Final report for GW20-207
Project Information
The leaffooted bug, Leptoglossus zonatus, is an important pest of tree nut crops in California. In pistachio, feeding from leaffooted bug can lead to significant damage and loss of nut crops. Given the pest’s rapid and unpredictable movement into orchards, and lack of effective monitoring tools to detect such migrations, growers often rely extensively on pesticide applications to control the pest, commonly at first perceived risk of pest activity. This management approach can be both economically costly for growers and environmentally unsustainable. In 2018-19, our group initiated a collaborative project with a large tree nut producer to explore the use of summer cover crops as an integrated pest management (IPM) strategy for large hemipteran pests. Over a two-year period, we assessed the influence of irrigated groundcover strips in the row middles on pest damage and biological control of leaffooted bug.
In this study, a combination of field and laboratory studies were used to evaluate the effect of flowering groundcovers on the reproductive fitness of the leaffooted bug egg parasitoid, Hadronotus pennsylvanicus, and the abundance of its host L. zonatus in pistachio. Evaluated groundcovers included oat (Avena sativa L.), cowpea (Vigna unguiculata L.), white mustard (Sinais alba L.), and buckwheat (Fagopyrum esculentum Moench). Under laboratory conditions, buckwheat provided the greatest benefits to female H. pennsylvanicus longevity. While all floral diets improved female fecundity under laboratory conditions, there was no difference in the total offspring produced per female. In field trials, flowering groundcovers did not influence the abundance of H. pennsylvanicus nor parasitism rates of L. zonatus. While the availability of diet can improve the lifetime reproductive fitness of H. pennsylvanicus, the addition of groundcovers in a pistachio orchard did not enhance populations of H. pennsylvanicus nor biological control of L. zonatus. Findings from this study suggest that the use of flowering groundcovers to improve biocontrol in pistachio is likely not a viable solution to sufficiently suppress L. zonatus populations and meet the current production demands of California tree nut production.
Objective 1: Evaluate egg parasitoid Hadronotus pennsylvanicus as a biological control agent to leaffooted bug Leptoglossus zonatus.
Objective 2: Measure the impact of diet quality on life parameters of leaffooted bug parasitoid Hadronotus pennsylvanicus.
Objective 3: Evaluate the influence of groundcovers on biological control of leaffooted bug in pistachio.
(Objective 3A) Monitor seasonal abundance of leaffooted bug and their key natural enemies in pistachio with and without groundcovers.
(Objective 3B) Assess parasitism and predation of leaffooted bug in plots with and without trap crops.
Aug 2020 - Feb 2021: Lab assays on host-plant preference and insect life parameters (Obj. 1-2).
Jan 2021: Meet with growers and coordinate seeded ground covers at field sites.
Feb 2021: Coordinate with growers on seeding identified ground covers into orchard.
Mar 2021 – Oct 2021: Field sampling to assess spatial and temporal insect abundance (Obj. 3A).
Mar 2021 – Oct 2021: Monitoring biological control using sentinel egg clusters (Obj. 3B).
Oct 2021 – Nov 2021: Determine spatial crop damage in sites during harvest (Obj. 3C).
Nov 2021 - Dec 2021: Clean, analyze, and visualize data.
Jan 2022: Communicate YR 1 trap crop data with collaborating growers.
Feb 2022: Coordinate and implement YR 2 cover crops with collaborating growers.
Jan 2022 – Apr 2022: Finalize analyses, write manuscript, and prepare report for growers.
Apr 2022 – Jul 2022: Disseminate results to growers through outreach and extension events.
Cooperators
- - Producer
- - Producer
- - Producer
- (Researcher)
Research
Insect colonies
Insect colonies were reared from specimens collected in Fresno County, CA. Leaffooted bugs Leptoglossus zonatus were fed a diet of zucchini (Cucurbita pepo) and green beans (Phaseolus vulgaris). Hadronotus pennsylvanicus were reared from host eggs of leaffooted bug with 1:1 honeywater diet. Wasps were reared in 50ml plastic centrifuge tubes sealed at open end with 250mm mesh. Colonies and laboratory bioassays were conducted in controlled conditions maintained at standardized 25°C ±1, 75 ±5% RH and 16:8 L:D.
