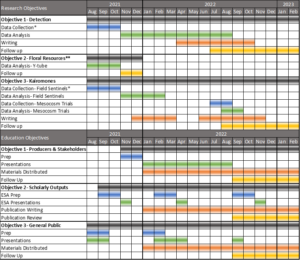
Final report for GW21-221
Project Information
The brown marmorated stink bug (BMSB, Halyomorpha halys Stål) is an invasive insect that feeds on specialty fruit and vegetable crops in North America. Current management of H. halys relies heavily on broad-spectrum insecticides which are only moderately effective and disrupt sustainable integrated pest management (IPM) programs by killing natural enemies and contributing to insecticide resistance and secondary pest outbreaks. The samurai wasp [Trissolcus japonicus (Ashmead)], a highly effective biocontrol agent native to the home range of H. halys, has shown strong promise in providing long-term and effective management of H. halys. While adventive populations of T. japonicus have been discovered in Utah, geographic distribution and climate models suggest this region is only marginally suitable. Utah’s high-elevation climate provides unique challenges to wasp establishment; therefore, conservation efforts are critical next steps to enhance T. japonicus populations. The objectives of this research were to answer: 1) What is the distribution of T. japonicus in northern Utah specialty crops and nearby urban areas, and how does the diversity of understory vegetation influence their abundance? 2) Which cover crops can increase wasp lifespan and fecundity? 3) How effective are stink bug host chemical cues (kairomones) in attracting the T. japonicus and increasing stink bug egg parasitism rates? Results were disseminated to specialty crop producers and other stakeholders through multiple presentations (webinars, grower meetings, and field days), one extension fact sheet, two newsletter articles, and two research journal publications (and one more in preparation). This research produced applicable information that supports improved sustainability of H. halys management and reduced economic losses to specialty crops.
My research focused on the status, conservation, and enhancement of an effective egg parasitoid, the samurai wasp, [Trissolcus japonicus (Ashmead)] of the invasive insect pest, brown marmorated stink bug (BMSB) (Halyomorpha halys Stål) in northern Utah by pursuing the following objectives:
- Objective 1- Determine the establishment and spread of the T. japonicus (e.g., overwinter survival) since its first detection in northern Utah in June, 2019, including assessment of the impacts of understory vegetation diversity on its occurrence and abundance.
- Objective 2- Determine cover crop floral resource candidates to attract and sustain the T. japonicus, including impacts on wasp lifespan and reproduction.
- Objective 3- Determine the viability of stink bug host chemical cues (kairomones) in attracting T. japonicus and increasing egg parasitism rates in both a field and mesocosm setting.
After reviewing preliminary results from Objective 2, research efforts were shifted towards a second experiment as a part of Objective 3 (mesocosm arena assessment of T. japonicus response to kairomones).
My educational objectives focused on increasing the knowledge of specialty crop producers, research and extension colleagues, the general public, youth, and other interested stakeholders on conservation and enhancement of the T. japonicus for biological control of the invasive brown marmorated stink bug (BMSB; Halyomorpha halys).
- Objective 1- Promote the T. japonicus for the sustainable management of BMSB to approximately 10,000 specialty crop producers and other relevant stakeholders.
- Objective 2- Add to the national H. halys research knowledge base and share project results with professional colleagues through two journal publications and three scientific conference presentations.
- Objective 3- Disseminate information on invasive species and sustainable pest management to the general public via varied outreach venues and formats.
Preliminary research and planning for this project began the summer of 2020 which resulted in the foundation of this proposal. Data collection for research objectives 1-3 began in May 2021 to take advantage of the active season for BMSB and samurai wasp and avoid loss of critical data. Student summer funding for 2021 was available to support this work from a USDA Utah Department of Agriculture and Food Specialty Crop Block Grant; however, this funding was insufficient to achieve this project’s goals. The funds received from this grant were used to support the completion of the research and outreach objectives as outlined above. Follow-up focused on assessing the impacts and success of each objective, as outlined in the Evaluation and Producer Adoption section of the narrative.
*Data collection began May 2021 before the grant funding cycle begins.
** After reviewing preliminary results from Objective 2, research efforts were shifted towards a second experiment as a part of Objective 3.
Cooperators
- (Educator)
- (Educator)
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
Research
Objective 1- Determine the establishment and spread of the T. japonicus (e.g., overwinter survival) since its first detection in northern Utah in June 2019, including assessment of the impacts of groundcover vegetation diversity on its occurrence and abundance.
