Final report for GW16-021
Project Information
Little research has been done to diagnose the causal agents of various diseases that affect peony. Modern extension bulletins and growers’ guides rely on nearly 100 year-old research to diagnose disease on this high-value cut flower and landscape crop. In order to provide growers with more information, samples of peonies were collected from 12 states in the United States during the summer of 2016 with the purpose of identifying various pathogens causing disease. Fungal plant pathogens were isolated from diseased tissue and identified by morphology and PCR and sequencing of various informative genes. A total of 10 fungal genera were identified, including five genera never before reported on peonies in the United States: a Botryosphaeria species, multiple Colletotrichum species, Mycocentrospora acerina, a Phoma species, and Pilidium concavum. In addition to those new to the United States, a number of new state-pathogen-host combinations were identified in the survey. A list of the state-pathogen combinations found as a result of this survey, combined with data from previous years that helped justify funding for this grant are displayed in Table 1. Koch’s postulates were performed for most first reports to confirm pathogenicity. The information gained from this survey will be valuable for growers and disease diagnosticians to make better diagnoses and subsequent disease management decisions. Additional diagnoses of diseases, including a visual diagnosis of Tobacco rattle virus from South Carolina and sooty mold on peonies from Alaska, and physiological disorders, such as normal fall dieback from Canada, were made via email and using a Facebook page which as of the writing of this report has over 275 followers. Lastly, educational materials were created as a result of this project, namely, a workshop presented to the Alaska Peony Grower's Association and two presentations at the same conference. A journal article with the findings of this project is in review and a disease diagnosis bulletin is in preparation and pending submission. The information gained from this project will be valuable for growers and disease diagnosticians to make better diagnoses and subsequent disease management decisions.
Objective 1. During the spring and summer of 2016 we will conduct surveys to obtain diseased foliage and solicit additional foliage to be collected by peony growers. Collections will focus on at least 30 growers in Alaska, Oregon, and Washington; however, they will extend to other Western states as identified during the course of the study.
Samples were collected from peonies from a total of 11 states: 1 farm in Connecticut, 2 farms in Maryland, 1 farm in New York, 1 farm in Pennsylvania, one farm in Virginia, 5 farms in Washington, 2 farms in Oregon, and 37 farms in Alaska and from landscape plantings in Indiana during the 2016 growing season. Surveys were also conducted on the plant material at the WSU Puyallup Research and Extension Center in Puyallup, WA. Samples processed from outside of the Western US were supported by another grant.
Objective 2. We will attempt to isolate causal organism of disease using traditional fungal and bacterial isolation methods. The isolated organisms will be maintained in-vitro in petri plates containing the appropriate growth media.
Isolations were performed to identify fungi or fungal-like organisms associated with disease symptoms. A total of 351 isolations were attempted by the graduate student and temporary employee from collected foliage using traditional fungal isolation techniques. Of these isolations, the majority were from Alaska (126), Washington (112), and Oregon (31). Figure 1 shows a comprehensive list of the states surveyed and the number of samples processed per state. With the exception of a root sample infected with Sclerotium rolfsii, from which sclerotia were taken from the surface of the infected root, a small piece of tissue was excised from the margin of a lesion and was surface sterilized in a 1% NaOCl solution and rinsed twice in sterile water. Surface sterilized tissue samples were then plated onto potato dextrose agar amended with streptomycin and chloramphenicol (PDA+s/c) at 10 mg/L each. Subsequent growth was transferred to a new PDA+s/c plate.
Attempts to isolate bacteria from samples that were unsuccessful at isolating a fungal pathogen were attempted for samples from Alaska, however, putative bacterial pathogens were not isolated from any samples. Subsequent growth of organisms was transferred to maintain pure cultures.
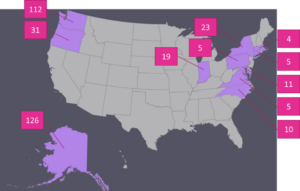
Figure 1. States from which samples of diseased peonies were collected during the 2016 growing season and the number of samples processed from each state.
Objective 3. We will use a combination of morphological and molecular methods (PCR and sequencing) to positively identify the suspected causal organisms of disease. If suspected pathogen is viral, samples will be submitted to a commercial virus screening laboratory for identification.
A combination of morphological and molecular techniques were used to identify fungal and fungal-like organism that were isolated from peony tissues. All fungal and fungal-like plant pathogens initially identified by morphology and PCR and sequencing of the internal transcribed spacer (ITS). DNA from isolates was extracted from isolates and used in PCR and sequencing of the ITS region of nuclear ribosomal DNA using primer pairs ITS6 and ITS4. Further identification by sequencing of the glyceraldehyde 3-phosphate dehydrogenase and the cytochrome c oxidase subunit 1 genes was used for the Colletotrichum and Sclerotinia and Phytophthora isolates, respectively. Sequences were compared with known sequences of fungal organisms published in the NCBI BLAST database. The sequences for all isolates used in pathogenicity trials were deposited into GenBank. Morphological traits used to identify fungi included colony and spore morphology and the production of resting structures. All fungi were identified to at least the genus level, with some identified to the species level.