Objective 1: Evaluation of Hadronotus pennsylvanicus as biocontrol agent to Leptoglossus zonatus
To measure adult longevity and fecundity of H. pennsylvanicus, newly emerged (<24 h) males and females (n=120) individuals were housed in mating pairs in 10ml plastic tubes closed with a mesh cap. Tubes with mating pairs were randomly assigned to one of the following treatments: a) water and 25 fresh (<72 h) L. zonatus eggs (“water & host”; n=20), b) honey-water solution and 25 fresh host eggs (“honey-water & host”; n=20), or c) honey-water solution and no host eggs (“honey-water”; n=20). Dietary treatments (water or honey-water solution) and host eggs were replenished every other day until the female wasp expired. A new male specimen was introduced to the tube to replace any expired males to maintain copulation potential throughout the study. Following 24hr exposure to the parasitoids, host eggs were removed and observed daily for the occurrence of parasitoid emergence or L. zonatus nymph hatch. Eggs that neither hatched nor yielded a parasitoid were dissected to look for the presence of a dead L. zonatus or dead H. pennsylvanicus, and were determined aborted if neither occurred. Observations on age-specific survival, longevity and fecundity were used to calculate life table parameters for H. pennsylvanicus on host L. zonatus (rm, intrinsic rate of increase; λ, finite rate of increase; T, mean generation time; Td, doubling time; R0, net reproductive rate; GRR, gross reproductive rate).
Objective 2: Diet quality on life parameters of Hadronotus pennsylvanicus
Effects of candidate trap crop species on the fecundity and longevity of H. pennsylvanicus was assessed (Fig.1). Newly emerged H. pennsylvanicus individuals, one male and one female, were placed together in a rearing chamber containing the inflorescence of one of the following diet treatments; oat (Avena sativa L.), cowpea (Vigna unguiculata L.), white mustard (Sinais alba L.), and buckwheat (Fagopyrum esculentum Moench), water-only, or honey-only. The stem of each inflorescence was submerged in a container of water with the rearing chamber, and replaced every other day to ensure nectar availability to wasp. After 24hr of exposure to diet treatment, a set of 25 leaffooted bug eggs (<72hrs old) were provided for parasitism. Eggs were replaced every other day until the female parasitoid expired. A new male specimen was introduced to the rearing chamber to replace any dead males and maintain copulation potential throughout bioassay. Following exposure, leaffooted bug eggs were removed and observed daily for the emergence of either parasitoids or leaffooted bug nymphs. Observations on age-specific survival, longevity and fecundity will be used to calculate life tables for H. pennsylvanicus.
Fig. 1 Egg parasitoid Hadronotus pennsylvanicus feeding on buckwheat nectar.
Objective 3A: Groundcover influence on leaffooted bug and natural enemies
After preliminary trials and conducting a literature review regarding attraction to hemipterans, beneficial biological control agents, as well as feasibility for use in pistachio systems, it was decided to test four different plant species in ground cover treatments: oat (Avena sativa L.), cowpea (Vigna unguiculata L.), white mustard (Sinais alba L.), and buckwheat (Fagopyrum esculentum Moench). Strips containing resident weedy vegetation were used as controls. To test the role of selected treatment groundcovers, we used a randomized block design containing five replicate blocks with six plots each (30 plots total). Each plot contained three consecutive rows wide with 180 trees per row (Fig. 2-3). All groundcover treatments were seeded at the beginning of March 2020. Sampling of insects took place every 2-weeks between April – October 2020. Ground covers were sampled with 15” sweep nets (5 sets of 30 sweeps per plot) and measurements of ground cover height, biomass, and phenological stage were recorded. Pest and natural enemy abundance in the canopy were monitored using beat sampling (5 sets of 60 beats per plot). Each beat sample consisted of an aggregate sample from 20 trees. Each tree was struck three times with a ½” PVC pipe while holding a sweep net below, where insects fall from the canopy into the sweep net. All insects from the sweep and beat samples were identified to genus or species. Yellow sticky-traps were also be used to monitor pest and natural enemy abundance. Three pairs of sticky-cards were used in each plot, and were positioned at every 20th tree along the center row of each plot. For each pair, one trap was positioned in the crop canopy (approximately 2.5m from the ground) and the other along the ground vegetation (approximately 0.25m from the ground). Traps were collected every two weeks and all insects identified to genus or species.
Fig. 2 Leaffooted bug, Leptoglossus zonatus, feeding on pistachio nut cluster in Coalinga, California.
Fig. 3 Experimental plot containing summer flowering groundcovers within pistachio orchard.
