Final report for GS21-243
Project Information
This project is an exploration of arbuscular mycorrhizal (AM) fungal species associating with tea (Camellia sinensis) roots under various soil, environmental, and management conditions in the United States. Species in the genus Camellia are known to associate with AM fungi in their native and historical ranges, but the community profile of AM fungi on tea roots in the United States has not yet been described (Bag et al., 2020). Given the growing interest in cultivating tea as a specialty crop in the United States, and especially in the Southeast, we are undertaking research to assess AM fungi-tea associations in the United States. This research will describe the AM fungal communities associating with tea in different soil types, environmental conditions, and management conditions. The project addresses these questions with two different approaches: In the first, AM fungal communities are described from across a wide geographical area within Florida and the greater Southeast; in the second, an experimental plot at the University of Florida (UF) Plant Science Research and Education Unit (PSREU) in Citra, FL is used to test AM fungal communities on early-stage establishing tea plants under different cover crop treatments. The results of these efforts will inform management practices for new tea growers in Florida and the Southeast.
1) Study and characterize AMF communities in association with tea roots in Central Florida, North Florida, and in the Southeast. This objective casts a wide net to capture the diversity of AMF-tea community composition according to geographical occurrence, including considerations of climate, soil physical properties, and local vegetative communities. AMF species and community profiles determined from this objective will inform Objective 2.
2) Evaluate AMF colonization and community profile in association with tea roots under several ground cover strategies for tea farms, including biotic ground cover and WBC. Correlations between AMF and soil physical properties, soil nutrient levels, tea plant health, and product quality metrics will be investigated.
3) Report the results of this research to inform BMPs for ground cover management in tea growing systems. In particular, the work is intended to address issues of cover crop species selection, effect on soil nutrients and soil physical properties. In addition, symbiotic interactions between cover crop, tea plant, and soil fungal communities, will be studied and described with relevance to tea plant health and management recommendations. This objective includes dissemination of findings through webinars, extension outreach, conference presentations, and research publications.
Research
Planting Materials:
This project focussed on a landrace tea variety that has shown good establishment and growth in the Gulf states. ‘Fairhope’ originated from three plants rescued from a defunct Lipton research farm in Fairhope, AL in the 1970s. These three plants, chosen for distinct morphological features from one another, were transplanted and allowed to cross breed. The resulting progeny were both numerous and vigorous; today they comprise all the plants at the Fairhope Tea Plantation in Fairhope, AL, as well as a great number of nursery plants available for retail sale throughout the Southeast.
This study assessed arbuscular mycorrhizal (AM) fungal relationships with tea plants. To this end, it comprised two facets: a survey study conducted across multiple states, and an experimental study gauging the effect of cover types on AM fungal-tea interactions (hereafter, CT).
Survey Site Descriptions
Survey sites were located in Florida, Mississippi, Louisiana, Oregon, and Pennsylvania (Table 1). The “RDG” site is a SARE partner grower farm (SARE LS18-297), Rain Drop Gardens in Reddick, FL, with 72 ‘Fairhope’ tea plants established in 2018 as 1 y/o seedlings at the same spacing and bed size as the Citra site. There are two living ground cover treatments with 6 plants x 6 replicates in each treatment: winter annual legume (crimson clover) and perennial legume (perennial peanut). The control plot is 3 WBC replicates. Tea plants are mulched and mowed as at Citra. This site also contains grower-established mature tea hedgerows, which will be included in the statewide survey.