Rationale
Preliminary research in 2020 found that the T. japonicus was primarily detected in urban landscapes, had successfully overwintered, and had expanded its distribution despite the unique climate challenges of northern Utah (Fig. 1). However, one year of overwinter survival is insufficient evidence of establishment; further study is necessary to fully understand the wasp’s overwintering capability, climate tolerance, and potential expansion, particularly within agricultural landscapes, in northern Utah. Comparing the effects of understory vegetation diversity on wasp abundance will provide further insight into foundational resource needs for the T. japonicus and methods to enhance its establishment in urban and agricultural landscapes.
Common methods of monitoring for parasitoid wasps are yellow sticky card deployments, collection of wild H. halys egg masses, and deployment of sentinel (lab-reared) H. halys egg masses. These methods proved effective in detecting T. japonicus in 2019 and 2020 (Holthouse et al. 2020; K. Richardson, unpublished data), were used to compare the abundance of T. japonicus and native parasitoids in yellow sticky card surveys (Fig. 2), and to analyze parasitism success on wild egg masses (Fig. 3). Results from the following proposed methods will add to 2020 data on abundance and parasitism rates and will be used to build distribution and climate/habitat suitability maps.
Materials and Methods
Surveys
Yellow sticky cards (YSC) (20 x 14 cm; Alpha Scents Inc., West Linn, OR) were deployed at 24 and 31 sites in 2019 and 2020, respectively (Tables 1-2). Sites were located in Box Elder, Cache, Davis, Salt Lake, Utah, and Weber counties of northern Utah. YSC were deployed starting 21 May and 29 May (2019 and 2020, respectively) and ending 1 October in 14 d (±1 d) intervals resulting in a total of nine deployment periods. At each site, a YSC was secured with two twist ties to a branch at approximately 2 m height on select ornamental and fruit trees based on past studies that support H. halys oviposition attraction to these hosts (Holthouse et al. 2021a).
In 2021, YSC surveys were conducted at 36 sites (Table 3) including some sites monitored in 2019-2020 and 24 orchards with groundcover types of interest. Orchard sites were selected based on understory vegetation categories of bare soil (cultivated and herbicide-managed), turfgrass (managed), or floral (with wildflowers, herbaceous weeds, or strip-cropped floral resources) with each understory type replicated eight times. Sites were monitored for consistency in groundcover categorization throughout the season and were assumed to have similar insecticide application patterns. A total of ten, 14 d (+1 d) YSC deployments were conducted from 19 May to 5 October as described for 2019-2020.
Surveys for H. halys were conducted over the same time period at the 24 orchard sites in 2021. Clear dual sticky panel traps (Trécé Inc., Adair, OK) attached to a wooden stake and positioned ~1.2 m above the ground were deployed at the orchard edge within 10 m of the YSC. Halyomorpha halys dual pheromone lures (Trécé Inc., Adair, OK) were secured to traps using twist ties with replacement after 12 wk. Halyomorpha halys (adults and nymphs) were recovered from traps every 14 d (+1 d).
Parasitoid Processing
Upon retrieval, YSC were examined under a stereomicroscope (Leica Stereozoom S9E, Leica Microsystems Inc.) with 98–880x magnification for the presence of Trissolcus spp. Wasps were initially removed by cutting out a small piece of YSC, then soaked in Histo-Clear II histological clearing fluid (National Diagnostics, Atlanta, GA) for ~5–7 min to dissolve the adhesive, and mounted on a white cardstock point with clear fingernail polish and insect pin to allow for species identification. Due to the capture of large numbers of Trissolcus spp. on 23 August to 11 October 2021, wasp specimens were directly identified on small pieces of YSC to reduce processing time, and only removed if required for species identification. All Trissolcus specimens were identified to species-level following the key to Nearctic Trissolcus (Talamas, Johnson, et al. 2015). Some Trissolcus specimens were unidentifiable beyond the genus level due to physical damage. Intact parasitoid wasps were labeled and stored in the Alston Lab, Department of Biology, Utah State University, Logan, UT. Voucher specimens were deposited in the Utah State University Insect Collection.
Data Analysis
Mean hourly temperature data were obtained each year from weather stations representative of each site, and accumulated degree days using a linear method based on averaging the daily maximum and minimum temperatures, a 1 January biofix, and 12 °C lower development threshold (LDT) (Qiu et al. 2007) were calculated for the end date of each deployment period.
Following methods of Christman et al. (2022), land cover values each year were obtained from USDA National Agricultural Statistics Service (NASS) CropScape and Cropland Data Layer (CDL) (USDA NASS CDL, 2019-2021). Urban land cover included all types of developed land. To assess urbanness of each site, a 1 km boundary was established, the number of pixels of urban land cover within the boundary counted, and the proportion of urban land cover quantified. Sites with 0-0.33, 0.33-0.67, and 0.67-1.00 urbanness were considered low, medium, and high, respectively.