Objective 3B: Trap crop influence on biological control
To assess the influence groundcovers have on biological control, sentinel leaffooted bug egg masses (< 72 hrs old) were placed in the crop canopy once a month (Fig. 4). After 3 days of exposure, sentinel eggs were collected and held under controlled laboratory conditions. All emerged parasitoids were identified to species and their abundance recorded. All sentinel eggs remaining were counted, and any destroyed eggs will be presumed to be damaged by a predator. Remaining eggs which neither hatched nor produced a parasitoid was dissected to verify whether they contained a dead parasitoid, dead leaffooted bug, or determined aborted.
Fig. 4 Leaffooted bug sentinel eggs glued to branch of pistachio tree within experimental plot to evaluate predation and parasitism rates.
Objective 3C: Trap crop influence on feeding damage
At harvest, individual plots with and without the ground covers were harvested and subjected to commercial grading in processing facility. This is considered the most realistic measure of crop damage and the most relevant to growers.
Objective 1: Evaluation of H. pennsylvanicus as biocontrol agent to L. zonatus
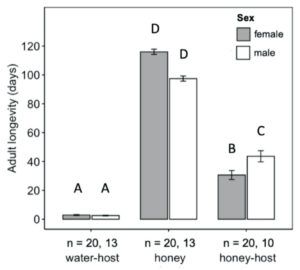
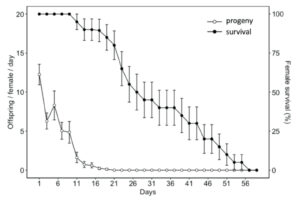
Adult diet significantly influenced the longevity of H. pennsylvanicus (χ2 = 16.05, df = 2, p < 0.001) (Fig. 5). Water-fed males and females lived an average of 2.54 ± 0.24 (SE) and 2.90 ± 0.29 (SE) days respectively. Honey-water fed females had a 74% reduction in their lifespan when provided host eggs for reproduction. Honey-water fed females lived an average 116.00 ± 1.81 (SE) days in the absence of host eggs and 30.60 ± 3.10 (SE) days when provided host eggs. Females lived significantly fewer days than males only when provided with honey-water diet and host eggs (post hoc Tukey test, z = 3.371, df = 5, p < 0.01). On average, 79% of all parasitism occurred within the first week of a honey-fed female’s lifetime (Fig. 6). The highest mean parasitism rates observed was on day 1 of the study, averaging 12.25 ± 1.32 (SE) progeny, accounting for 49.00 ± 5.00% (SE) of total eggs available. Overall fecundity showed relatively consistent declines over the first three weeks, with a < 50% reduction in parasitism by day 5, and a complete absence of progeny following day 19.
Fig. 5 Mean longevity (± SE) of adult female (grey bars) and male (white bars) Hadronotus pennsylvanicus when reared with water and host eggs, honey-water and Leptoglossus zonatus host eggs, or honey-water only. All bars indicating different letters are significantly different from one another (p < 0.05).
Fig. 6 Mean age-specific fecundity (white dots) (± SE) and female survival rate (black dots) (± SE) of Hadronotus pennsylvanicus when provided Leptoglossus zonatus host eggs and fed honey-water ad libitum.
Objective 2: Hadronotus pennsylvanicus life parameters on host plant
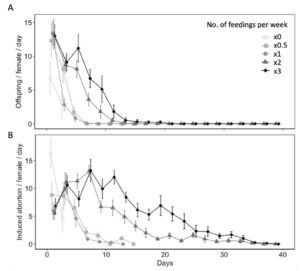
Diet availability significantly influenced reproductive and non-reproductive host mortality ( χ2 = 35.26, df = 4, P < 0.001 for offspring produced per female per day; χ2 = 30.45, df = 4, P < 0.001 for induced abortions per female per day) (Fig. 7A). All parasitism occurred within first 15 days of the females lifetime, with the highest mean parasitism rates occurring on day 1 of study. On day 1, water-fed females produced fewer offspring (6.70 ± 2.38) than females provided honey-water diet once (13.40 ± 1.69) or three times per week (13.00 ± 1.64) (z = 2.84, P< 0.05 for x1; z = 2.74, P < 0.05 for x3), however was not significantly different than females fed honey-water twice (13.30 ± 2.16) or every other week (12.30 ± 2.50) (z = 2.66, P= 0.06 for x1; z = 2.29, P = 0.15 for x0.5). Fecundity showed relatively consistent declines over the first three weeks for all diet treatments. However, females provided honey-water twice or three times per week maintained higher fecundity rates on days 5 (x2, 8.10 ± 1.27; x3, 11.20 ± 2.04), 7 (x2, 3.60 ± 0.92; x3, 6.70 ± 1.33), and 9 (x2, 1.80 ± 0.93; x3, 5.10 ± 1.96) in comparison to individuals fed once per week, every other week, or a water-only diet. There was a significantly greater proportion of aborted host eggs observed when L. zonatus eggs were exposed to a female wasp than those unexposed ( χ2 = 31.62, df = 1, P < 0.001). In the absence of a parasitoid, the average proportion of eggs determined aborted was 0.70 ± 0.26. Diet availability also influenced non-reproductive host mortality over the course of a females lifetime ( χ2 = 146.17, df = 4, P < 0.001 for induced egg abortions per female per day) (Fig. 7B).