Fungi and fungal-like organisms from 10 genera, plus at least one unidentified powdery mildew species were isolated and identified by morphology from the peony samples collected (Table 1). These pathogens were associated with a range of symptoms including leaf spots, stem spots or cankers, shoot blight, and root rot (Figure 2). The samples included five genera never before reported on peonies in the United States: a Botryosphaeria species, multiple Colletotrichum species, Mycocentrospora acerina, a Phoma species, and Pilidium concavum. Additional novel host-pathogen-state combinations were identified as described in Table 1. The most common disease identified on peonies, as determined by the number of states from which the disease was identified, was Botrytis gray mold caused by Botrytis spp. The second most common disease was measles, caused by Graphiopsis chlorocephala.

Samples of virus diseases were not collected during this study, thus, were not submitted to virus indexing. We were able to punitively diagnose Tobacco rattle virus on peonies in South Carolina and Alaska based on visual symptoms from photos provided by growers using the Facebook page developed as an outreach portion of this grant.
Table 1. Fungal and fungal-like diseases associated with peony by state in surveys conducted from 2013-2016. Diseases in bold represent first reports in the state in which they were found.
|
State |
Disease (causal organism)x |
|
Alaska |
Botrytis gray mold (multiple Botrytis spp.) Leaf spot (Sclerotinia sclerotiorum) Phoma (Phoma spp.)y Licorice spot [proposed, see discussion] (Mycocentrospora acerina)y |
|
Connecticut |
Anthracnose (Colletotrichum spp.)y |
|
Indiana |
Botrytis gray mold (unidentified Botrytis spp.)z Measles (Graphiopsis chlorocephala) Powdery mildew (unidentified spp.)z |
|
Maryland |
Anthracnose (Colletotrichum spp.)y Botrytis gray mold (unidentified Botrytis spp.)z Measles (Graphiopsis chlorocephala) White stem rot (Sclerotinia sclerotiorum) |
|
Michigan |
Leaf spot (unidentified Alternaria spp.)z |
|
Missouri |
Leaf spot (unidentified Alternaria spp.)z |
|
New York |
Botrytis gray mold (unidentified Botrytis spp.)z Leaf spot (Botryosphaeria spp.)y Leaf spot (unidentified Alternaria spp.) Powdery mildew (unidentified spp.)z |
|
North Carolina |
Botrytis gray mold (unidentified Botrytis spp.) Tan-brown leaf spot (Pilidium concavum)y White stem rot (Sclerotinia sclerotiorum) |
|
Oregon |
Anthracnose (Colletotrichum spp.)y Botrytis gray mold (multiple Botrytis spp.) Tan-brown leaf spot (Pilidium concavum)y Leaf spot (unidentified Alternaria spp.) Root rot (Phytophthora cactorum) |
|
Pennsylvania |
Leaf spot (unidentified Alternaria spp.)z Measles (Graphiopsis chlorocephala) Powdery mildew (unidentified spp.)z |
|
Virginia |
Tan-brown leaf spot (Pilidium concavum)y Measles (Graphiopsis chlorocephala) Powdery mildew (unidentified spp.)z |
|
Washington |
Anthracnose (Colletotrichum spp.)y Botrytis gray mold (multiple Botrytis spp.) Tan-brown leaf spot (Pilidium concavum)y Leaf spot (unidentified Alternaria spp.) Measles (Graphiopsis chlorocephala) Powdery mildew (unidentified spp.)z Licorice spot [proposed, see discussion] (Mycocentrospora acerina)y Southern blight (Sclerotium rolfsii) |
xDisease name is followed by causal organism in parentheses.
yIndicates pathogen represents first report in the United States.
zIndicates pathogenicity trials not conducted in this study.
Objective 4. We will perform Koch’s postulates on live plants and detached peony tissues to determine pathogenicity of the organisms.