Table 1. Sites used in the survey study of AM fungi associated with tea roots. Six sites were located within Florida, and one site each was located in Mississippi, Louisiana, Oregon, and Pennsylvania. Young farms are in a production setting established in the last 10 years.
|
Site name |
Town, State |
Latitude |
Percent Sand* |
Annual Precipitation (in.)† |
Setting |
|
Glen St. Mary Nursery |
Macclenny, FL |
30.26007
|
70-86 |
53.66 |
Forest |
|
Raindrop Gardens, Peanut Cover |
Reddick, FL |
29.38687
|
86-100 |
54.21 |
Young farm |
|
Raindrop Gardens, WBC |
Reddick, FL |
29.38687
|
86-100 |
54.21 |
Young farm |
|
Citra Diversity Garden |
Citra, FL |
29.40774
|
86-100 |
54.21 |
Varietal garden |
|
Mississippi State University |
Starkville, MS |
33.48080
|
50-70 |
64.06 |
Experimental farm |
|
Minto Island Tea |
Salem, OR |
44.90689
|
20-52 |
44.54 |
Established farm |
|
Bartow |
Bartow, FL |
27.53333
|
70-86 |
51.45 |
Backyard garden |
|
Louisiana State University |
Hammond, LA |
30.50468
|
50-70 |
66.52 |
Experimental farm |
|
Morris Arboretum |
Philadelphia, PA |
40.08759
|
20-52 |
51.18 |
Botanical garden |
|
Tallahassee |
Tallahassee, FL |
30.51936
|
70-86 |
59.71 |
Young farm |
*Obtained from USDA Soil Series descriptions and USDA soil textural class triangle.
†Obtained from NOAA.
Cover type study (CT):
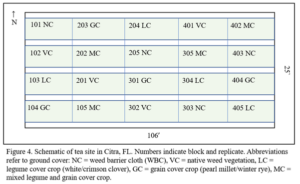
Citra: The site is maintained by UF’s Plant Science Research and Education Unit (PSREU) in Citra, FL (Figure 3). It houses 240 ‘Fairhope’ tea plants arranged in a randomized block design. The plants were installed in September, 2020 and will be established for 1 year at the time of this proposal’s start. The plants were 3 year-old seedlings at the time of installation. This site is managed with a summer/winter annual cover crop rotation (Figure 4). In 2021-2023, legumes and cereals were sown in warm/cool season rotations which comprised hairy indigo (Indigofera hirsuta) and pearl millet (Pennisetum glaucum) in warm seasons and crimson clover (Trifolium incarnatum) and winter rye (Secale cereale) in cool seasons. Each rep contains 12 tea plants, spaced 60 cm apart in beds 4’ wide. Tea plants are mulched with pine bark 16” wide on center, to prevent competition from cover crops. Ground cover height is managed by mowing as needed to maintain 4-6” tall.
Objective 1
Objective 1 is to characterize AM fungal colonization and diversity across a diverse geographical range. This phase of the project was performed in Years 1 and 2 at the 10 Survey sites (6 sites in Florida, and one each in Mississippi, Louisiana, Oregon, and Pennsylvania). A detailed and standardized protocol for sampling procedures was distributed to project partners prior to any sample collection. AM fungal community assemblages were assessed for each site using Illumina sequencing of the rDNA small subunit (SSU) region. Two rounds of amplification using AM fungal-specific primers were performed prior to barcoding and sequencing (Table 2). Sequences generated by Illumina sequencing were analyzed in RStudio using the ‘DADA2’ pipeline (Callahan et al., 2016) and ‘phyloseq’ (McMurdie and Holmes, 2013) shiny application. Beta diversity of AM fungal community assemblages associating with tea were analyzed against site and soil properties with a non-metric dimensional scaling (NMDS) plot. Alpha diversity was calculated using Shannon's Diversity Index and Simpson's Diversity Index.
Table 2. Cycling conditions for three PCR reactions performed anterior to Illumina MiSeq sequencing of AM fungal amplicons. *Touchdown PCR performed from 5°C above annealing temperature to 2°C below annealing temperature, with a stepwise temperature reduction of 0.2°C per cycle.