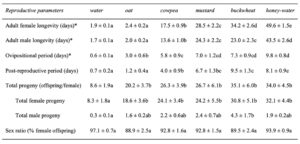
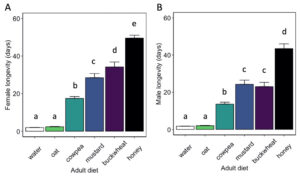
Adult diet significantly influenced the longevity of female ( χ2 = 3662.8, df = 5, P < 0.01) and male ( χ2 = 1500, df = 5, P< 0.01) H. pennsylvanicus (Fig. 8). Females provided a buckwheat diet lived longer (34.2 ± 2.6 d) than those provided a mustard (z = -3.95, P < 0.01), cowpea (z = -12.53, P < 0.01), or oat (z = -21.77, P < 0.01) diet. However, there was no significant difference in male longevity when provided a buckwheat (23.0 ± 2.3 d) or mustard diet (24.2 ± 2.2 d). Female and males wasps lived longer when provided a cowpea diet (17.5 ± 0.9 d for females; 13.6 ± 1.0 d for males) than an oat diet (z = -15.80, P < 0.01 for females; z = -9.80, P < 0.01 for males). Oat-fed females lived on average 2.4 ± 0.2 d, whereas males lived 2.0 ± 0.2 d. There was no significant difference in longevity when provided water or oat diet for either females or males. Parasitoids survived for the longest duration when provided a honey-water diet (49.6 ± 1.5 d for females, z = 9.12, P < 0.01; 43.5 ± 2.6 d for males, z = 9.56, P < 0.01).
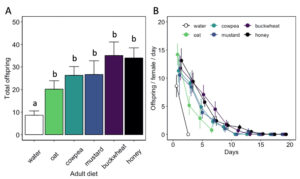
Adult diet also influenced the duration of female oviposition ( χ2 = 115.91, df = 5, P < 0.01) and post-reproductive ( χ2 = 104.31, df = 5, P < 0.01) periods, as well as lifetime fecundity ( χ2 = 32.94, df = 5, P < 0.01) (Table 1). Females fed a cowpea (5.8 ± 0.9 d), mustard (7.0 ± 1.2 d), buckwheat (7.3 ± 0.9 d), or honey diet (9.8 ± 0.8 d) exhibited longer ovipositional periods than individuals fed an oat diet (z = -3.08, P < 0.05 for cowpea; z = -4.01, P < 0.01 for mustard; z = -4.56, P < 0.01 for buckwheat; z = -6.43, P < 0.01 for honey). However, there was no significant difference in ovipositional period between cowpea-, mustard-, buckwheat-, or honey-fed females. Similarly, females fed a buckwheat (9.5 ± 1.3 d) or honey diet (8.1 ± 0.9 d) maintained the longest post-reproductive periods. Water-fed females produced less offspring (8.6 ± 1.9 offspring) than individuals fed a floral diet (z = -2.27, P < 0.05 for oat; z = -3.04, P < 0.05 for cowpea; z = -2.99, P < 0.05 for mustard; z = -4.96, P < 0.01 for buckwheat) or honey diet (z = -4.98, P < 0.01) (Fig. 9). However, there was no significant difference in female fecundity when provided oat (20.2 ± 3.7 offspring), cowpea (26.3 ± 3.9 offspring), mustard (26.7 ± 6.1 offspring), buckwheat (35.1 ± 6.0 offspring), or honey diet (34.0 ± 4.5 offspring). There was also no difference in the sex ratio of progeny between diet treatments.
