Final report for GW19-200
Project Information
We investigated the frequency of secondary exposure to rodenticides in raptors that provide natural pest control on farms in the Western United States. In the agricultural landscape, the majority of a raptor’s diet consists of rodent pests, that can reduce yields and damage infrastructure. Integrated pest management (IPM) strategies for rodent pests frequently incorporate natural pest control provided by raptors, however, rodenticides are often applied concurrently. Rodenticide exposure can cause lethal or sub-lethal secondary poisoning in raptors, ultimately lowering the pest control they provide and increasing farmers’ need for rodenticide use.
We have little understanding of how rodenticide exposure in raptors varies across seasons and between species in agriculture. Furthermore, studies to date have focused primarily on spring and summer months for both understanding the role that raptors play in providing pest control and for monitoring rodenticide exposure—whereas both occur year-round. To address these knowledge gaps, we investigated rodenticide exposure rates in hawks and owls across multiple seasons and as a function of proximity to rodenticide application sites to answer two critical questions: 1) How does barn owl rodenticide exposure and diet vary over an annual cycle? 2) How do rodenticide exposure rates compare between diurnal and nocturnal raptors?
To accomplish this task, we collaborated with ongoing barn owl research projects (SW18-063), pest management specialists, raptor biologists, and producers who are interested in developing data-driven management recommendations for raptors and natural pest control in agriculture. We conducted extensive outreach to academics, professionals, farmers, and the general public, all who stand to benefit from the results of this study. We directly reached over 250 farmers and agricultural professionals with management recommendations and worked alongside of multiple land managers to develop and/or enhance barn owl box networks with the goal of increasing natural pest control in agriculture. Our ultimate objective is to enhance the understanding of simultaneous use of raptors and rodenticides in agriculture on a year-round basis and communicate IPM recommendations to a wide audience.
We tested the blood of hawks and owls for circulating anticoagulant exposure to detect recent sublethal exposure (from the past 1-2 weeks depending on dose and compound). The results of our study indicated low rates of acute anticoagulant rodenticide exposure in barn owls across seasons, although we also documented low rodenticide use at our study sites during the study period. We found that non-breeding red-tailed hawks were exposed to anticoagulant rodenticides more frequently than barn owls at our study site, with red-tailed hawks across all years having a 15% (5/34) positivity rate. While these results suggest low acute exposure rates to anticoagulant rodenticides in raptors on farms, these results must be interpreted with caution. Because we wanted to study the interaction between raptors and the normal pest management approaches on farms, we did not influence the applications of rodenticides, and therefore sampling did not necessarily align with locations or timing that baiting occurred. Further research is needed where sampling is closely coordinated with the timing and locations of field-scale baiting or in landscapes with higher prevalence of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
Objectives:
- Quantify differences in rodenticide exposure frequencies in wintering and breeding barn owls.
- Quantify differences in rodenticide exposure frequencies between wintering diurnal (red-tailed hawks) and nocturnal raptors (barn owls).
- Describe the diet of overwintering barn owls.
- Create stakeholder-verified recommendations for the use of rodenticides and raptors for effective IPM systems for rodent pests, in conjunction with Kross et al. ongoing barn owl–rodenticide research.
- Publicize findings through to the scientific community, producers, and the general public through presentations, publications, and field workshops.
Our project is closely coordinated and in collaboration with an ongoing Western SARE project (SW18-063) investigating impacts of barn owls and rodenticide exposure on sustainable agriculture in the breeding season. Our project adds two additional components to the ongoing project. First, it will help provide a complete year-round picture of the interaction of rodenticides and raptors in an agricultural context. Second, it will provide a comparison of rodenticide exposure rates in a second group of raptors (diurnal red-tailed hawks). Diurnal raptors provide a large portion of pest control services on farms, especially in winter months, and may experience different rodenticide exposure rates than nocturnal raptors.