For those fungal species that represented first reports on peony either in the United States or in the state in which the original sample was collected, pathogenicity trials were conducted using a representative isolate for each state-pathogen combination (Table 2). For all trials, isolates were grown up on PDA at 20C in the dark for 3-40 days, depending on species, with the exception of the Phytophthora cactorum which was grown up on V8 agar. Peony leaves, stems, or roots (depending on the site of original pathogen recovery) were removed from potted peony plants and surface sterilized in a 1% NaOCl solution for 3-5 minutes and then rinsed twice in sterile water. Plugs 5mm in diameter were cut from the actively growing region of the fungal colony and placed mycelium side down on the host tissue. For trials that took place on foliage, plugs were inoculated onto the abaxial side of the leaf. Both wounded and unwounded tissues were inoculated for most fungal species trials except for those that remained unwounded according to the results of preliminary pathogenicity trials and those conducted on stems and roots which were all wounded by pins used to hold plugs securely onto host tissue. For all trials, an uncolonized plug was used as a control. Inoculated and mock inoculated tissues were placed into a plastic bin containing wire racks suspended over moistened paper towels. A lid was placed on the bin and the bin was placed in a plastic bag to maintain high humidity conditions. Inoculated tissues were incubated at 20C under dark conditions. After sufficient observable symptom development (3-40 days depending on fungal species), plant material was removed from the incubator and the fungus was re-recovered from the tissue as described in the original fungal isolation protocol above. The identity of the re-isolated pathogen was confirmed by morphological analysis. A total of five replicates were performed per treatment. Most trials were conducted using the peony cultivar ‘Sarah Bernhardt.’ Trials for Graphiopsis chlorocephala were conducted on peony cultivar ‘Kansas’ due to the prior observation that this cultivar appeared highly susceptible to this pathogen, and those for a Colletotrichum spp. isolate from Connecticut took place on both ‘Sarah Bernhardt’ and ‘Kansas’ due to observations of varying susceptibility during pathogenicity trials.
Pathogenicity trials confirmed the ability of all organisms identified as first reports (Tables 1 and 2) to cause disease on peony consisting of symptoms like those observed on the original sample (Figure 2). A list of the isolates used for pathogenicity trials including GenBank accession numbers can be found in Table 2. In most cases, 5 of 5 inoculated tissues (wounded and unwounded) displayed symptoms, with successful re-isolation from the majority of lesions (data not shown). Lesions only developed on 4 of the 5 replicates for both wounded an unwounded tissues for the Colletotrichum spp. isolate from Oregon (HAM02) and the Botryosphaeria spp. isolate from New York (NY12). Lesions were produced on only 4 of 5 unwounded tissues for the Alternaria isolate from Washington (FR01) (pathogenicity tests were not conducted on wounded tissues). The Colletotrichum spp. isolate from Connecticut (CT03) appeared to have differences in virulence depending on peony cultivar. On ‘Kansas,’ CT03 was able to cause lesions characteristic of anthracnose infections on 4 of 5 wounded tissues and 2 of 5 unwounded tissues after 20 days, whereas symptoms only developed on 2 of 5 wounded and 1 of 5 unwounded tissues after 40 days. Lesions developed on tissue regardless of wounding for all isolates and wounding did not appear to influence the frequency of infection.
Table 2. Fungi isolated from diseased peonies and used in pathogenicity trials by state. All isolates were sequenced and sequences deposited in GenBank.
|
State |
Species |
Isolate Code |
Plant tissuez |
GenBank Accession Number(s) |
|
AK |
Sclerotinia sclerotiorum |
CC04 |
Foliage |
MF776031 |
|
Mycocentrospora acerina |
PE06 |
Stem |
MF776041 |
|
|
Phoma spp. |
RR06 |
Stem |
MF776043 |
|
|
CT |
Colletotrichum spp. |
CT03 |
Foliage |
MF776032; MF780699 |
|
IN |
Graphiopsis chlorocephala |
IN03 |
Foliage |
MF776048 |
|
MD |
Graphiopsis chlorocephala |
SF01c |
Flower bud |
MF776044 |
|
Colletotrichum spp. |
SF02b |
Stem |
MF776045; MF780702 |
|
|
NC |
Pilidium concavum |
NC10 |
Foliage |
MF776037 |
|
NY |
Alternaria spp. |
NY12b |
Foliage |
MF776039 |
|
Botryosphaeria spp. |
NY12 |
Foliage |
MF776038 |
|
|
OR |
Alternaria spp. |
ADE02 |
Foliage |
MF776028 |
|
Colletotrichum spp. |
HAM02 |
Foliage |
MF776036; MF780700 |
|
|
Pilidium concavum |
OP83 |
Foliage |
MF776040 |
|
|
Graphiopsis chlorocephala |
OR78b |
Stem |
MF776050 |
|
|
Phytophthora cactorum |
OP94 |
Tuberous root |
MF776049; MF780698 |
|
|
VA |
Pilidium concavum |
VA04 |
Foliage |
MF776047 |
|
Graphiopsis chlorocephala |
VA02 |
Foliage |
MF776046 |
|
|
WA |
Alternaria spp. |
FR01 |
Foliage |
MF776035 |
|
Sclerotium rolfsii |
DG92 |
Tuberous root |
MF776034 |
|
|
Pilidium concavum |
AR92 |
Foliage |
MF776030 |
|
|
Mycocentrospora acerina |
AR61 |
Stem |
MF776029 |
|
|
Colletotrichum spp. |
PUY45c |
Flower bud |
MF776042; MF780701 |
|
|
Graphiopsis chlorocephala |
WBC12 |
Stem |
MF776051 |
zPlant tissue from which the pathogen was originally isolated.