Fig. 7 Age specific fecundity (A) and induced egg abortions on host (B) (mean ± SE) of Hadronotus pennsylvanicus when provided Leptoglossus zonatus host eggs and fed honey-water diet once (x1), twice (x2), or three (x3) times per week, every other week (x0.5), or fed a water-only diet (x0).
Table 1 Reproductive attributes (mean ± SE) of Hadronotus pennsylvanicus reared on Leptoglossus zonatus eggs at 25C ± 1, 75 ± 5% RH and 16:8 L:D, fed oat, cowpea, mustard, buckwheat floral provisions, or a water or honey-water diet ad libitum. Asterisks (*) indicate where adults were not provided host eggs for oviposition.
Fig. 8 Hadronotus pennsylvanicus female (A) and male (B) longevity (mean ± SE) when fed floral resources, water, or a honey diet. All bars indicating different letters are significantly different from one another (p < 0.05).
Fig. 9 Hadronotus pennsylvanicus lifetime fecundity (A) and progeny produced over time (B) (mean ± SE) when provided Leptoglossus zonatus host eggs every other day and fed a floral resource, water, or honey diet. All bars indicating different letters are significantly different from one another (p < 0.05).
Objective 3A: Trap crop influence on leaffooted bug and natural enemies
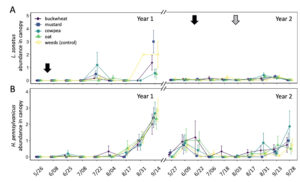
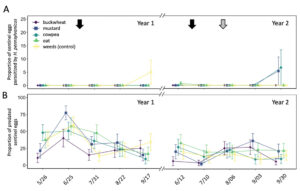
In total, 180 L. zonatus adults were recorded in the pistachio orchard over the course of this 2-year study. Adult L. zonatus were more abundant in the crop canopy then on groundcovers (z = -6.24, P < 0.01), with 90% of individuals collected from the crop canopy. In canopy samples, there was a significant difference in total L. zonatus abundance across the season ( χ2 = 272.65, df = 18, P < 0.01), with the greatest counts observed in August – September (Fig. 10A). However, there was no difference in L. zonatus abundance recorded in the crop canopy between groundcover treatment plots in either year of the study. While L. zonatuspresence on groundcovers followed similar abundance trends as those observed in the crop canopy, similarly, there were no significant differences in L. zonatus abundance on groundcovers between treatment plots (Fig. 11A).
In total, 550 H. pennsylvanicus individuals were collected from sticky card traps over the course of the study. Hadronotuspennsylvanicus were more abundant in the crop canopy than on groundcovers (z = -12.18, P < 0.01), with 97% of individuals collected from canopy traps. Hadronotus pennsylvanicus abundance in the crop canopy changed throughout successive seasons ( χ2 = 952.39, df = 18, P < 0.01) (Fig. 10B). There was a notable increase in H. pennsylvanicus abundance in the crop canopy later in the growing season (August). During the 2021 growing season, H. pennsylvanicus abundance increased early in the growing season (June – July), with a similar peak in abundance later in the season (August). Despite fluctuations in H. pennsylvanicus abundance, there were no significant differences in the number of parasitoids present in the canopy between groundcover treatments. The seasonal abundance of H. pennsylvanicus observed within groundcovers followed similar trends to those found in the crop canopy, with populations increasing later in the growing season (Fig. 11B). Hadronotus pennsylvanicus abundance within groundcovers was greatest early (June) in Year 2 of the study. However, there were no significant differences in H. pennsylvanicus abundance in crop canopy or within groundcovers between the groundcover treatments. Minute pirate bugs Orius spp. Wolff (Hemiptera: Anthocoridae), green lacewings Chrysoperla spp. Schneider (Neuroptera: Chrysopidae), and ladybird beetles Hippodamia spp. Dejean (Coleoptera: Coccinellidae) were the most common predatory insects collected from sampling (1838, 174, and 410 total individuals collected, respectively).
Fig. 10 Mean abundance (± SE) of Leptoglossus zonatus (A) and Hadronotus pennsylvanicus (B) in the canopy of pistachio containing groundcover treatments. Arrows on the top indicate a treatment with pyrethrin (black arrow; 3 July 3 2020 & 20 June 20 2021) and mowing of the groundcover (grey arrow; 2 August 2021).