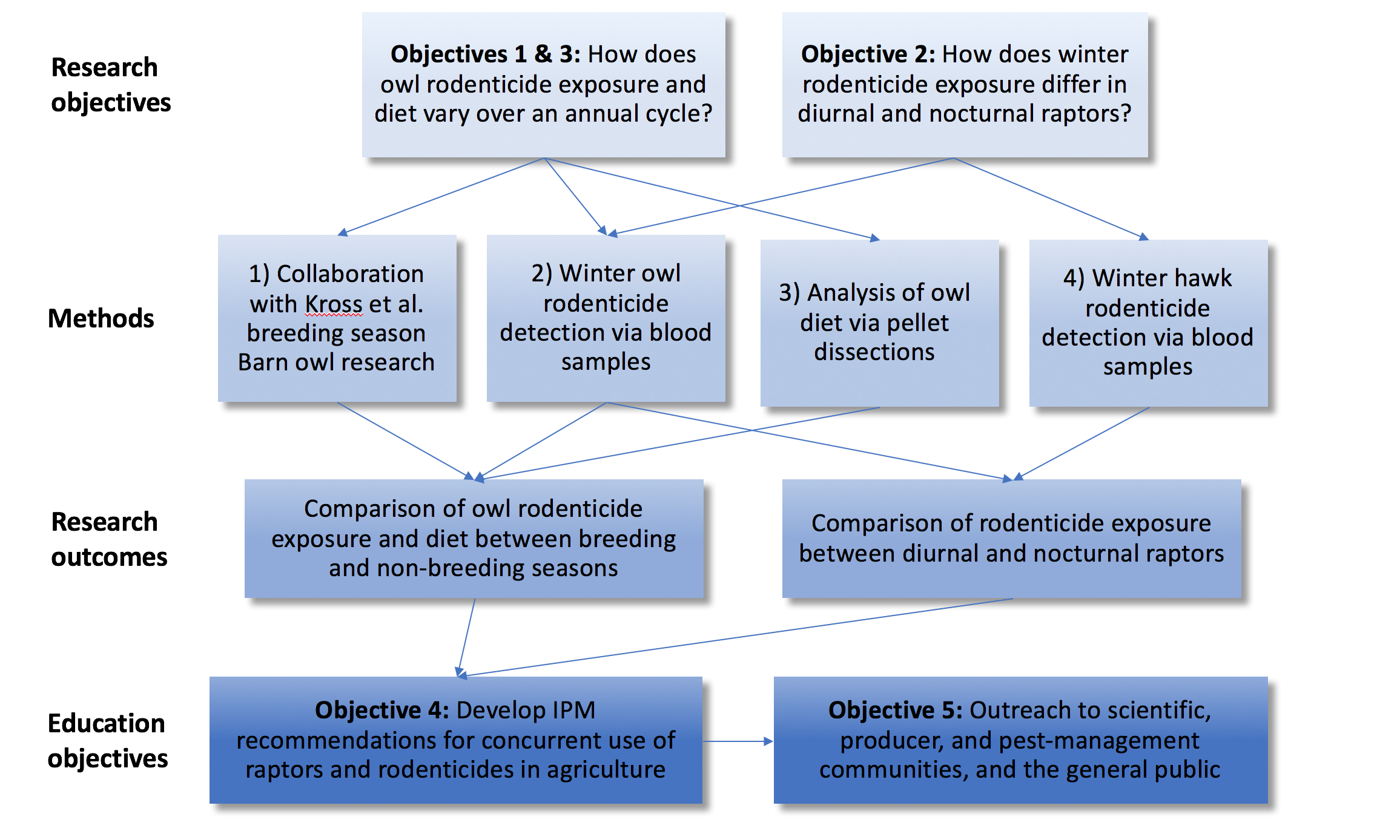
Figure 2: Flowchart of interaction between project objectives, methods, and outcomes.