Objective 5. We will prepare and disseminate information regarding the peony pathogens. Such educational material will include an extension bulletin published through Washington State University and/or the University of Alaska Fairbanks, a workshop conducted in Alaska, and first reports published in peer-reviewed journals.
As a result of this project, two extension bulletins, one peer-reviewed paper, and one workshop have been prepared. One extension bulletin on Tobacco rattle virus has been published in collaboration with the University of Alaska Fairbanks, as described in a future section, and one on disease diagnosis and management is pending submission. A peer-reviewed journal article has been submitted to the journal Plant Disease and is in review. A workshop on general disease management and disease management in peonies was conducted in 2017 at the Alaska Peony Grower's Association Conference and is described in a future section. In addition, we conducted over 50 field visits to do field collections and meet with growers to discuss surveying for disease and disease diagnosis and management.
Research
Sample Collection
Peonies showing symptoms of disease on above or below-ground tissues were collected both commercial and landscape plantings of peonies from a total of 11 states. Samples from the Pacific Northwest states of Washington, Oregon, and Alaska, were largely collected by the authors during in-field surveys. Fields were sampled at various times throughout the growing season from emergence to senescence. In-field surveys were not conducted in a systematic fashion, rather, fields were walked to identify and collect samples with a range of disease symptoms present. Quantitative data on incidence and severity of disease were not collected. For some Pacific Northwest samples and for the remainder of the survey locations, samples were collected by peony growers and university extension collaborators, double-bagged in plastic, and sent in by mail.
Fungal Isolations and Identification
Isolations were performed to identify fungi or fungal-like organisms associated with disease symptoms. With the exception of a root sample infected with Sclerotium rolfsii, from which sclerotia were taken from the surface of the infected root, a small piece of tissue was excised from the margin of a lesion and was surface sterilized in a 1% NaOCl solution and rinsed twice in sterile water. Surface sterilized tissue samples were then plated onto potato dextrose agar amended with streptomycin and chloramphenicol (PDA+s/c) at 10 mg/L each. Subsequent growth was transferred to a new PDA+s/c plate.
Isolates were transferred to PDA+s/c overlaid with sterile cellophane membrane and grown up at room temperature conditions. Hyphae were removed from the cellophane using a sterile tool and transferred into a 2mL Eppendorf tube containing several 3.5mm glass beads. Mycelium was frozen at -80C and then mechanically homogenized into a paste. A DNeasy Plant Mini Kit (Qiagen) was used to extract DNA from homogenized mycelium according to the manufacturer’s instructions.
DNA from isolates was used in PCR and sequencing of the internal transcribed spacer (ITS) region of nuclear ribosomal DNA using primer pairs ITS6 and ITS4 (White et al. 1990). ITS PCRs were carried out in 50 μL reactions containing: 1X buffer (Genescript, Nanjing, China), 0.2 μM of each dNTPs (Genescript), 0.24 μM of each primer, 1.5 mg/mL BSA (New England Biolabs, Ipswich, Massachusetts), 3 U Taq DNA polymerase (Genescript), and variable amounts of DNA template (not quantified). Additional sequencing of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene and the cytochrome c oxidase subunit 1 (COI) were also performed for the Colletotrichum spp. and Phytophthora spp. isolates, respectively. The GAPDH PCR reactions were carried out using a modified protocol from Weir et al. (2012) and the COI reactions were carried out using a modified protocol from Robideau et al. (2011). PCR products were cleaned up using ExoSAP-IT Express (Affymetrix, Santa Clara, California) as per the manufacturer’s instructions, and sequenced in both directions (Genewiz, South Plainfield, New Jersey) with the same primers used in amplification.
ITS, GAPDH, and COI sequences for selected isolates were obtained and compared to results in the BLAST search engine in GenBank. BLAST searches were performed to compare the sequences from the peony fungi to fungi listed in the NCBI database. Fungal isolates were identified to genus or species based on sequence similarity to fungi in the database. Colony characteristics such as colony color, sclerotia development, and growth habit and microscopic structures such as conidia and chlamydospore production of the fungi were compared against morphological descriptions of the fungi to confirm BLAST results.