|
Primers |
Initial Denaturation |
Denaturation |
Annealing |
Extension |
Cycles |
Final Extension |
|
AML1/AML2 |
5 min. (95°C) |
30 sec. (95°C) |
30 sec. (57.4°C)* |
60 sec. (72°C) |
35 |
5 min. (72°C) |
|
AMV4.5NF/AMGDR |
5 min. (95°C) |
30 sec. (95°C) |
30 sec. (50.6°C)* |
60 sec. (72°C) |
35 |
5 min. (72°C) |
|
UDI Indexing |
3 min. (95°C) |
30 sec. (95°C) |
30 sec. (55°C) |
30 sec. (72°C) |
8 |
5 min. (72°C) |
Objective 2
Research for Objective 2 occurred at Citra. Colonization rates were determined by root staining and arbuscule counting using the gridline intersect method, which estimates colonization by examining roots with a gridded microscope objective and counting arbuscules at the gridline intersections (Giovanetti and Mosse, 1980). AM fungal species identification and community assemblage analysis was performed as described in Objective 1 (Table 1). Plant size index (PSI) was used to estimate plant vigor as biomass accumulation, by measuring three plant dimensions to calculate volume. Data were analyzed using generalized linear mixed models.
Objective 3
Results were disseminated via webinars with tea growers and academic meetings. A manuscript containing our findings is in preparation for peer-reviewed publication.
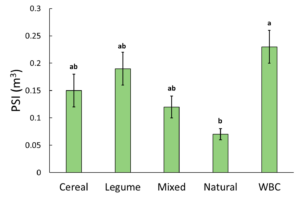
PSI ranged from 0.01 m3 to 0.46 m3 . Analysis by generalized linear mixed model and Fisher's LSD found a significant relationship between cover type and PSI when alpha=0.05. The highest PSI was measured in the WBC treatment, and the lowest was in the Natural treatment (Figure 1). This result is in line with the recommendation that establishing seedlings should not be subjected to competition with other plants, since the treatments with other vegetation excluded had the largest biomass response. It also suggests that managing vegetation as a single species may be a viable alternative to fabric weed barriers (WBC), since the legume treatment had the next highest PSI.
Survey Site Diversity
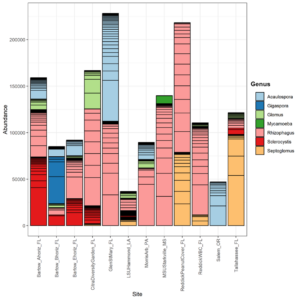
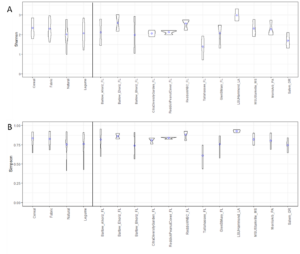
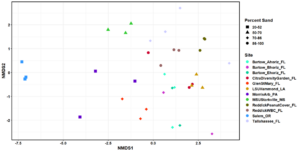
In total, 530 OTUs and 10 AM fungal genera were detected in the survey study. The top 50 grouped taxa included 283 OTUs and 7 AM fungal genera, (Rhizophagus, Acaulospora, Septoglomus, Sclerocystis, Gigaspora, Glomus), and one amoeba genus, Mycamoeba (Figure 2). Acaulospora was the only genus detected in the Salem, Oregon samples. Samples from the forested Glen St. Mary site showed a nearly even split of Rhizophagus and Acaulospora assignments, as well as the highest number of Acaulospora taxa in Florida soils. Alpha diversity was highest at Louisiana State University and lowest at the Loeb Farm in Tallahasse, FL, based on both Shannon (2.98 and 1.38, respectively) and Simpson (0.93 and 0.61, respectively) diversity indices (Figure 3). Beta diversity visualized with an NMDS plot suggested a relationship between AM fungal community diversity and percentage of sand in the soils, with sites belonging to the lowest percentage of sand clustering separately from other sand percentages (Figure 4).