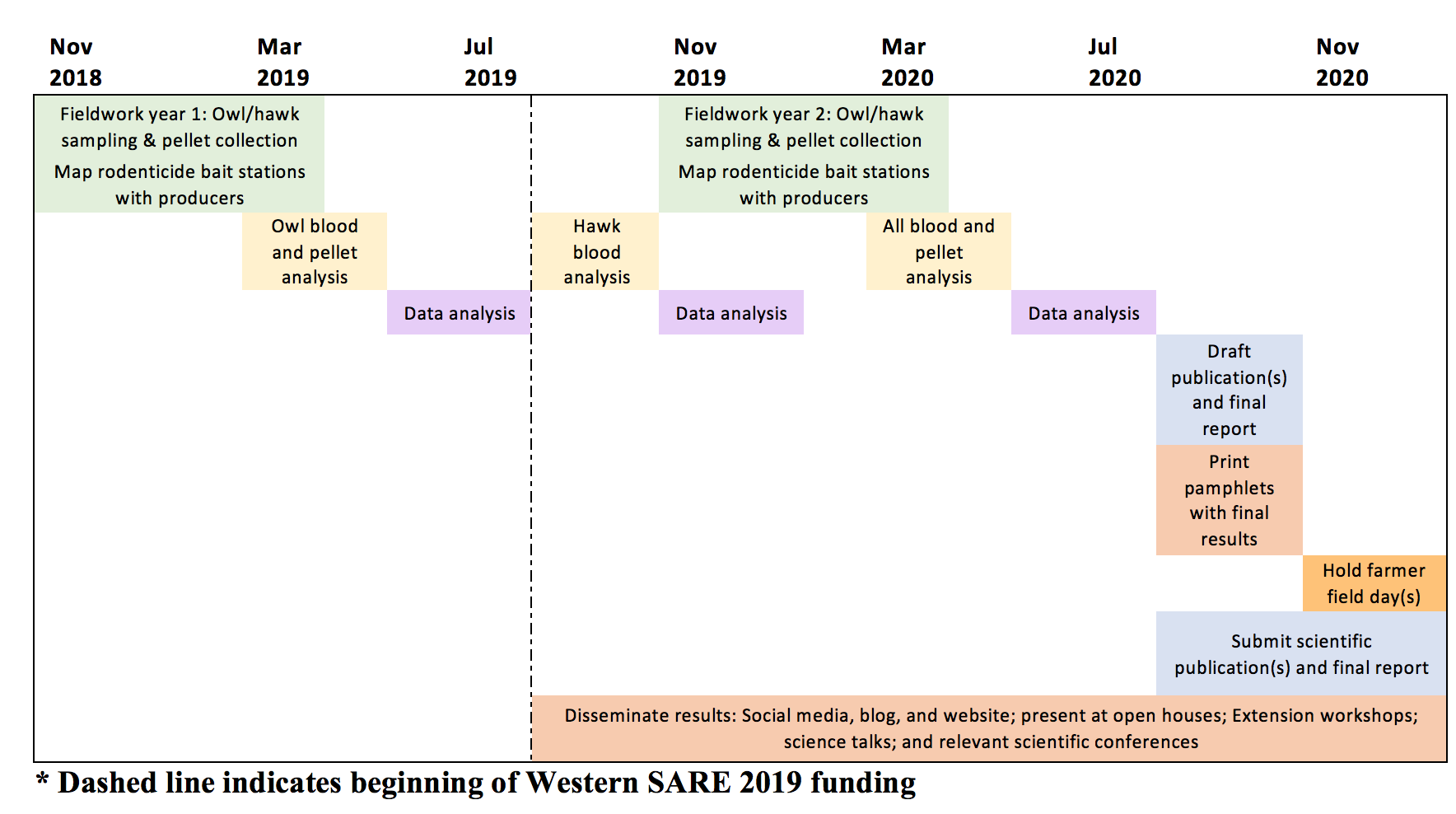
My proposed starting and ending dates are September 1, 2019 through December 31, 2020. This project will use samples collected over two field seasons, however the funding from the Western SARE Graduate Student Grant will be used to cover all costs from the second field season in addition to the cost to screen hawks for rodenticide exposure from the first field season. The vertical dashed line in the timeline below indicates the approximated beginning of the funding period in 2019.
- During fall 2019, hawk samples will be sent to the lab for rodenticide screening and supplies for the second field season will be purchased. Data analysis will begin once results are received.
- From mid-November to mid-March a second year of field work will be conducted including blood sampling from both hawks and owls and owl pellet collection. During this time, we will also work with producers to map the date, location, and types or rodenticides applied to the landscape.
- Spring and summer 2020 will consist of rodenticide screening, pellet analysis, and data analysis. Once results are finished, these will be used to develop scientific publications, final report, and pamphlets/fact sheets.
- In winter 2020 official farmer field day will be held, publication(s) and the final report will be submitted. Throughout the duration of the project, research will be publicized though outlets described Education Outreach Plan.
Figure 3: Timeline for completing proposed project.
Cooperators
- - Technical Advisor
- - Technical Advisor
- - Producer
- - Producer
- - Producer
Research
All work was conducted under appropriate federal (# 23406) and state permits (# 1259). Field sampling protocols were approved by UC Davis Institutional Animal Care and Use Committee (IACUC # 20516). Rodenticide screenings took place at Texas A&M Veterinary Medical Diagnostics Laboratory via measurement by liquid chromatography/mass spectrometry (LC/MS), which detects occurrence and measures quantity of first- and second-generation anticoagulant rodenticides (Warfarin, Bromadiolone, Brodifacoum, Diphacinone, Chlorophacinone, Coumatetralyl, Difenacoum, and Difethialone).