Fig. 11 Mean abundance (± SE) of Leptoglossus zonatus (A) and Hadronotus pennsylvanicus (B) within groundcover treatments in organic pistachio.
Objective 3B: Trap crop influence on biological control
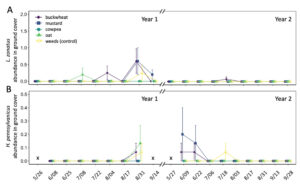
Parasitism on sentinel L. zonatus eggs primarily occurred late in the growing season (September) (Fig. 12A). A total of 7 of the 762 egg clusters deployed were parasitized over the 2-year study, with H. pennsylvanicus accounting for 57% of parasitoids to emerge from eggs, followed by Ooencyrtus spp. Ashmead (Encyrtidae), Anastatus spp. Motschulsky (Eupelmidae) accounting for the remaining 29% and 14%, respectively. Despite parasitism occurring late in the growing season, there was no significant difference in the number of parasitoids to emerge from sentinel eggs between each sample period, nor between groundcover treatment plots. In the field, the average proportion of eggs that failed to yield a parasitoid or L. zonatus nymph (i.e. was determined non-viable) was 6.2 ± 0.6. Sentinel eggs in the canopies of buckwheat (8.9 ± 1.6%) exhibited a greater proportion of non-viable eggs than those in plots containing cowpea (5.6 ± 1.1%; z = -3.25, P < 0.05), mustard (5.6 ± 1.1%; z = -3.25, P < 0.05), and oats (4.8 ± 1.1%; z = -4.18, P < 0.01). However, there was no significant difference in the proportion of non-viable eggs between buckwheat and weed plots. The proportion of non-viable eggs from unexposed (control) sentinel eggs (3.09 ± 0.62%) was significantly less than those placed in the orchard (z = -5.78, P < 0.01 for buckwheat; z = 3.44, P < 0.01 for weeds; z = -3.03, P < 0.05 for cowpea; z = -3.03, P < 0.05 for mustard).
Groundcover treatment had a significant influence on the number of sentinel eggs taken by predators ( χ2 = 51.61, df = 4, P < 0.01) (Fig. 12B). Plots containing oat groundcovers maintained the greatest total number of predated eggs (z = -2.95, P < 0.05). However, there was no significant difference in the total number of eggs eaten by a predator between cowpea, mustard, and weedy control groundcover treatments. Plots containing buckwheat had the fewest total number of eggs eaten by a predator through both years of the study (z = 3.70, P < 0.01). While there were seasonal differences in the total number of sentinel eggs eaten by a predator ( χ2 = 369.61, df = 9, P < 0.01), few dates exhibited consistent differences between groundcover treatments.
Fig. 12 Mean proportion (± SE) of sentinel Leptoglossus zonatus eggs parasitized by Hadronotus pennsylvanicus (A) or predated egg clusters (B) in pistachio canopy within each groundcover treatment. Arrows on the top indicate treatment with a pyrethrin (black arrow; 3 July 2020 & 20 June 2021) and mowing of the groundcover (grey arrow; 2 August 2021).
Objective 3C: Trap crop influence on feeding damage
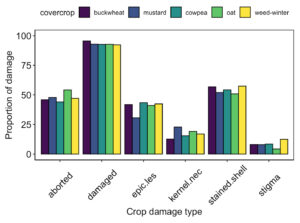
While we were unable to collect data on crop in year 2 of the study, there was no significant difference in crop damage at harvest between groundcover treatments in year 1 of the study (Fig. 13).
Fig. 13 Proportion of pistachio nut damage between groundcover treatments at harvest following Year 1 of the study.
Research Outcomes
In some instances, the use of groundcovers has been demonstrated to improve weed control, soil health, and aid in water retention in orchards. If there are added benefits of groundcovers for crop protection, there may likely be greater adoption from the California nut industries. However, the efficacy of such groundcovers may often depend on multiple characteristics of the crop and groundcover plants, as well as the diversity, abundance, and behavior of insect pests and their natural enemies throughout the growing season. For growers, the adoption of such management tactics aimed to enhance biocontrol is contingent on economic viability, reliability in reducing crop damage, and ultimately, whether such tactics successfully increase revenue and profitability. It is also important to consider that the establishment and maintenance of groundcovers requires additional labor, fuel and material costs for growers, all of which is a recurring expense given that groundcovers must be re-sown each year. Therefore, understanding the potential prospects and limitations of conservation biocontrol within a given crop system must be thoroughly evaluated prior to its implementation. Here, working closely with growers ensures that the recommended practices are not only feasible, but compatible with current orchard management practices, therefore providing greater opportunity to be applied and adopted long-term.