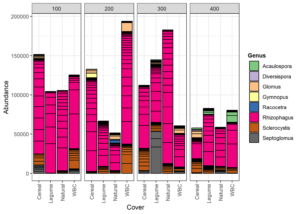
Cover Type (CT) Study:
In the CT study, 318 OTUs and 12 AM fungal genera were detected. The top 50 grouped taxa included 160 OTUs with assignments in 7 AM fungal genera: Acaulospora, Diversispora, Glomus, Racocetra, Rhizophagus, Sclerocystis, and Septoglomus (Figure 5). One basidiomycete genus was included as well: Gymnopus. Rhizophagus was the most represented genus and was present in every replicate. Sclerocystis was the second most frequent genus across all replicates. Acaulospora appeared only in the 400 block. Racocetra showed the highest abundance in the Natural treatment of the 200 block, while Septoglomus was most abundant in the legume treatment of the 300 block, and Gymnopus was highest in the Cereal treatment of the 200 block. Colonization rate was not affected by treatment (p=0.24) (Table 1). Alpha diversity was highest in the WBC and Cereal treatments based on Simpson diversity’s index, with Shannon’s diversity index showing the highest value for the Cereal treatment (Figure 3). The Natural treatment showed the lowest diversity in both indices. Analysis by generalized linear model showed no difference in alpha diversity between treatments for either index (Shannon p=0.028, Simpson p=0.28). A regression analysis of PSI against alpha diversity showed a weak linear relationship between mycorrhizal diversity and plant size (Shannon R2 = 0.07, Simpson R2 = 0.05) (Figure 6).
Table 1. Percent colonization of AM fungi on plants in the CT experiment. Colonization rate was not affected by cover type as determined by generalized linear mixed model. Values are reported as percentage, and dispersion is reported as the standard error of the mean.
|
Cover Type |
Percent Colonization |
|
Cereal |
72±5.1 |
|
Legume |
78±4.1 |
|
Natural |
79±4.0 |
|
Mixed |
80±3.9 |
|
WBC |
68±4.7 |

This study detected a total of 12 genera of AM fungi symbiotic with tea roots growing in various contexts, suggesting a high diversity of AM fungi associated with tea plants in the US. By focusing on DNA extracted from roots instead of the rhizosphere, this study targeted only AM fungi which form associations with tea. This study was broader in scope than other studies published examining this particular symbiosis which examined tea plants growing in a single location or region (Singh et al., 2008; Sharma et al., 2013; Wu et al., 2019).
All of the sites included in the survey study are managed to some degree, with the notable exception of the Glen St. Mary site in Macclenny, FL. This site was part of a commercial nursery that was abandoned sometime in the 1960s, and at the time of this writing the area is completely forested. However, tea nursery stock was left behind when the area fell into disuse. The tea plants growing there at the time of sampling are potentially original to the site, or instead are offspring of the abandoned plants. The AM fungal assemblage associated with these plants is potentially representative of a natural or wild tea-AM fungal community. The Glen St. Mary samples showed a nearly even split of Acaulospora and Rhizophagus assignments, which agrees with previously published work on "wild" tea-AM fungal assemblages (Singh et al., 2008). The presence of Acaulospora in the tea roots may indicate a host preference in an unmanaged setting.
These findings affirm that natural vegetation should be managed in establishing tea plantations, either by limiting diversity with living mulches or by excluding vegetation entirely with fabric weed barriers. The findings also provide direction for experiments on inoculation of tea seedlings with single-species or multi-species assemblages of AM fungi in the context of soil type.
Sharma, C., Gupta, R. K., Pathak, R. K., & Choudhary, K. K. (2013). Seasonal Colonization of Arbuscular Mycorrhiza Fungi in the Roots of Camellia sinensis (Tea) in Different Tea Gardens of India. International Scholarly Research Notices, 2013, e593087. https://doi.org/10.1155/2013/593087
Singh, S., Pandey, A., Chaurasia, B., & Palni, L. M. S. (2008). Diversity of arbuscular mycorrhizal fungi associated with the rhizosphere of tea growing in ‘natural’ and ‘cultivated’ ecosites. Biology and Fertility of Soils, 44(3), 491–500. https://doi.org/10.1007/s00374-007-0231-9
Wu, Q.-S., Shao, Y.-D., Gao, X.-B., Xia, T.-J., & Kuäœa, K. (2019). Characterization of AMF-diversity of endosphere versus rhizosphere of tea (Camellia sinensis) crops. The Indian Journal of Agricultural Sciences, 89(2). https://doi.org/10.56093/ijas.v89i2.87097
Educational & Outreach Activities
Participation Summary:
Grower Presentations:
Clarke, C. (8 June 2023). “Tea Varieties: Global and Domestic.” UF/IFAS Extension Field Day. Plant Science Research and Education Unit. Citra, FL.