1) Collaboration with Kross et al. (SW18-063) breeding season research (Objective 1)
To investigate differences between rodenticide exposure in the breeding and non-breeding season of barn owls utilizing nest boxes on farms, we worked in collaboration with project SW18-063 which operated in the years 2018-2021. Together we worked to collect over 250 rodenticide screening samples from breeding barn owls on northern California’s agricultural landscapes. The rodenticide exposure rates gathered from these tests will allow us to compare rodenticide exposure rates between breeding and non-breeding barn owls. Additionally, diet analyses of breeding barn owls done under SW18-063 will allow us to compare to the non-breeding barn owl diet studied as part of this project.
2) Winter barn owl rodenticide exposure (Objectives 1 & 2)
To investigate the rate at which non-breeding barn owls (Tyto furcata) on farms are exposed to rodenticides, we searched nest boxes for roosting barn owls during the months of September through February in the years 2018-2019 and 2019-2020 at the same sites that research took place for SW18-063. The study sites were in Yolo County comprised of large-scale vineyard and olive orchards with remnants of natural habitat (grassland or riparian) in and around the farm fields. Wild barn owls were not sampled in the 2020-2021 winter due to limited access and staffing due to COVID-19 restrictions.
We monitored nest boxes using a wireless camera (SONY Action Cam) on an extendable pole to look for roosting adult barn owls. For female owls found roosting with males, we palpated their abdomen to check for signs of breeding, if egg development was detected the individual was not sampled. We banded all birds that were screened for rodenticide exposure with the appropriate federal USGS leg band for identification. We did not sample the same individual twice within 14 days.
We tested for circulating rodenticide exposure by sampling whole blood and measured with LC/MS. By testing whole blood (as opposed to liver tissue) we can investigate sub-lethal exposure in living birds that has occurred within the past 1-2 weeks approximately, depending on dose consumed and the compound type. We collected blood samples from non-breeding owls via medial metatarsal venipuncture and drew 1-1.25mL of blood (well below the maximum amount allowed by IACUC guidelines). Blood samples were stored in EDTA tubes on ice in the field and transferred to cryotubes for short term storage in a freezer before shipping to the lab for screening.
We opportunistically collected carcasses of any barn owls found near roads adjacent to our study sites. These barn owls were sent to the California Department of Fish and Wildlife, Wildlife Investigations Laboratory for necropsy and sample collection. We collected heart blood, if present and liver tissue samples. Additionally, due to obtaining small sample sizes from wild populations, we partnered with rehabilitation facilities to get blood samples from barn owl patients (upon stabilization) that were collected from agricultural areas.
To learn about rodenticide usage at our study sites, we spoke with land managers to document anticoagulant rodenticide use (location, timing, and compounds) at the building- and field-scale.
3) Winter hawk rodenticide exposure (Objective 2)
In non-breeding months (November through February) in the years 2018-2019, 2019-2020, 2020-2021, we sampled red-tailed hawks (Buteo jamaicensis) found foraging in agricultural fields at our study sites. We chose to only conduct hawk rodenticide screenings in winter, as the diurnal raptor population increases during these months due to an influx of migratory and over-wintering raptors on the landscape. Red-tailed hawks were categorized as immature (birds hatched the previous spring/summer) or adult (birds that had hatched in a year before the previous spring/summer). Age class was discerned by evaluating plumage, those with brown tails were considered immatures and those with red tails were considered adults. In this species, brown tail feathers are replaced with the characteristic red tail feathers after an individual’s first winter.
We tested for circulating rodenticide exposure in living individuals via testing whole blood, opportunistically collected carcasses, and partnered with rehabilitation facilities with the same methods used for barn owls (see above). Rodenticide use information was also collected in the same way as listed above.
4) Winter barn owl diet analysis (Objective 3)
We categorized the diet composition of barn owls in non-breeding months to elucidate rodenticide exposure pathways and to investigate differences between the non-breeding and breeding diet of barn owls in collaboration with SW18-063. To analyze non-breeding barn owl diet, we collected fresh pellets found inside nest boxes of roosting owls that were sampled for rodenticide exposure. We dissected pellets and used prey remains to determine species composition and the minimum number of individual prey items in a pellet.