Abundance detection maps were built in ArcGIS Pro (version 3.0.2). To compare T. japonicus and total Trissolcus spp. captures among urbanness categories, data from each year were pooled across sample dates and analyzed using the Kruskal-Wallis test followed by the Bonferroni corrected Dunn’s test. For the 2021 groundcover study, only the 24 sites with designated groundcover and monitored for H. halys were included in the analysis. Captures of T. japonicus and native Trissolcus spp. were compared across groundcover types for both season-long data and peak season (>900 DD12℃) using the Kruskal-Wallis test followed by the Bonferroni corrected Dunn’s test and were separately analyzed by the Kruskal-Wallis test alone for the effect of the presence or absence of H. halys. All statistical comparisons were run using R software (R version 4.1.1; R Core Team 2021) and were considered significant at p < 0.05.
One bare ground orchard site in Box Elder County was removed from all statistical analyses due to extremely high numbers of T. euschisti (Ashmead) uncharacteristic to this groundcover type and other sites in the area. This site was only surveyed in 2021 and was in close proximity to commercial berry fields which may have contained unusually high numbers of native stink bug hosts, and thus, high numbers of parasitoid wasps. Excluding this site allowed for statistical comparisons that met model assumptions.
Objective 2- Determine cover crop floral resource candidates to attract and sustain T. japonicus, including impacts on wasp lifespan and reproduction.
Rationale
Research has shown that non-crop host plants can be critical in providing essential food and habitat resources to beneficial insects, such as predators and parasitoids (Baggen and Gurr 1998; Hickman and Wratten 1996). Effectiveness and longevity of parasitoid wasps can significantly benefit from floral resources such as nectar and pollen (Jervis et al. 1992; Lee et al. 2004). This study will focus on cover crops because of their benefits and appropriateness to agricultural production systems. Many growers already use cover crops for the benefits of soil conditioning, weed and erosion management, and attraction of beneficial insects.
Although McIntosh et al. (2020) explored the impact of eight flowering plant species on T. japonicus survival in the laboratory, only two of the previous plants were tested in this study (buckwheat and sweet alyssum). The remaining flowers in their study (dill, cilantro, marigold, crimson clover, yellow mustard, and phacelia) are either more appropriate for small garden plantings than large commercial specialty crop production fields or are inappropriate for the Intermountain West region. No other studies have explored the use of cover crop floral resources, appropriate to intermountain regions, for enhancement of T. japonicus.
Material and Methods
Here, the attractiveness of four cover crops: alfalfa [Medicago sativa (L.)], buckwheat [Fagopyrum esculentum (Moench)], red clover [Trifolium pratense (L.)], and sweet alyssum [Lobularia maritima (L.) Desv.] to T. japonicus females were assessed using a Y-tube olfactometer. A single, mated, unfed female wasp (24-48 hr old) from the USU T. japonicus colony (originating from females collected in 2019 from Salt Lake City, Utah) was placed in a Y-tube olfactometer, made from a glass tube body (90 mm stem, 80 mm arms, 15 mm internal diameter) (Fig. 4), with approximately 0.5 g of floral material on one side of the Y-arm and a blank control on the other (following similar methods outlined in Bertoldi et al. 2019; Salerno et al. 2002; Zhong et al. 2017). Flowers were grown from seed in the USU research greenhouse and charcoal filtered airflow was maintained at a rate of 90-110 cc/min. Wasps were carefully inserted into the Y-tube entrance of the central stem and observed for five minutes. Residence time in each arm of the olfactometer was recorded. The olfactometer was rotated after every fifth parasitoid trial to negate a possible left vs right side bias and cleaned with laboratory detergent and deionized water between each flower species.
Objective 3- Determine the viability of stink bug host chemical cues (kairomones) in attracting T. japonicus and increasing egg parasitism rates in a field setting.
Rationale
Though sentinel H. halys egg masses are a common way to assess wasp parasitism rates, studies have shown that lab-reared egg masses underestimate parasitism rates and attract fewer wasps than wild egg masses. This is thought to be because T. japonicus uses volatile chemicals released by H. halys during host feeding and oviposition (Abram et al. 2017; Holthouse et al. 2020; Jones et al. 2014). Research by Malek et al. (in review) found that combining the H. halys host kairomones of n-tridecane and (E)-2-decenal at a ratio of 4:1 was highly attractive to T. japonicus adults in a Y-tube olfactometer experiment. However, another study found the ratio of 9:1 used in a field experiment demonstrated little success, possibly due method of delivery (filter paper) and rapid volatility of the chemicals (J. Kaser, personal communication). There is evidence of wasp attraction to these kairomones in a lab setting, but more research is needed to understand how they perform in agricultural field settings.