Pathogenicity Trials
For those fungal species that represented first reports on peony either in the United States or in the state in which the original sample was collected, pathogenicity trials were conducted using a representative isolate for each state-pathogen combination. For all trials, isolates were grown up on PDA at 20C in the dark for 3-40 days, depending on species, with the exception of the Phytophthora cactorum which was grown up on V8 agar. Peony leaves, stems, or roots (depending on the site of original pathogen recovery) were removed from potted peony plants and surface sterilized in a 1% NaOCl solution for 3-5 minutes and then rinsed twice in sterile water. Plugs 5mm in diameter were cut from the actively growing region of the fungal colony and placed mycelium side down on the host tissue. For trials that took place on foliage, plugs were inoculated onto the abaxial side of the leaf. Both wounded and unwounded tissues were inoculated for most fungal species trials except for those that remained unwounded according to the results of preliminary pathogenicity trials and those conducted on stems and roots which were all wounded by pins used to hold plugs securely onto host tissue. For all trials, an uncolonized plug was used as a control. Inoculated and mock inoculated tissues were placed into a plastic bin containing wire racks suspended over moistened paper towels. A lid was placed on the bin and the bin was placed in a plastic bag to maintain high humidity conditions. Inoculated tissues were incubated at 20C under dark conditions. After sufficient observable symptom development (3-40 days depending on fungal species), plant material was removed from the incubator and the fungus was re-recovered from the tissue as described in the original fungal isolation protocol above. The identity of the re-isolated pathogen was confirmed by morphological analysis. A total of five replicates were performed per treatment. Most trials were conducted using the peony cultivar ‘Sarah Bernhardt.’ Trials for Graphiopsis chlorocephala were conducted on peony cultivar ‘Kansas’ due to the prior observation that this cultivar appeared highly susceptible to this pathogen, and those for a Colletotrichum spp. isolate from Connecticut took place on both ‘Sarah Bernhardt’ and ‘Kansas’ due to observations of varying susceptibility during pathogenicity trials.
References:
Robideau, G.P. De Cock, A.W., Coffey, M.D., Voglmayr, H., Brouwer, H., Bala, K., Chitty, D.W., Désauliners, N., Eggertson, Q.A., Gachon, C.M., Hu, C.H., Küpper, F.C., Rintoul, T.L., Sarhan, E., Verstappen, E.C., Zhang, Y., Bonants, P.J., Ristaino, J.B., and Lévesque, C.A. 2011. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Molecular Ecology Resources 11:1002-1011.
Weir, B.S., Johnston, P.R., and Damm, U. 2012. The Colletotrichum gleosporioides species complex. Studies in Mycology 73:115-180.
White, T.J., Bruns, T.D., Lee, S.B., and Taylor, J.W. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: M.A. Innis, D.H. Gelfand, J.J. Sninsky, T.J. White (eds.) PCR Protocols — a Guide to Methods and Applications, Academic Press, San Diego, CA.
Results
Fungal Isolations and Identification
Fungi and fungal-like organisms from 10 genera, plus at least one unidentified powdery mildew species were isolated and identified by morphology from the peony samples collected (Table 1). These pathogens were associated with a range of symptoms including leaf spots, stem spots or cankers, shoot blight, and root rot (Figure 2).
Botrytis spp. (multiple species including B. cinerea and B. paeoniae, data not shown), Graphiopsis chlorocephala, and Alternaria spp. were isolated from the most states represented in our surveys. Out of the 11 states from which samples were acquired, 7 states had at least one sample infected with a Botrytis spp., 5 with G. chlorocephala, and 5 with an Alternaria spp. (Table 1). There was no clear pattern in symptoms associated with the different Botrytis species in our surveys, however, the symptoms of Botrytis gray mold resembled those well documented in the literature. All above-ground parts of the peony, including the foliage (Figure 2F), stems, flower, and flower bud, were observed as being infected with Botrytis, and prolific sporulation was often observed on infected host tissue. The symptoms associated with G. chlorocephala ranged from flecking on the stems and leaves to large expanding, purple-red lesions (Figure 2C, M). G. chlorocephala was also observed infecting the sepals of a flower bud. These symptoms also match those described in the literature. Conidia of G. chlorocephala could often be observed being borne from symptomatic leaf tissue, appearing charcoal to olive green in color. The symptoms associated with the Alternarias were variable and ranged from dark to light brown leaf spots (Figure 2I).
Other pathogens were not as commonly isolated in our surveys, such as Sclerotium rolfsii and Phytophthora cactorum which were only found in Washington and Oregon, respectively. Plants infected with S. rolfsii did not emerge uniformly in the field and coarse white mycelium and orange sclerotia could be observed on root tissue (Fig. 2O). P. cactorum was observed causing large chocolate brown lesions on the tuberous root tissue (Figure 2E) expanding to the root crown causing above-ground, blackish-brown blight of shoots. Colletotrichum spp. were isolated from peonies from four states (Table 1 and 2), and appear to represent 3 distinct species based on GAPDH sequence similarity to isolates reported in GenBank. The Colletotrichum spp. isolates from Washington and Oregon show the most sequence similarity to C. godetiae, the isolate from Maryland shares sequence similarity with C. nymphaeae, and the isolate from Connecticut appears to belong to the C. gleosporioides complex, most closely related to C. aenigma (phylogenetic analysis not shown). The symptoms associated with the Colletotrichum species ranged from expanding stem cankers to purple leaf spots with ashy gray centers (Figure 2A-B, L). Salmon colored conidiomata were often observable within stem cankers (Figure 2B) and formed in concentric rings in lesions on foliage during pathogenicity trials.