To aid in estimates of biomass consumed, we measured jaw bones of voles and gophers, as individuals from different age classes are frequently consumed by barn owls. We categorized prey into the following groups: mouse (Mus musculus, Peromyscus maniculatus, or Reithrodontomys megalotis), vole (Microtus californicus), gopher (Thomomys spp.), rat (rattus spp.), shrew (Sorex spp.), songbird (Passeriformes), and field cricket (Gryllus spp.). We used the following calculations to determine biomass: mouse x 20.2g (the average mass of mice trapped on site, Phillips unpublished data), vole: ln(vole mass) = 3.154 ln (mandible length) – 5.015 (for degraded jaws the average mass was used), gopher: log (gopher mass) = 3.49 log(mandible length) – 2.73 (for degraded jaws the average mass was used), shrew x 5.8g, bird x 60.5g, and cricket x 2g (Kross et al. 2016).
Because red-tailed hawks do not readily produce pellets that can be collected to determine diet and incidental observations cannot be compared in a systematic way, we collected beak and talon swab samples from red-tailed hawks, a proven method to determine recent prey items of a raptor by analyzing trace prey DNA with metabarcoding techniques (Bourbour et al. 2019). However, the processing of these diet swabs is not currently funded and are stored until further funding can be secured. The information gained by analyzing these swab samples can be used to compare winter hawk and owl diet on the same landscapes and reveal potential rodenticide exposure pathways.
Rodenticide screening results:
We tested non-breeding barn owls for circulating anticoagulant rodenticides and did not detect exposure to any anticoagulant rodenticide compounds in 54 samples taken across two winter field seasons. In collaboration with SW18-063, we tested over 250 breeding barn owls and only detected one positive sample in a barn owl nestling (trace levels of Chlorophacinone, Phillips unpublished data). Chlorophacinone, a first-generation anticoagulant rodenticide often used in or along field edges, was not used during the wintering sampling months at our study sites.
We sampled the blood of one barn owl from a rehabilitation facility and tested the paired liver and heart blood from three adult owl carcasses found on roads adjacent to our study site. There were no positive results detected in any of the blood or tissues from barn owl carcasses nor in the individual sampled from the rehabilitation facility. Sample sizes of wild, rehab, and carcasses are listed below.
We tested for circulating anticoagulant rodenticides in a total of 34 wild red-tailed hawks hunting on farms in the same locations barn owls were sampled. In 2018-2019 one or multiple second-generation anticoagulant rodenticide compounds were detected in the blood of 5 wild individuals at trace levels (<5 ppb). In the 2018-2019 winter, 36% (5/14) hawks tested positive for 1-2 second-generation anticoagulant rodenticides. Bromadiolone, a second-generation anticoagulant rodenticide, was the only compound we documented use of at our study sites and was present in three of the positive individuals. Two other second-generation compounds were present in three of the positive individuals, indicating exposure off site. Two individuals were positive for two compounds which may represent two separate exposure events per individual.
One carcass of a juvenile red-tailed hawk was collected and tested for exposure to anticoagulant rodenticides with liver tissue and heart blood. This individual was negative in the heart blood, but positive in the liver tissue for Bromadiolone at 51 ppb, suggesting exposure occurred more than 1-2 weeks prior to death. The results of the necropsy indicated that anticoagulant rodenticide exposure was not the apparent cause of death. We tested the blood of 5 red-tailed hawks in rehabilitation facilities and had two positive detections. One individual tested positive for trace amounts of Difethialone, and a second blood sample 7 days later yielded a negative result. The second individual was identified as a suspected poisoning case by veterinarians on site. Despite being tested 15 days after admission, the individual was positive for trace levels of Brodifacoum. The sample sizes, years, ages, and compounds found are listed below.
Land managers of field sites where wild raptor sampling took place indicated that Bromadiolone was administered year-round by professional pest control companies in and around all buildings (offices and processing facilities). Buildings were sparse on the sites, with buildings clustered in one spot on approximately 2,000-5,000 acres of cultivated and natural lands. Field-scale anticoagulant rodenticide use did not take place during the months and years that this study took place. Field-scale baiting typically occurred in spring and/or summer on an as needed basis. Unknown anticoagulant rodenticide use on neighboring properties was likely occurring, as two second generation compounds not applied at the properties we worked at were present in 3/5 of the positive red-tailed hawk samples.