Materials and Methods
Field Trials
To assess the attractiveness of kairomone chemicals in a field setting, custom gray rubber septa lures loaded with 10 mg of test compounds were developed by Trécé, Inc., (Adair, OK). This field study included four lure treatments with varying ratios of n-tridecane to (E)-2-decenal: 100% n-tridecane, 90% n-tridecane, 80% n-tridecane, and hexane (control). Lab-reared H. halys sentinel egg masses were deployed adjacent to kairomone lures as hosts for parasitoid wasps.
Trials (n=6) were conducted from 24 June to 27 August 2021 in a strip of residential Catalpa speciosa trees in Salt Lake City, UT (40.772480, -111.854975). Catalpa trees were selected due to consistent populations of H. halys observed on leaves and pods. Treatments were replicated in four trees (three in the final deployment due to a lack of egg masses) with a blank buffer tree between each treatment tree. Each replicate tree (3 m wide canopy) contained four H. halys egg masses and one of each lure-treatment with one mass and lure placed approximately 2 m laterally from the trunk of the tree at each cardinal direction. Egg masses of H. halys were attached to small rectangles of white cardstock (2 cm by 3 cm) and clipped with a lure to the underside of tree leaves (Fig. 5). Treatments were placed at approx. 2 m height above the ground with cardinal direction of treatments randomized for each tree in each deployment.
H. halys egg masses (n=92) were ~48 hr old and produced in a laboratory colony at the Oregon Department of Agriculture (Salem, OR). Sentinel egg masses were deployed with kairomone treatment lures in the field for approximately 96 hr and returned to the laboratory for evaluation. Any wasps found guarding egg masses were collected into a 9-dram plastic vial (Thornton Plastics, Salt Lake City, UT) using a WHO (World Health Organization) in-line tube aspirator (Bioquip, Compton, CA) for later identification. After eggs were incubated at 25–27℃ for 14 days in the laboratory, they were evaluated for parasitism incidence and intensity (proportion of parasitized eggs per mass), and emerged wasps were identified to species using the key to Nearctic Trissolcus (Talamas, Johnson, et al. 2015). Egg masses were observed again 14 days later (4 weeks after collection) to identify late-emerging wasps or eggs with partially developed wasps or stink bugs. Individual egg fate was recorded as emerged or undeveloped parasitoid, hatched or unhatched H. halys nymph, predated, sunken, empty, or missing.
Mesocosm Trials
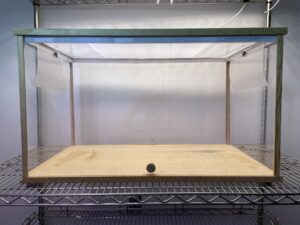
In order to mitigate multiple uncontrollable factors in a field setting, a second experiment was conducted in a mesocosm-scale lab-based experimental system in 2022. In a 0.5 m height x 0.5 m depth x 0.8 m length plexiglass observation cage with a mesh lid, a clear panel trap (Alpha Scents, Inc, 30.5 cm X 30.5 cm) and lure attached via a clothespin to the upper portion of the card were hung at either end of the cage (1 m apart) (Fig. 6). Four to five female T. japonicus that were 1-20 day-old post-eclosion (according to colony availability), and honey fed from the Utah State University T. japonicus colony (originating from females collected in 2019 from Salt Lake City, Utah) were introduced to the center, bottom of the cage via a small access hole (stoppered by a cork) and allowed up to 30 min to select between the control and treatment lure by landing on the adjacent clear panel sticky trap (2-way choice). Treatments consisted of four combinations of chemical attractant to repellent ratios and load rates: 5 mg–100% n-tridecane, 10 mg–100% n-tridecane, 10 mg–90% n-tridecane, and 10 mg–80% n-tridecane.
Each treatment was tested against a hexane control lure and trials were replicated until each treatment had a minimum of 32 individuals make a choice. The 10 mg–100% lure was replicated more heavily to test experimental setup. Wasps that did not make a choice within the 30 min experimental period were not included in the analyses. The observation cage was cleaned etween lure types and treatment vs control lures were rotated every other trial to account for side bias. Trials were conducted in a temperature controlled rearing room at 21-27°C and 30-50% RH.
Statistical Analyses
To analyze field trial results, data were pooled across sample dates and each egg fate category was analyzed using the Kruskal-Wallis test to compare differences across treatment lure types. In mesocosm trials the null hypothesis that T. japonicus showed no preference between the control and treatment lure (an overall choice of 50:50) was analyzed with a Chi-square goodness of fit test. All statistical comparisons were run using R software (R version 4.1.1; R Core Team 2021) and were considered significant at p < 0.05.