Clarke, C. (26 April 2023). “Agroforestry Practices in Tea Cultivation.” Webinar. US League of Tea Growers Webinar Series. Recording: https://www.youtube.com/watch?v=bD6YLFORIhA
Clarke, C. (21 October 2022). “Tea Research Update: Constructing a Sense of Place.” US League of Tea Growers Annual Meeting. October 21-23. Williams, CA.
Clarke, C., Richter, B.S., Rathinasabapathi, B. (1 October 2021). “Tea Research Update: Camellia sinensis in North Central Florida.” US League of Tea Growers Annual Meeting. September 30 – October 2. Amite, LA.
Academic Presentations:
Clarke, C., Lam, R., Spakes-Richter, B., and Rathinasabapathi, B. (4 August 2023). “Freezing as a Novel Method for Black Tea (Camellia sinensis) Processing. Poster Presentation at the American Society for Horticultural Science Annual Conference. July 31-August 5. Orlando, FL.
Clarke, C. (6 March 2023). “Growing tea in Florida: Characterization of Camellia sinensis germplasm for regional adaptability, quality, and mycorrhizal interactions.” Departmental seminar. UF Horticultural Sciences Department. Gainesville, FL.
Project Outcomes
The most important contribution garnered from this work is the conclusion about managing herbaceous vegetation in establishing tea farms. In the course of this work, I have met tea growers who are allowing native vegetation to grow as an understory to their tea plants; our results support that they may see better establishment if this vegetation is managed as a single-species cover crop, ideally a legume. Management of tea understory with a legume cover will help reduce fertilizer costs and improve soil quality (economic and environmental). This work also leads to further research assessing species for use in mycorrhizal inoculation of tea seedlings. Mycorrhizal inoculation of seedlings has been shown to improve plant vigor and establishment in both habitat restoration and agricultural contexts. Improving establishment will lead to financial benefits in the form of reduced plant loss, and plants that reach economic maturity more quickly. Finally, during this research we placed high importance on communication of our work with the dedicated core of US tea growers, especially the US League of Tea Growers (USLTG). This work allowed us to connect with 50 tea growers or potential tea growers, and led us to new collaborations with established tea growers as well as tea researchers in other states. By having a public-facing engagement with tea growers, we have become a touch point for growers looking for information on tea cultivation, which has allowed us to bridge connections between more and less experienced growers.
Studying the literature about AM fungi increased my knowledge about these organisms and their interactions with plants. It was very instructive to gain an ecological perspective on agricultural research, including some of the principles governing biodiversity and productivity in agricultural systems. Overall, our perspectives on raising perennial plants in the context of latitude, geography, and existing plant and soil microbe communities were altered. For example, in the course of our investigations, we discovered that tea roots in our area are parasitized by two types of nematode, which underscored the complexity of introducing new species of crop into existing systems. We became familiar with methods for studying the root endophytic complement of fungi and other microorganisms, including methods in microscopy. I learned molecular methods for fungal species identification like PCR cloning, Sanger sequencing, and how to execute next-gen (Illumina) metabarcoding studies. I gained computation skills in community DNA data analysis and interpretation. Finally, this research led to me investigate and understand statistical methods necessary for analyzing ecological data, which I would not have otherwise encountered in my Ph.D. research. The collective experience will enable me to approach agricultural sciences from an ecological perspective in future studies, from hypothesis generation, to experimental design and analysis.
Future studies should encompass research on AM fungal assemblages for tea seedling inoculation and their effect on seedling establishment.