Barn owl samples
2018-2019 wild blood samples: 30
2019-2020 wild blood samples: 24
Rehab blood samples: 1
Carcass paired liver and blood samples: 3
Red-tailed hawk samples
2018-2019 wild hawks sampled: 9 immature, 5 adults
2019-2020 wild hawks sampled: 5 adults
2020-2021 wild hawks sampled: 13 immature, 2 adults
Rehab blood samples: 5 mixed age
Carcass paired liver and blood: 1 immature
Compounds detected in red-tailed hawks by age – (Concentrations found in trace amounts <5 ppb unless indicated)
Wild:
Bromadiolone: 2 immatures
Difethialone: 1 immature
Bromadiolone + Difethialone: 1 immature
Brodifacoum + Difethialone: 1 adult
Rehab:
Difethialone: 1 immature (tested negative 7 days after intake)
Brodifacoum: 1 immature (positive after 15 days in rehab facility)
Carcass:
Bromadiolone: 1 immature (51 ppb liver; negative heart blood)
Summary of wild red-tailed hawk blood samples screened for rodenticide exposure by year and age class. Number of positive tests in each category indicated, with percent positive in bold.
|
|
Adult |
Immature |
Total |
|
2018-19 |
20% 1/5 |
44% 4/9 |
36% 5/14 |
|
2019-20 |
0/5 |
-- |
0/5 |
|
2020-21 |
0/2 |
0/13 |
0/15 |
|
Total |
8% 1/12 |
18% 4/22 |
15% 5/34 |
Winter barn owl diet results:
We collected 226 whole pellets, 198 from 2018-2019 and 28 from 2019-2020. The most abundant prey items detected were mice, making up 51% of the total biomass consumed. The second and third largest groups were voles and rats, making up 20% and 18% of the total biomass consumed, respectively. These results will be compared to the breeding diet being analyzed for SW18-063.
Barn owl breeding season diet published in Kross et al. 2016, a study conducted in the same county as our study sites, is presented for preliminary comparisons. We found that compared to Kross et al. 2016, the proportion of mice and rats consumed was higher and the proportion of voles and gophers consumed was lower in non-breeding months than in the breeding season.
Summary of number of prey items found from each group, estimated biomass, and proportion of biomass in diet. Proportion of biomass found in the breeding season analyses in Kross et al. 2016 shown for comparison.
|
Prey species |
Min # of individuals |
Est. biomass (g) |
Prop biomass |
Breeding prop biomass (Kross et al. 2016) |
|
Mice |
604 |
10056.6 |
0.51 |
0.38 |
|
Vole |
87 |
3923.6 |
0.20 |
0.37 |
|
Gopher |
11 |
988.5 |
0.05 |
0.13 |
|
Black rat |
10 |
3550.0 |
0.18 |
0.04 |
|
Shrew |
2 |
11.7 |
0.00 |
0.001 |
|
Field cricket |
81 |
162.0 |
0.01 |
0.003 |
|
Bird |
14 |
847.0 |
0.04 |
0.08 |
Discussion:
One of our main objectives was to investigate whether barn owl rodenticide exposure varied over the annual cycle on the agricultural landscape in collaboration with SW18-063. We detected low exposure rates across seasons and documented low rodenticide use at our study sites. While these results suggest low acute exposure to anticoagulant rodenticides in barn owls nesting on farms, these results must be interpreted with caution. For example, we know that there was low usage of field-scale rodenticides at our study sites and baiting that occurred at the field-scale did not always align with the locations or times that barn owls were sampled. We intended to study the interaction between raptors on farms and the normal pest management approaches taken by land managers, therefore, we did not influence the applications of rodenticides. Further research should be done where sampling occurs simultaneously with field-scale baiting or in landscapes with higher prevalence of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
Our second main objective was to investigate whether differences in rodenticide exposure rates were present in nocturnal and diurnal raptors that hunt on farms. We sampled non-breeding barn owls (nocturnal) and red-tailed hawks (diurnal) and found that red-tailed hawks were exposed to anticoagulant rodenticides more frequently than barn owls at our study site. Red-tailed hawks across all years of the study had a 15% (5/34) positivity rate. We also found that the positivity rate was higher in the immature age class, as 4/5 positive samples came from immature hawks. Vulnerability to anticoagulant rodenticide exposure in younger hawks may be due to their inexperience with hunting and targeting easier prey that may be weakened from exposure to rodenticides. Differences in anticoagulant rodenticide exposure rates between barn owls and red-tailed hawks could arise from differences in foraging location, foraging time, diet composition, physiology, or changes in prey behavior after consuming rodenticides.