Tables and Figures
Table 1. Sites surveyed in 2019: county location, urbanness measure (proportion of surrounding 1 km habitat with developed land), latitude and longitude.
|
Site ID |
County |
Urbanness |
Latitude |
Longitude |
|
BE1 |
Box Elder |
0.029 |
41.73761 |
-112.28079 |
|
BE2 |
Box Elder |
0.090 |
41.72079 |
-112.14402 |
|
DA1 |
Davis |
0.327 |
41.06271 |
-112.01766 |
|
DA3 |
Davis |
0.585 |
41.03606 |
-111.90633 |
|
DA4 |
Davis |
0.925 |
41.02282 |
-111.93749 |
|
DA6 |
Davis |
0.963 |
41.02065 |
-111.93025 |
|
DA9 |
Davis |
0.629 |
40.97439 |
-111.90437 |
|
DA10 |
Davis |
0.938 |
40.90257 |
-111.87136 |
|
SL1 |
Salt Lake |
0.925 |
40.78039 |
-111.89626 |
|
SL2 |
Salt Lake |
0.921 |
40.78004 |
-111.89545 |
|
SL3 |
Salt Lake |
0.998 |
40.77311 |
-111.86849 |
|
SL4 |
Salt Lake |
0.921 |
40.77078 |
-111.85519 |
|
SL5 |
Salt Lake |
1.000 |
40.76951 |
-111.86596 |
|
SL6 |
Salt Lake |
0.888 |
40.76735 |
-111.85298 |
|
SL7 |
Salt Lake |
0.823 |
40.76367 |
-111.85029 |
|
SL8 |
Salt Lake |
0.960 |
40.75231 |
-111.87353 |
|
SL9 |
Salt Lake |
0.968 |
40.74897 |
-111.85457 |
|
SL11 |
Salt Lake |
0.962 |
40.63577 |
-111.86400 |
|
UT1 |
Utah |
0.989 |
40.34708 |
-111.71357 |
|
UT8 |
Utah |
0.045 |
40.04023 |
-111.86118 |
|
UT13 |
Utah |
0.152 |
39.99747 |
-111.82487 |
|
WE1 |
Weber |
0.518 |
41.32905 |
-112.00984 |
|
WE2 |
Weber |
0.904 |
41.31532 |
-111.95860 |
|
WE3 |
Weber |
0.914 |
41.18553 |
-112.04079 |
Table 2. Sites surveyed in 2020: county location, urbanness measure (proportion of surrounding 1 km habitat with developed land), latitude and longitude.
|
Site ID |
County |
Urbanness |
Latitude |
Longitude |
|
BE1 |
Box Elder |
0.029 |
41.73761 |
-112.28079 |
|
CA3 |
Cache |
0.126 |
41.58166 |
-111.84779 |
|
DA1 |
Davis |
0.327 |
41.06271 |
-112.01766 |
|
DA2 |
Davis |
0.910 |
41.06028 |
-111.97000 |
|
DA3 |
Davis |
0.585 |
41.03606 |
-111.90633 |
|
DA4 |
Davis |
0.926 |
41.02282 |
-111.93749 |
|
DA5 |
Davis |
0.961 |
41.02142 |
-111.93200 |
|
DA6 |
Davis |
0.970 |
41.02065 |
-111.93025 |
|
DA7 |
Davis |
0.939 |
41.02036 |
-111.93600 |
|
DA8 |
Davis |
0.499 |
40.99549 |
-111.88500 |
|
DA9 |
Davis |
0.628 |
40.97439 |
-111.90437 |
|
DA10 |
Davis |
0.938 |
40.90257 |
-111.87136 |
|
SL1 |
Salt Lake |
0.926 |
40.78039 |
-111.89626 |
|
SL2 |
Salt Lake |
0.922 |
40.78004 |
-111.89545 |
|
SL3 |
Salt Lake |
0.998 |
40.77311 |
-111.86849 |
|
SL4 |
Salt Lake |
0.921 |
40.77078 |
-111.85519 |
|
SL5 |
Salt Lake |
1.000 |
40.76951 |
-111.86596 |
|
SL5 |
Salt Lake |
1.000 |
40.76951 |
-111.86600 |
|
SL6 |
Salt Lake |
0.889 |
40.76735 |
-111.85298 |
|
SL7 |
Salt Lake |
0.822 |
40.76367 |
-111.85029 |
|
SL8 |
Salt Lake |
0.960 |
40.75231 |
-111.87353 |
|
SL9 |
Salt Lake |
0.968 |
40.74897 |
-111.85457 |
|
SL10 |
Salt Lake |
0.998 |
40.69306 |
-111.84800 |
|
SL11 |
Salt Lake |
0.962 |
40.63577 |
-111.86400 |
|
UT4 |
Utah |
1.000 |
40.31310 |
-111.71038 |
|
UT5 |
Utah |
0.976 |
40.26861 |
-111.65600 |
|
UT6 |
Utah |
1.000 |
40.22972 |
-111.66389 |
|
UT8 |
Utah |
0.044 |
40.04023 |
-111.85337 |
|
UT13 |
Utah |
0.156 |
39.99747 |
-111.75843 |
|
WE1 |
Weber |
0.518 |
41.32905 |
-112.00984 |
|
WE2 |
Weber |
0.904 |
41.31532 |
-111.95860 |
|
WE3 |
Weber |
0.914 |
41.18553 |
-112.04079 |
Table 3. Sites surveyed in 2021 by groundcover designation (bare, floral, turf, and unspecified). Twelve sites surveyed in 2021 were not included in the groundcover study and were not monitored for groundcover type (unspecified). County location, urbanness measure (proportion of surrounding 1 km habitat with developed land), latitude and longitude are provided.
