Final report for GS23-280
Project Information
Resistant sorghum varieties and resident natural enemies play a crucial role in suppressing the sorghum aphid (Melanaphis sorghi) and enhancing profitability and sustainability of sorghum growing operations. This economically relevant pest has invaded over 90% of sorghum-growing states and caused millions of dollars of damage. To continue to develop and deploy resistant varieties, it is critical to understand which resistance traits can effectively control pests. Plant volatiles, or chemical compounds that assist natural enemies in locating their prey, are plant traits that have yet to be incorporated into resistant varieties. Our previous work has identified one variety that both decreases aphid reproduction (antibiosis) and produces plant volatiles to attract natural enemies. Our preliminary field experiments have shown that this dually resistant variety not only maintains lower aphid abundances but also attracts significantly more natural enemies compared to varieties without this trait. The main objectives of the project are: 1) screen a broad range of sorghum varieties for both antibiotic and volatile traits and 2) assess if varieties with enhanced resistance will also have enhanced yield and suppress aphids across multiple growing regions. We will test both experimental and commercially available hybrids. This will allow us to identify genetic sources of different resistance traits for future crop breeding and inform growers on which varieties best suppress pests for their growing region. Using dually resistant varieties will allow growers to control aphids using both host plant resistance and biological control, thereby decreasing the need for insecticide applications, increasing yield, and increasing profitability.
Project Objectives:
Objective 1: Screen 10 varieties of sorghum for antibiotic and volatile resistance traits. We predict that sorghum varieties will vastly differ in their propensity to decrease aphid population growth and attract natural enemies.
Objective 2: Measure the effectiveness of 5 dually resistant sorghum varieties at suppressing aphids and producing high yields in several growing regions of Texas. We predict that varieties with both antibiotic and volatile traits will better protect sorghum from sorghum aphid than varieties with only antibiotic traits. Dually resistant varieties will keep aphid populations from growing and kill remaining aphids via attraction of natural enemies such as insect parasitoids and predators. In addition, we predict that dually resistant varieties will protect sorghum in different growing regions, making it effective for a broad range of growers. We also predict that dually resistant varieties will have enhanced yield, owing to their genetic background and enhanced resistance to aphid damage.
Cooperators
- (Researcher)
Research
Objective 1:
Ten varieties will be screened for antibiotic and volatile traits (see Table 1). Five experimental and five commercial varieties were selected based on known resistance/susceptibility to the aphid, pedigree, and agronomic qualities.
Plants will be reared in the laboratory to maintain sorghum aphid colonies for laboratory and field experiments and to complete volatile and antibiosis assays. One seed will be planted per pot in growing medium and will be grown in a greenhouse at 28-32 ℃ with supplemental light and maintained in insect-proof tents. Sorghum aphid colonies will be established with aphids collected from sorghum in College Station, Texas in June of 2020. Colonies will be maintained on susceptible sorghum plants in a growth chamber at 30℃, 40% RH, and 12:12 light:dark cycle. Aphids from laboratory colonies will be used to infest plants in volatile assays, antibiosis assays, and the field experiment.
We will use previously established methods to collect volatiles from infested sorghum plants to identify and quantify volatile compounds produced by different sorghum varieties. Ten sorghum varieties will be grown in the fashion as described above. The sorghum plants will either be inoculated with 50 aphids 48 h prior to volatile collection or remain aphid-free. There will be at least 10 replicates of each treatment per variety. Collections will be performed in walk-in growth rooms. Volatiles from leaves of individual plants will be collected in heat-resistant oven bags. Air will be filtered and pumped into the oven bags while a vacuum pump will pull air out of the oven bags at the same time. Volatiles will be pulled into a volatile filter trap. Volatiles will be eluted from the volatile filter traps. These volatiles will be quantified using a gas chromatograph and mass spectrometer using established techniques. To identify volatile compounds, we will compare mass spectra and retention times with published libraries of volatile data. We will use ANOVAs to compare amounts of individual volatile compounds among varieties.
To determine if sorghum varieties affect aphid reproduction via antibiotic traits, we will measure aphid population growth rate on sorghum plants. Plants will be grown as described above and infested with 50 aphids 48 h prior to the start of the experiment. There will be at least 10 replicates per variety. Aphid numbers on each plant will be counted each day for seven days total. Population growth rate will be calculated, and ANOVAs will be used to compare the strength of antibiosis among varieties.
Objective 2:
From our volatile and antibiosis assays, we will select a subset of five varieties that will vary in resistance traits. We expect to have some varieties with both antibiotic and volatile traits, some varieties with only antibiotic traits, and some varieties that are susceptible to sorghum aphid. Our preliminary screening of a few varieties has shown that all these combinations exist among the varieties tested. Varieties will be planted at experimental farms belonging to Texas A&M in the following locations: College Station, Amarillo, and Corpus Christi. These locations are in the panhandle and central plains, where most of the sorghum production occurs in Texas. Plots will be eight rows wide and ~12 m (40 ft) long and will be arranged in a randomized complete block design. Plots will be treated with an herbicide to reduce weed growth, but no insecticides will be applied to the field. Sorghum will be watered monthly through flood irrigation.