All rodenticides detected in red-tailed hawks were second-generation compounds, which are typically used around buildings for commensal rodents and are not approved for use at the field-scale. These findings suggest that commensal rodents (i.e., mice and rats) may be the pathway to exposure for wintering hawks in this system when other prey is less common, such as California ground squirrels. While we do not know the diet composition of red-tailed hawks in this study, we do know that non-breeding barn owls consumed a higher proportion of mice and rats in the non-breeding season compared to what they might consume in the breeding season. This may be due to lower abundances of dispersing juvenile gophers and voles in winter months. These findings may indicate differences in anticoagulant rodenticide exposure between non-breeding barn owls and red-tailed hawks are a result of differences in hunting location, time, or rodent behavior, rather than diet, although we did not directly test these hypotheses in this study.
This study was designed to detect acute anticoagulant rodenticide exposure in living populations of hawks and owls on the agricultural landscape. With our study design, we expected to find lower rates of anticoagulant rodenticide exposure than studies that tested liver tissue from carcasses or rehabilitation birds. Studies utilizing carcasses or rehabilitation birds have found much higher rates of exposure due to the length of time that exposure can be detected in the liver compared to the blood, and/or by sampling a from a group of individuals that have suffered a trauma or have symptomatic signs of poisoning. We cannot infer that individuals in this study had not been previously exposed to rodenticides nor can we infer that they did not suffer any negative sub-lethal effects as a result of exposure, as this was not tested in the study. Our study provides valuable information on the interaction between raptors and rodenticides used in agriculture and will be informative to future research in similar systems.
We intend to develop a set of management recommendations based on these results in conjunction with SW18-063. The results of this study will be included in my dissertation in the Ecology program at UC Davis and will be submitted to a scientific journal for peer review.
Research Outcomes
Education and Outreach
Participation Summary:
I have been able to reach a broad audience from academics and agricultural professionals, to a diverse array of farmers with preliminary results from this study through various presentations and demonstrations. I have also been able to reach farmers and members of the general public with information about boosting natural pest control on farms through presentations, consultations, and open houses. Due to COVID-19 several talks and open houses were canceled, postponed, or moved to a virtual platform. Despite restrictions and changes, I was able to hold a small field demonstration and present virtually at international conferences, and at community educational events.
Other ways I have been disseminating information include a Western SARE Quick Guide on natural vertebrate pest control, a factsheet about barn owls created for and distributed to winery customers, and a short video in partnership with Wild Farm Alliance in place of several canceled talks (See below in Education and Outreach Activities for a full list of activities). The results of this work will be included in my dissertation in the Ecology program at UC Davis and will be the focus of a publication in a peer reviewed journal. Our findings will be presented in conjunction with any final field-day activities associated with SW18-063.
Another important part of my educational goals included creating space to mentor students on aspects of the project and having students participate in research and education activities. During the scope of this project I worked with 7 undergraduate students from Sacramento State and UC Davis as field interns. The opportunities they had working on this project allowed them to develop their interests, skills, and experience which was critical for them to obtain related jobs and acceptance to graduate and vet school.
Consultations:
- 7/2020 – Checked occupancy and status of local land owner’s Barn owl boxes we advised about installation last winter. Discussed natural pest control, species consumed by Barn owls, and our current research.
- 12/2021 – Spoke with a grower relations representative for Napa Valley winegrowers about best practices to integrate barn owl boxes on the properties he represents. Discussed nest box number, placement, monitoring, and natural pest control.
Curricula, factsheets, educational tools:
- 5/2020 – Helped develop a factsheet/coloring sheet on barn owl be distributed to winery customers to educate their customers on the importance of barn owls on their property (over 100 customers).
- 3/2021 – Western SARE How-To Quick Guide: Welcome in Barn Owls to Provide Rodent Control. Summarizes information for farmers and land managers that want to encourage barn owl to nest on their property. https://western.sare.org/resources/welcome-in-barn-owls-to-provide-rodent-control/
Journal articles:
- 2020 – eOrganics journal article published: Promoting beneficial raptors: identification, pest control services, and management. Contributed by co-authoring sections on barn owls and red-tailed hawks. https://eorganic.org/node/34052
- 2021 – Journal of Wildlife Management journal article in review: Banding records of nestling barn owls inform timing for nest box management in California.