Furthermore, multiple isolates of the same Phoma species were isolated from stem lesions from peonies grown in Alaska. The stem lesions from which the Phoma isolates were recovered were elongate, silver to ashy gray in color with a relatively wide, dark purple-red margins (Figure 2J). These isolates share sequence similarity to Phoma exigua (syn. Boremia exigua).
Lastly, Mycocentrospora acerina and Pilidium concavum were found on peonies in two and four states, respectively. Samples from which M. acerina was isolated from typically had small (1mm long), reddish-purple stem lesions scattered irregularly across stem tissue (Figure 2P), however, samples from one farm in Alaska had lesions caused by M. acerina that were more expanded and had dry gray centers (Figure 2K). M. acerina was also isolated from red spots on leaf tissue and from the sepals surrounding a closed bud. P. concavum was isolated from tan brown lesions from foliar tissue. Tan-orange to pink conidiomata could sometimes be observed within the lesion in concentric rings (Figure 2H), becoming black to dark brown upon tissue senescence.
Pathogenicity trials
Pathogenicity trials confirmed the ability of all organisms identified as first reports (Tables 1 and 2) to cause disease on peony consisting of symptoms like those observed on the original sample (Figure 2). A list of the isolates used for pathogenicity trials including GenBank accession numbers can be found in Table 2. In most cases, 5 of 5 inoculated tissues (wounded and unwounded) displayed symptoms, with successful re-isolation from the majority of lesions (data not shown). Lesions only developed on 4 of the 5 replicates for both wounded an unwounded tissues for the Colletotrichum spp. isolate from Oregon (HAM02) and the Botryosphaeria spp. isolate from New York (NY12). Lesions were produced on only 4 of 5 unwounded tissues for the Alternaria isolate from Washington (FR01) (pathogenicity tests were not conducted on wounded tissues). The Colletotrichum spp. isolate from Connecticut (CT03) appeared to have differences in virulence depending on peony cultivar. On ‘Kansas,’ CT03 was able to cause lesions characteristic of anthracnose infections on 4 of 5 wounded tissues and 2 of 5 unwounded tissues after 20 days, whereas symptoms only developed on 2 of 5 wounded and 1 of 5 unwounded tissues after 40 days. Lesions developed on tissue regardless of wounding for all isolates and wounding did not appear to influence the frequency of infection.
Discussion
From all 12 states from which samples were collected, at least one organism represented a first report on peonies in the state, with five of the genera collected representing first reports in the United States (Table 1). Although many of the isolates of Botrytis spp., G. chlorocephala, and Alternaria spp. represent first reports in the states in which they were found, these findings were not unexpected, as these diseases have been well known in the United States and were the focus of peony disease research in the 1930s and 40s. These pathogens are also regularly cited in modern extension bulletins and grower’s guides in the United States (see Missouri Botanical Garden 2012 as an example). Multiple species of Botrytis have been reported previously on peony in the United States and globally, however Botrytis isolates collected during this survey were not identified to species nor were Koch’s postulates performed as the diversity of Botrytis species is the subject of another project. The most recent literature describing G. chlorocephala on peonies, cause of the diseases “measles,” was reported in 1936. At that time, G. chlorocephala (formerly Cladosporium paeoniae) had been widely reported in 20 states and the District of Columbia. While reported in the United States, the only comprehensive pathogenicity trials for Alternaria spp. on peony prior to this study have been completed on tree peony (P. suffruticosa) in China. These studies involved three distinct species of Alternaria. Further studies are warranted on Alternaria spp. in the United States to determine which species are causing disease and their diagnostic symptoms, as the symptoms associated with Alternaria species in this study appeared quite variable in the field. Morphological examination of the Alternaria species indicated that there may be potentially more than one species may be present, as there were marked differences in spore size and colony color and growth habit.
New pathogen-host-state combination first reports were identified for a number of additional fungal and fungal-like pathogens including P. cactorum, S. sclerotiorum, and S. rolfsii. Although these pathogens have previously been reported in the United States on peony, reports are few. Furthermore, many of these reports were included in host-pathogen indices which did not include completion of Koch’s postulates. The exception was a Phytophthora spp. reported on peony in Pennsylvania in 1921. Although pathogenicity trials were conducted, this Phytophthora was not identified to species, so it was unclear whether or not it was the same species that was found in the present survey. Therefore, although the pathogens have been previously reported, the pathogenicity trials performed in this study represent the first time Koch’s postulates have been completed for these organisms on peony in the United States. These new state reports also help provide more resolution regarding the geographical distribution of these pathogens on peony, which is essential information for regional disease management and epidemic avoidance.
