project.
Other important educational outcomes
During the course of this project, we were able to provide training to undergraduate students working towards these pecan-related efforts. These efforts included not only practical aspects of managing agroecosystems but also, research-based experience (i.e., experimental design, data collection, and visualization). As a result, other projects arose from these undergraduate student’s interest in learning ecological processes in agroecosystems and how they may be affected by agricultural management decisions. For example, one of the University of Georgia’s undergraduate students, James Winston Cornish, aided with this grant and is currently pursuing two additional projects with our assistance. Winston also applied and received additional funding through the University of Georgia to pursue his independent work under the supervision of Pedro Toledo.
Additionally, Cristopher Bryce Vaughn (from Abraham Baldwin Agricultural College), another undergraduate student who helped with this project, is currently pursuing an additional goal under our guidance and will work on an independent project in the spring of 2024. He is also helping with processing samples. Each undergraduate student received training in field methods, identification methods, data management, and molecular ecology techniques.
Project Outcomes
In perennial systems, finding ways to establish natural enemy communities is important for stable and long-lasting crop protection. In the case of pecan aphids, reducing reliance on pesticide use and harvesting more naturally occurring biological control appears promising. Previous studies show that insecticide applications in pecan orchards can induce aphid outbreaks, which is partially explained by reducing natural enemies that are often killed or displaced by chemical exposure. In addition, it is recognized that with routine use of some pesticide products (e.g., neonicotinoids), aphid populations are reported to display resistance in Georgia. Therefore, incorporating further knowledge of natural enemy communities into management packages should help with lowering the use or frequency of pesticide applications. While pesticides offer short-term efficacy, they can contribute to decreased environmental health, pose risks to human health, and fail to provide long-term regulation of pests.
Our project focused on assessing the presence and activity of aphid predators in the field, specifically their temporal and spatial distribution as well as their potential efficacy in aphid management. While the immediate impact on farmers' lives is currently not directly addressed, this project represents a crucial initial effort to the future implementation of sustainable strategies. The future aim is to reduce not only chemical reliance for pest control but also, the economic burden to farmers when buying crop protection products over the years. This project is part of a larger study where we have been honing in the ecological principles that are necessary for designing more assertive biological control strategies. Here, by showcasing the potential services provided by predators, we aim to empower farmers to make informed choices that optimize resource utilization and contribute to long-term sustainability. It is important to keep in mind that pecan trees take at least five years to bear nuts and offset the establishment costs. While our study primarily focuses on mature pecan trees, the outcomes could significantly benefit younger orchards, where aphid management costs are present, and profits are nonexistent.
In conclusion, this graduate student grant has provided the funds necessary to better understand the natural biological control providers in pecan to help build future conservation biological control in pecan systems. The project engaged growers, undergraduate students, and multiple scientists, and provided the opportunity for Pedro Toledo to advance knowledge of natural enemies and biological control with the pecan system. Over the next two years, using data generated during this project, we will begin to disentangle future benefits, emphasizing the interconnected nature of the three pillars of sustainability in pecans and other perennial crop systems. There is a lot of potential for improving the “growth” of natural pest management within perennial systems like pecans because, unlike annual systems, the communities of service providers can grow with the production system.
In the course of our project, which was integrated into the broader study on pecan tree crops (SARE Project LS20-340 – Initiated by Dr. Angelita Acebes), my advisor (Dr. Jason Schmidt) and I focused on unexplored areas of the sustainability package for pecan systems. Specifically, it attempts to tease apart the consequences of current management on arthropod communities of beneficial and pests within the context of tall tree systems. The challenges of implementing sustainable arthropod management in agroecosystems became apparent, particularly in tall tree crops such as pecans, where a multitude of management procedures and environmental background variations intersect during each season. This complexity certainly influences the ecology of arthropods and the success of biological control.
During the project, I also learned the critical importance of collaborative efforts with researchers specializing in diverse facets of agricultural systems. Notably, our collaboration extended to other experts, including Dr. Cristina Pisani, who studies horticultural parameters, and Dr. Clive Bock, who specializes in pecan disease. Publications arising from this collaborative work will enable a more comprehensive understanding of barriers and opportunities to improving biocontrol practices in tall tree systems.