|
Groundcover |
Site ID |
County |
Urbanness |
Latitude |
Longitude |
|
Bare |
BE4 |
Box Elder |
0.180 |
41.44748 |
-112.03740 |
|
BE6 |
Box Elder |
0.180 |
41.42223 |
-112.03153 |
|
|
BE7* |
Box Elder |
0.399 |
41.41284 |
-112.03087 |
|
|
DA3 |
Davis |
0.605 |
41.03606 |
-111.90632 |
|
|
UT3 |
Utah |
0.983 |
40.33722 |
-111.71865 |
|
|
UT4 |
Utah |
0.999 |
40.31310 |
-111.71038 |
|
|
UT7 |
Utah |
0.050 |
40.04556 |
-111.86118 |
|
|
WE2 |
Weber |
0.920 |
41.31532 |
-111.95857 |
|
|
Floral |
BE5 |
Box Elder |
0.185 |
41.42383 |
-112.03349 |
|
CA1 |
Cache |
0.192 |
41.81379 |
-111.80955 |
|
|
UT8 |
Utah |
0.054 |
40.04023 |
-111.85337 |
|
|
UT9 |
Utah |
0.048 |
40.02905 |
-111.83839 |
|
|
UT12 |
Utah |
0.122 |
40.00388 |
-111.82487 |
|
|
UT14 |
Utah |
0.135 |
39.99692 |
-111.83255 |
|
|
UT16 |
Utah |
0.104 |
39.98245 |
-111.82606 |
|
|
WE3 |
Weber |
0.941 |
41.18553 |
-112.04066 |
|
|
Turf |
BE3 |
Box Elder |
0.004 |
41.69340 |
-112.00984 |
|
CA2 |
Cache |
0.616 |
41.72535 |
-111.80753 |
|
|
CA3 |
Cache |
0.149 |
41.58166 |
-111.84779 |
|
|
DA5 |
Davis |
0.961 |
41.02142 |
-111.93194 |
|
|
UT10 |
Utah |
0.082 |
40.02306 |
-111.70732 |
|
|
UT11 |
Utah |
0.105 |
40.01267 |
-111.77738 |
|
|
UT15 |
Utah |
0.141 |
39.99258 |
-111.76879 |
|
|
WE1 |
Weber |
0.542 |
41.32905 |
-112.00984 |
|
|
Unspecified |
CA4 |
Cache |
0.147 |
41.57325 |
-111.81705 |
|
DA11 |
Davis |
0.928 |
40.74598 |
-111.85400 |
|
|
SL1 |
Salt Lake |
0.931 |
40.78039 |
-111.89626 |
|
|
SL4 |
Salt Lake |
0.982 |
40.77078 |
-111.85519 |
|
|
SL7 |
Salt Lake |
0.976 |
40.76367 |
-111.85029 |
|
|
SL8 |
Salt Lake |
0.969 |
40.75231 |
-111.87353 |
|
|
SL9 |
Salt Lake |
0.974 |
40.74897 |
-111.85457 |
|
|
DA11 |
Salt Lake |
0.990 |
40.74598 |
-111.85400 |
|
|
SL10 |
Salt Lake |
1.000 |
40.69306 |
-111.84800 |
|
|
UT2 |
Utah |
0.997 |
40.34708 |
-111.71357 |
|
|
UT13 |
Utah |
0.001 |
39.99747 |
-111.75800 |
|
|
UT17 |
Utah |
0.275 |
39.98220 |
-111.80317 |
*Site was dropped from all statistical analysis.