In addition to the state first reports, a number of pathogens were discovered that have never before been reported in the United States on peony. Pathogens reported here for the first time in the United States include: a Botryosphaeria sp., multiple Colletotrichum spp., M. acerina, a Phoma sp., and P. concavum. All of these pathogens have previously been reported on Paeonia elsewhere in the world, however, the number of reports are limited and often do not include symptom descriptions or formal pathogenicity trials. B. dothidea has been reported causing stem cankers in tree peonies in China and an unidentified Botryosphaeria has also been identified in tree peonies in Korea. As these reports are from tree peony, the present survey also represents the first report of a Botryosphaeria species causing leaf spots on the herbaceous peony (P. lactiflora). The pathogenicity of the single Botryosphaeria isolate collected from New York was confirmed using mycelial plugs, however the distribution and epidemiology of this pathogen under field conditions remains unclear.
Unidentified Colletotrichum spp. have been found in Korea and in Japan on tree peonies, but information is not available on the specific species of Colletotrichum causing disease or symptomatology associated with this pathogen. To our knowledge, pathogenicity trials for Colletotrichum species have not been performed on peony prior to the present study. Severe anthracnose was observed on the leaves, stems, and flowers of samples collected in Oregon and Connecticut during this survey, therefore, Colletotrichum spp. appear to be pathogens capable of causing significant economic damage in the United States. The most severe case of anthracnose observed during this survey was seen causing 100% losses in a block of ‘Edulis Superba’ peonies in Oregon (Fig 2A). Initial observations among the Colletotrichum spp. in the pathogenicity trials suggest that there may be varying abilities of these species to cause infection on peony that may be also cultivar- or phenology-dependent.
Pilidium concavum was isolated from multiple states in our surveys (Table 1). Some isolates appeared to be aggressive pathogens in our pathogenicity trials, with variable aggressiveness among isolates (data not shown). Despite their aggressiveness in the pathogenicity trials, P. concavum did not appear to be causing significant economic damage in any of the fields surveyed. First reports from China indicate P. concavum can cause a high incidence of foliar damage in tree peony, therefore, it may be important for growers and extension plant pathologists to monitor for the development of disease caused by P. concavum in the United States.
Of all the known pathogens of peony found in this survey, M. acerina is the one most recently discovered. M. acerina was first reported causing damage on herbaceous peonies in late 2015 in Chile. In their report, the Chilean scientists indicate the ability of this pathogen to infect all above and below-ground parts of the peony. Only above-ground infections were observed during the present survey, characterized by red spots on foliage, stems, and flower buds. In our survey, stems were often the most severely infected part of the plant (Fig 2K and 2P), however the reason is unclear. Stem infections could have been a result of inadequate fungicide coverage as compared with foliage or infection prior to full leaf expansion. High levels of M. acerina infection were identified at multiple locations in Alaska and Washington causing yield losses. The ability for this pathogen to infect the root means there may be potential for long-distance movement of the pathogen, therefore, monitoring for this disease, especially in rootstock production areas, may be important for maintaining the health of peony cut flower operations.
Phoma spp. have been reported on P. suffruticosa in China and Japan and on P. lactiflora in Korea. In the case of the Phoma spp. isolated from peonies in Alaska, the pathogen did not appear to be causing widespread yield losses, despite the obvious symptoms on some stems. The field from which the Phoma spp. was isolated was a relatively young (two year-old) field, therefore it is possible disease pressure could increase with inoculum build-up over time and increased canopy development and could become a problem in this field.
Many of the fungal pathogens identified on peony during this survey have relatively broad host ranges, therefore, numerous wild, cultivated, and landscape plant species could serve as alternative hosts and sources of inoculum. Species with wide host ranges include: S. sclerotiorum, S. rolfsii, M. acerina, P. cactorum, Phoma spp., Botryosphaeria spp., and Botrytis spp. Other fungal pathogens identified in this survey have relatively narrow host ranges, such as G. chlorocephala which has only been reported on Paeonia spp. Although the powdery mildews collected in this survey were not identified to species, powdery mildews are also typically considered host-specific. Due to this apparent host specificity, G. chlorocephala and powdery mildew species inoculum was likely to have originated on nearby peonies. Other fungi have been reported on a moderate number of hosts, such as the Colletotrichum spp. and P. concavum, which have been found on horticultural crops such as Malus, Prunus, Pelargonium, Vitis, and Fragaria, any of which could serve as alternative hosts. The source of inoculum for Colletotrichum on peonies may be species-specific. Reports of these pathogens are mostly from Europe with few in the United States, however, it is likely that the geographical and host distribution of many of these fungi are larger than indicated in the literature.