At boot stage, 20 plants in the center of the plots will be artificially infested with 50 sorghum aphid per leaf using leaf clippings from laboratory colonies. Plants will be re-infested as needed to ensure that all plants have approximately the same aphid densities. Yield will be measured for all varieties both years. Each variety will be replicated four times (n=20 total plots per site).
To determine the effect of sorghum variety on sorghum aphid suppression and natural enemy attraction, we will count sorghum aphids, parasitoid mummies, parasitoid adults, and predators on two leaves of the plants within the plots (one upper and one lower leaf). Measurements will be taken once a week from boot stage until plant maturity. Parasitoid mummies will be brought back to the laboratory to confirm identification. All natural enemies will be identified to the species level. Aphid densities above 50 will be approximated by counting in increments of 50, as aphid population growth can quickly grow to large sizes such that actual counts are not possible.
To estimate how much the attracted resident natural enemies contribute to aphid suppression, we will use exclusion cages. Within the experimental plots, another twelve plants at the boot stage will randomly be selected, with six plants being assigned to each treatment. The two treatments will be: 1) caged plants and 2) uncaged plants. Caged plants will exclude natural enemies, which will help us estimate how much antibiotic traits alone contribute to sorghum aphid suppression. Conversely, natural enemies will be able to access sorghum aphid on uncaged plants. The frame of cages (1 sq. m wide and 1 sq. m tall) will be constructed from PVC and a fine mesh sleeve will be placed over the PVC frame to exclude all natural enemies. Once plants reach the boot stage, we will infest each leaf with 40 sorghum aphids and all sorghum aphids will be sourced from the laboratory colony. Cages will then be placed over the assigned plants and after a one-week period, cages will be removed, and sorghum aphid densities will be counted. Estimates of sorghum aphid densities at the end of the experiment will be used to calculate percent suppression attributable to natural enemies.
For the field experiment, mixed model ANOVAs will determine whether changes in sorghum aphid density, natural enemy density, and days below the action threshold (50 aphids/leaf) are because of plant resistance traits. For the field cage study, we will use ANOVAs to compare sorghum aphid densities among varieties. We will also compare our results across sites to determine if varieties performed similarly.
Results for Objective 2
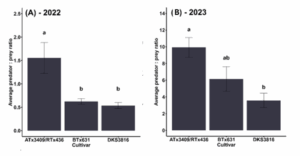
During two growing seasons, we tracked the number of natural enemies and aphids on three sorghum cultivars. We found a diverse array of natural enemies feeding on aphids in the field, including parasitoid wasps, lacewings, syrphid flies, and lady beetles. In 2022, there were an average of 1.5 natural enemies per aphid on the sorghum variety ATx3409/RTx436, which was three times higher than the other varieties (p<0.01) (Fig 1A). In 2023, nearly ten natural enemies per aphid were observed on ATx3409/RTx436 on average throughout the growing season (p<0.01) (Fig. 1B).
Figure 1: Natural enemy recruitment to aphid-infested plants. Letters above bars indicate significant differences among varieties (p<0.05).
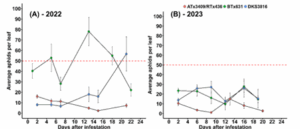
In 2022, the only sorghum variety to remain below the action threshold was ATx3409/RTx436 (Fig. 2A). On average, this cultivar had approximately 8 aphids per leaf throughout the growing season, whereas other cultivars like BTx631 had an average of 46 aphids per leaf. In 2023, aphid densities were unexpectedly low and none of the cultivars reached the action threshold.
Figure 2: Sorghum aphid populations over time. The red dotted line depicts the action threshold of 50 aphids per leaf.
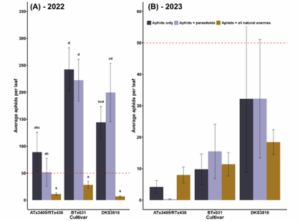
In both years of our field study, we used field cages to exclude natural enemies and determine the relative contributions of antibiosis and volatile traits. Cages that excluded all natural enemies, for instance, could only rely on antibiosis traits to reduce aphid abundance. In 2022, natural enemies reduced aphid populations by an estimated 83% across all sorghum varieties (Fig. 3A). It should be noted that our exclusion experiments tracked insect populations for only a week and that as the growing season continued, aphid suppression was not consistent across all varieties (Fig. 2A). We hypothesize that both predators (lady beetles, lacewings, minute pirate bugs, and syrphid flies) and parasitoid wasps play a role in this suppression, as the parasitoid-only treatments did not significantly reduce aphid populations (p<0.05). In 2023, natural enemy exclusion treatments did not affect aphid densities (p=0.8) (Fig. 3B). However, ATx3409/RTx436 did maintain the lowest aphid densities overall (p<0.01).