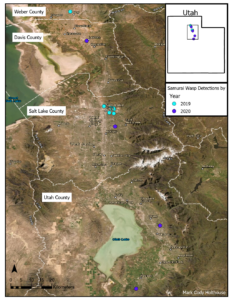
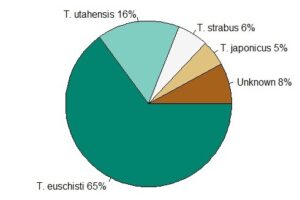


Fig. 5. Deployed laboratory reared Halyomorpha halys egg mass with adjacent rubber septa containing the kairomone treatment on the underside of a northern catalpa leaf in Salt Lake City, UT, 24 June to 27 August 2021.
Fig. 6. Mesocosm experiment set up with a 0.5 m height x 0.5 m depth x 0.8 m length plexiglass observation cage with a mesh lid, a clear panel trap (Alpha Scents, Inc, 30.5 cm X 30.5 cm) and lure attached via a clothespin to the upper portion of the card were hung at either end of the cage (1 m apart).
Objective 1:
Results
Trissolcus japonicus Detections
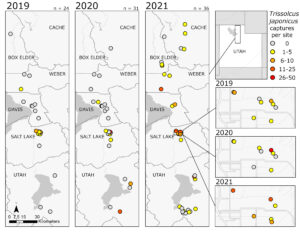
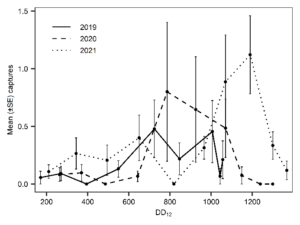
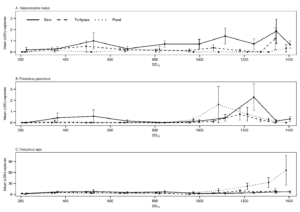
In 2019, T. japonicus was detected in two of five counties surveyed (24 total sites; Salt Lake and Weber) throughout the sampling period. Seasonal detections occurred from 171 to 1043 DD12℃. In 2020, detections occurred in three of five counties surveyed (31 total sites; Davis, Salt Lake, and Utah) and from 262 to 1149 DD12℃. Detections in 2021 were present in all six counties surveyed (36 total sites; Box Elder, Cache, Davis, Salt Lake, Utah, and Weber) and occurred throughout the entire sampling period (210- 1367 DD12℃). By the end of September 2021, T. japonicus was detected 85 km both north and south of the original 2019 detection site in Salt Lake City (Fig. 1). In all years, detections began increasing around 600 DD12℃, peaking from 700 to 1,000 DD12℃ in 2019 and 2020, and from 1,000 to 1,200 DD12℃ in 2021(Fig. 2). Mean T. japonicus captures per YSC increased each year with 1.61 (±0.58), 2.23 (±1.34), and 3.51 (±0.84) in 2019, 2020, and 2021, respectively.
Landscape Effects on Trissolcus japonicus Abundance
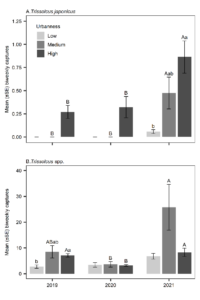
In the first two years of surveys, T. japonicus only occurred at sites with highly urban surroundings (Fig. 3A). In 2021, 8% of detections were in low urban sites, 13% in medium urban sites, and 79% of captures occurred in high urban sites. Urbanness was a significant effect each year (2019: χ2 = 7.65, df = 2, p = 0.022; 2020: χ2 = 7.38, df = 2, p = 0.025; 2021: χ2 = 46.0, df = 2, p < 0.001). Pairwise comparisons were not significant in 2019 and 2020. In 2021, T. japonicus was more abundant at high than low urban sites (p < 0.001). Across years, there were no significant differences in T. japonicus abundance in low urbanness sites (χ2 = 3.41, df = 2, p = 0.182). However, in medium and high sites the effect was significant (medium: χ2 = 12.1, df = 2, p = 0.002; high: χ2 = 40.2, df = 2, p < 0.001) and there were higher captures in 2021 than in 2019 (medium: p = 0.023; high: p < 0.001) and 2020 (medium: p = 0.006; high: p < 0.001) (Fig. 3A).
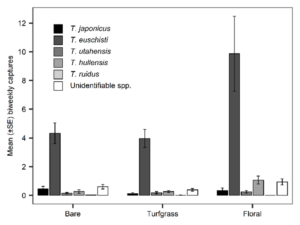
Groundcover type significantly affected T. japonicus abundance in the 24 orchard sites in 2021 (χ2 = 6.96, df = 2, p = 0.031); there were significantly more captures in bare ground orchards than turfgrass orchards (p = 0.029). Other pairwise comparisons were not significant. A total of 64 T. japonicus were captured; 31 (48%) in bare ground orchards, 9 (14%) in turfgrass, and 24 (38%) in floral (Table 1). Trissolcus japonicus experienced a similar peak (1000-1300 DD12℃) across all groundcover types in detections (Fig. 4B) and peak season analysis did not demonstrate any significant differences in abundance across groundcover types (χ2 = 3.03, df = 2, p = 0.22).