Although this survey provides valuable information on the range of pathogens present on peonies in the United States along with descriptions of disease symptoms, many questions remain about the epidemiology of these pathogens on peony. Further research on the biology and infection processes of these pathogens on peony are warranted to better inform disease management decisions, such as timing and mechanisms of spore dispersal, susceptible stages of the host, susceptibility of different cultivars, the efficacy of fungicides and fungicide application timing, and effective cultural control practices.
We have included the results of our study, however, the question of comparison with conventional systems does not apply for this project.
Research Outcomes
Education and Outreach
Participation Summary:
Consultations: Over 100 consultations with peony growers were made over the course of the study in the field, by email, telephone, or via the WSU Peony Research Facebook page (https://www.facebook.com/WSUpeonies/). The majority of the consultations were to answer questions that growers had about the health of their peony crop. Questions answered included disease diagnosis, diagnosis of physiological issues and injury to crops, and disease management recommendations.
Curricula, factsheets or educational tools: Three formal educational tools were developed as a result of this project. One factsheet on Tobacco rattle virus in peonies has already been published and can be found for free online at: http://extension.wsu.edu/publications/pubs/fs284e/. The bulletin has been shared publicly through our Facebook page (described above). Another factsheet on general plant disease management and diagnosis and management of peony diseases has been prepared and is in final preparation for submission to Washington State University Extension. The dissemination of this bulletin will be the same as that for the Tobacco rattle virus bulletin. Furthermore, a peer-reviewed journal article describing the results of this study has been prepared and is in review in the journal Plant Disease. The publication of this article will assist university-based extension personnel with peony disease diagnosis and monitoring. This project also resulted in a number of informal educational tools that were handed out to growers during field tours and extension presentations such as a factsheet on Mycocentrospora acerina, a fungus discovered in the United States on peonies as a result of this project and copies of PowerPoint presentations with color photographs of common disease symptoms on peonies and management recommendations.
On-farm demonstrations: The number of on-farm demonstrations is difficult to quantify, but exceeds 40 farms. In 2017 alone, more than 38 farms were visited in Alaska, 4 in Washington, 1 in Oregon. Additional on-farm demonstrations were made in 2016. On-farm demonstrations for this project includes visits with growers to scout fields for disease and discuss disease symptoms, development, and management strategies. These numbers do not include field tours, described below.
Online trainings: No online trainings were developed as a result of this project, however, online material is available for growers in the form of extension bulletins and the Facebook page (both described above).
Published press articles, newsletters: Two press articles have been made about our research, expanding the scope of knowledge about our research. The Washington State University College of Agricultural, Human, and Natural Resources team developed a news article related to the research conducted for this project. The article can be seen here: http://news.cahnrs.wsu.edu/blog/article/wsu-scientists-species-discovery-helps-save-colorful-peony-from-ugly-disease/. Furthermore, we have had an interview with Steve Elliott, communication coordinator at the Western Integrated Pest Management Center and another article is forthcoming.
Tours: We participated in the Arctic Alaska Peonies and Mat-Su Valley Peony field tours during the 2017 field season. At each field tour, we gave two presentations to two groups of approximately 50 people each regarding the results of our surveys of peony fields and introducing the bulletin we and collaborators produced on Tobacco rattle virus in peonies (described above). At these field tours, we also walked fields with growers to collect and show samples of specific diseases and physiological disorders which helps them to identify diseases independently.
Webinars, talks and presentations: We attended the Alaska Peony Grower's Association meeting in January of 2017 in Fairbanks, AK. During the main conference, at which there were approximately 100 participants, we also gave two research updates on the survey conducted as a result of this project and an update on ongoing fungicide trials on peony taking place in our lab at Washington State University. We also attended the American Phytopathology Society Pacific Division Meeting in Riverside, CA in June 2017 and gave a presentation entitled: "Multiple New Pathogens of Peony in the United States." This presentation was a part of the Graduate Student Competition at the meeting and was awarded first place which means that this presentation has been requested to be given again at the 2018 American Phytopathology Society meeting which will take place in Portland, OR. We also hosted and presented the results of this project at the 2017 Northwest Peony Society Conference ("Foliar Nematodes and Other Emerging Diseases of Peony") and the 2017 Wilbur-Ellis Professional Markets Technical Seminar ("2016 Peony Disease Survey Results").
Workshop/field day: We attended the Alaska Peony Grower's Association meeting in January of 2017 in Fairbanks, AK. At this conference, we gave a pre-conference workshop entitled "Peony Disease Identification and Management." The workshop lasted three hours and there were 25 participants. The workshop included the following topics: Plant Diseases 101, Fungicides: Understanding Efficacy, Learning to Read a Pesticide Label, and Common Peony Diseases in Alaska and Their Management.
Other: Described above.