Figure 3: Natural enemy exclusion effects on sorghum aphid populations. “Aphids only” treatments were caged plants that prevented all natural enemies (both parasitoids and predators) from accessing aphids. “Aphids + parasitoids” treatments were caged plants with mesh that allowed only parasitoids to access aphids. “Aphids + all natural enemies” plants were uncaged plants. The red dotted line depicts the action threshold of 50 aphids per leaf.
Discussion for Objective 2
Our work demonstrated that volatile-based resistance traits can play an important role in pest suppression in sorghum. We found that ATx3409/RTx436 recruited higher densities of natural enemies in the field compared to other sorghum varieties. The sorghum variety ATx3409/RTx436 attracted up to 9 natural enemies per aphid. In our previous work, we showed that a single lady beetle or green lacewing larvae could consume 20-40 aphids within a single day (Hewlett et al. 2019). Thus, highly attractive sorghum varieties can quickly reduce aphid populations. In our experiments, aphid populations on ATx3409/RTx436 remained below the action threshold, eliminating the need for insecticide sprays for control. Reliance on alternative pest management tactics can reduce the risk of insecticide resistance developing in aphid populations and protect pollinators (EPA 2016).
Our natural enemy exclusion studies also showed that while caged ATx3409/RTx436 can directly reduce aphid populations, this protection is only partial and natural enemies are needed to control aphid populations. We posit that both antibiosis and volatile-based resistance traits are important for future sorghum improvement and breeding. Other studies in maize and mustard plants have also shown that dually resistant plants are better protected against pests (Fatouros et al. 2014, Mutyambai et al. 2014). Moreover, based on our laboratory findings from Obj. 1, we predict that methyl salicylate (MeSA) is a volatile that plays a role in attracting natural enemies in the field. Because of logistical constraints, we could not directly test specific volatiles using dispensers, but similar work in maize and cabbage have found a positive correlation between MeSA and parasitoid attraction (Degen et al. 2012, Poelman et al. 2009). Further work must be done to understand how volatile profiles change with plant ontogeny and what specific blend of volatiles increases attraction to natural enemies.
In 2023, we found that aphid populations were near zero throughout the growing season, regardless of the sorghum variety. This was the second-hottest summer ever recorded in Texas, with an average temperature of 29.6℃ (Douglas et al. 2023). Not only can extreme temperatures cause fluctuations in volatile emissions (Liu et al. 2020), but this can be detrimental to sorghum aphid survival (Hinson 2022). Further work must be done to understand how temperature and drought can affect volatile-based resistance traits.
Overall, our work shows that laboratory assays to find and assess resistance traits can reliably predict natural enemy attraction and pest suppression in the field. This may serve as an alternative resistance screening tool that reduces cost, labor, and time investments. To the best of our knowledge, only a handful of researchers are investigating volatile-based resistance traits for pest management and crop improvement (Tamiru et al. 2020). Our work shows that these traits can be implemented successfully.
Educational & Outreach Activities
Participation summary:
This work was presented at a Texas A&M Research Forum, as well as at invited talks at the University of Maryland and a Master Gardeners meeting. I also served as a mentor for a USDA NIFA-funded REEU (Research Education and Experience for Undergraduates), working with a student from a Hispanic-Serving Institution in Texas. This student presented work on beneficial insect (a predaceous lacewing) attraction to different sorghum varieties at the Texas A&M University Summer Undergraduate Research Symposium.
Project Outcomes
Project Outcomes: This project has allowed myself and my collaborators to publish two peer-reviewed articles about sorghum resistance traits and applications in the field. This work was included in my Ph.D. dissertation, which is to be made available online in a year. Additionally, the University of Maryland wrote a publicly available web article about this work in sorghum. Ultimately, this two-year field experiment shows that resistance traits involved in the attraction of beneficial insects can result in economically important pest suppression. Traits associated with antibiosis (direct effects on aphid reproduction) also contribute to control. The dually defended variety remained below the action threshold for the entire growing season, eliminating the need to apply any insecticides for control. Laboratory assays of both trait types could reliably predict aphid and natural enemy responses in the field, making resistance screens less time-consuming and more cost-effective. Sorghum breeders can use the variety ATx3409/RTx436, which is now known to directly and indirectly defend against aphids, to uncover genes associated with these traits that can be incorporated into future, improved varieties.
Knowledge Gained: This project is one of the few studies that have tested the importance of volatile-mediated biological control for crop plants. An unexpected yet interesting outcome of this work was the impact of environmental conditions on aphid and natural enemy populations. We suspect that extreme temperatures not only impacted insect populations, but also expression of resistance traits. Identification of vulnerabilities in this pest management tactic can be the focus of future research. In terms of technical knowledge gained, I learned how to plant sorghum plots, irrigate plots, and apply herbicides with backpack sprayers with the help of David Kerns, Ph.D. (Texas A&M University). Six undergraduate students participated in data collection and insect rearing, giving them hands-on experience and job skills in entomology and agronomy.