Findings from this study would suggest that the use of flowering groundcovers aimed to improve biocontrol is likely not a viable solution to sufficiently suppress L .zonatus populations and meet the current production demands of California tree nut production. While nutrition may be a major influence on the reproductive fitness of H. pennsylvanicus, diet may represent only one of the many resources required by H. pennsylvanicus in order to persist in agricultural landscapes. For instance, parasitoids often require additional non-crop resources, such as overwintering habitat or alternative hosts. In the case of California pistachio orchards, the quantity and quality of such habitats and hosts may prove to be a limiting factor in the biocontrol potential of H. pennsylvanicus, rather than the availability of floral resources. Therefore, understanding the overwintering ecology and habitat requirements of H. pennsylvanicus in relation to host L. zonatus may prove critical for understanding the seasonality and synchronicity of this pest-parasitoid relationship in orchard systems.
The California tree nut industries are all highly motivated to promote sustainable pest management practices to reduce the environmental impacts of crop production. Here, we evaluated the potential of one specific practice (spring/summer groundcovers) and determined that it was insufficient to augment biological control of L. zonatus, a key pest of almonds and pistachios. Findings from this project are relevant to industry since many growers continue to experiment with the use of groundcovers to enhance a variety of ecosystem services. Knowing what doesn’t work is just as important as knowing what works, and in this way our work allows growers and researchers to move forward in the search for sustainable pest management practices that are ecologically appropriate, economically feasible and agronomically viable.
Education and Outreach
Participation Summary:
Highlights of this project were covered in popular trade journals, such as National Nut Grower and West Coast Nut, including articles titled "Summer Trap Crop Evaluations" (https://www.wcngg.com/2019/09/01/summer-trap-crop-evaluations/) and "Trap Crops in Pistachio" (https://www.wcngg.com/2020/12/03/trap-crops-in-pistachio/). With over 2,000 online views, the results of this study were disseminated across growers and pest control advisors. Project Co-PI Kent Daane and collaborator Judith Stahl also discussed project efforts in the UC Cooperative Extension podcast Growing the Valley (https://www.growingthevalleypodcast.com/podcastfeed/2022/4/12/plant-bugs-with-judith-stahl-and-kent-daane). Online publications such as the magazine articles and podcast episode provided a broad reach to grower audiences, with the journal West Coast Nut alone advertising a readership of >15,000 subscribers online and in print (see https://mediakit.jcsmarketinginc.com/west-coast-nut/).
Additionally, Robert Straser co-organized a symposium with PI Houston Wilson titled “Habitat diversity to enhance ecosystem services in perennial cropping systems” at the 2021 Entomological Society of America, Pacific Branch Conference virtual symposium. At this conference, Straser presented finding on the role nutrition plays on parasitoid fitness in the oral presentation titled “Floral resource provisioning to enhance biological control of the leaffooted bug, Leptoglossus zonatus, in tree nut crops”. Moving forward, we aim to publish UC Extension bulletins and semi-technical pamphlets to disseminate our findings further to the grower community. Information generated will be shared with other researchers through presentations at other professional meetings such as the regional and national meetings of Entomological Society of America.
Findings from this study were also published in peer-reviewed journals. Assessments on the life parameters of natural enemy H. pennsylvanicus on host L. zonatus have been published in the journal Biocontrol, titled “Evaluation of egg parasitoid Hadronotus pennsylvanicus as a prospective biological control agent of the leaffooted bug Leptoglossus zonatus” (https://link.springer.com/article/10.1007/s10526-022-10131-z). Evaluations on the role diet has on parasitoid reproduction and parasitism behavior were published in the journal Scientific Reports, titled "Food deprivation alters reproductive performance of biocontrol agent Hadronotus pennsylvanicus" (https://www.nature.com/articles/s41598-022-11322-5). Project findings on the role parasitoid nutrition has on lifetime reproduction, as well as the prospects of orchard groundcovers in mitigating feeding damage from L. zonatus are pending submission for publication.