- Planned 2022 – Planned peer-reviewed publication and dissertation chapter for results of this project: Anticoagulant rodenticide exposure rates in non-breeding raptors that provide natural pest control on farms.
On farm demonstrations:
- 5/2019 – Group of 12 6th graders from Woodland, CA came to one of vineyard study sites to learn about barn owl natural history and role in natural pest control in agriculture. We were accompanied by the vineyard manager who also talked about pest control and growing vines from his perspective.
- 12/2019 – Visiting international pest management research professional from the Julius Kühn-Institut in Germany came to learn about Barn owl boxes, placement, and role of Barn owls and other raptors as natural pest control in agriculture.
- 6/2020 – 7 grade school aged children from Sacramento, CA visited our study site and learned about barn owl natural history and role as natural pest control in agriculture.
- 6/2020 – Local land owner/farmer from Dixon, CA came to our study site to discuss barn owl box management, placement, and role of barn owls and other raptors as natural pest control in agriculture.
Online trainings - 0
Published press/newsletters:
- 10/2021 – Interviewed for an article in Bay Nature regarding barn owls and rodenticide exposure. https://baynature.org/article/raptors-rather-than-rodenticide/
Tours - 0
Webinars, talks, presentations:
- 3/2019 – Presentation: UC Merced/UC Agriculture and natural resources, Continuing Education for Pest Management Professionals, Merced, CA - The use of barn owls and other raptors in rodent control (75 participants) http://cemerced.ucanr.edu/Continuing_Education_for_Pest_Management/
- 11/2019 – Poster presentation: The Wildlife Society, Wildlife Disease & Toxicology Workshop, Sacramento, CA. Sublethal anticoagulant rodenticide exposure in red-tailed hawks (100 participants) https://tws-sacshasta.org/media/pdf/W/i/l/Wildlife-Disease-Agenda-Abstracts-booklet_final-Google-Docs.pdf
- 6/2019 – Presentation: American Ornithological Society conference, Agroecosystems Symposium, Anchorage, AK. Raptors and vertebrate pest control in agriculture https://americanornithology.org/media/pdf/A/O/S/AOS2019_AbstractBooklet_v3-1.pdf
- 7/2020 – Virtual presentation: North American Congress for Conservation Biology. Raptors, Rodenticides, and Rain in California’s Agroecosystems http://scbnorthamerica.org/index.php/naccb-2020/
- 8/2020 – Virtual presentation: North American Ornithological Conference, invited Lightening symposium: Protecting and restoring bird habitat in the agricultural matrix: net benefits for birds and farmers. Raptors, Rodenticides, and Rain in California’s Agroecosystems https://naocbirds.org/
- 9/2020 – Wild Farm Alliance short video: Farming with nature is the solution. Video details how farmers can support birds and benefit from natural pest control services. (773 views) https://www.wildfarmalliance.org/farming_with_nature_is_the_solution
- 5/2021 – Virtual presentation: California Raptor Center Virtual Open House– Raptors and non-toxic vertebrate pest control. (65 participants)
- 3/2021 – Virtual presentation: Wild Farm Alliance Virtual Field Day– Raptors and non-toxic vertebrate pest control in agriculture. (95 participants)
- 10/2021 – Virtual presentation: Raptor Research Foundation annual conference– Anticoagulant rodenticide exposure in raptors in California’s agroecosystems.
- 12/2021 – Virtual presentation: University of California Agriculture and Natural Resources continuing education workshop– Managing burrowing rodents on the organic farm. Barn owls and raptors as natural pest control in agriculture. (85 participants) https://files.constantcontact.com/76b51227701/8fb43088-443e-415e-956a-20e063addd07.pdf
Workshop/field day:
- 6/2019 – AOS Agroecosystems workshop, Anchorage, AK, discussed net benefits of ecosystem services provided by raptors in agriculture and future research directions and possibilities.
- 3/2020 – Field day with Sonoma County Wildlife Rescue, Sonoma, CA, to discuss and teach field research techniques and protocols for rodenticide screening samples from wild Barn owls for collaboration on breeding work.
Other educational activities:
- 5/2019 – California Raptor Center open house booth with material discussing Barn owls and other raptors on farms. (approx. 225 participants)
- 2/2020 – Peregrine Elementary School, booth at Women in Science, Davis, CA – Discussed the beneficial role of raptors in agriculture and perspectives on being a woman scientist. (approx. 250 participants)