Mean captures of H. halys were low with 0.72 (±0.15), 0.23 (±0.08), and 0.06 (±0.03) adults per biweekly for bare, turfgrass, and floral, respectively (Table 1). Halyomorpha halys demonstrated similar patterns of peak abundance to T. japonicus (Fig. 4A) and mean T. japonicus captures per YSC in commercial orchards with H. halys presence were significantly higher compared to mean detections at sites with H. halys absence (χ2 = 11.5, df = 1, p < 0.001).
Landscape Effects on Native Trissolcus spp. Abundance
Of native Trissolcus spp. detected, T. euschisti was by far the most abundant (70%), followed by T. utahensis (Ashmead) (9%), T. hullensis (Harrington) (4%), T. ruidus (Johnson) (<.1%), T. strabus (Johnson) (<.1%), and T. parma (Johnson) (<0.1%) (Table 2). The exotic T. japonicus made up only 3-7% of Trissolcus spp. detected in northern Utah each year. An average of 13% of Trissolcus captures were unidentifiable beyond genus due to damaged specimens.
Urbanness was a significant effect in captures of native Trissolcus species in 2019 (χ2 = 8.42, df = 2, p = 0.015), but not in 2020 (χ2 = 0.567, df = 2, p = 0.753) or 2021 (χ2 = 4.68, df = 2, p = 0.096). Only pairwise comparisons between low and high urbanness in 2019 were significant (p = 0.015) and no clear trends across years were present (Fig. 3B). Across years there were no significant differences in Trissolcus abundance in low urbanness sites (χ2 = 3.91, df = 2, p = 0.142). However, in medium urbanness sites the effect was significant (χ2 = 14.6, df = 2, p < 0.001) with higher captures in 2021 than 2020 (p < 0.001). In high urbanness sites there was also a significant effect of year (χ2 = 31.3, df = 2, p < 0.001) with lower captures in 2020 than in either 2019 (p < 0.001) or 2021 (p = 0.002) (Fig. 3B).
In 2021, orchard groundcover type did not significantly influence captures of native Trissolcus spp. combined throughout the season (χ2 = 2.07, df = 2, p = 0.356); however, response of native Trissolcus only started to diverge after 900 DD12℃ and significant effects occurred during peak abundance, from 900 DD12℃ through the end of sampling (χ2 = 10.3, df = 2, p = 0.006). A total of 1,688 Trissolcus were captured in the 24 orchard sites: 394 (23%) in bare ground, 386 (23%) in turfgrass, and 908 (54%) in floral (Table 1). Pairwise comparison demonstrated that peak season native Trissolcus spp. abundance was significantly higher in floral orchards than bare (p = 0.007) and higher with borderline significance in floral than turfgrass (p = 0.0518). Trissolcus spp. abundance was similar between bare and turfgrass groundcovers and showed no significance in pairwise comparisons. Trissolcus spp. proportions were similar across mean biweekly captures for each groundcover type (Fig 5).
In contrast to T. japonicus, peak total Trissolcus spp. captures were misaligned with H. halys detections (Fig. 4A-C), and its presence or absence did not have a significant effect on captures (χ2 = 0.779, df = 1, p = 0.377).
Discussion
Despite the unique geographic challenges of extreme climate and high elevation, Trissolcus japonicus has successfully established in northern Utah since its initial detection in 2019 (Holthouse et al. 2020). Northern Utah consists of dry, semi-arid, and desert climates with average low temperatures below the estimated minimum threshold for T. japonicus development (12.2° C; Qiu et al. 2007) for up to eight months of the year (Utah Climate Center 2022). As adventive T. japonicus populations continue to persist in this harsh climate, it is crucial to understand its phenology in order to support conservation efforts in the Intermountain West.
The peak season of T. japonicus detections observed in this study falls in the months of July and August and is similar to the results Quinn et al. (2021) observed in the eastern U.S. In 2021, the peak season of T. japonicus was later than in previous years, possibly due to extreme drought conditions in Utah. The 18 months from January 2020 to June 2021 were the driest 18 month period on record for northern Utah (NCEI 2022). Some studies have shown that precipitation may play a greater role than temperature in some insect taxa occurrence in North America (Gutiérrez Illán et al. 2020), including the occurrence of T. japonicus’ primary host H. halys (Illán et al. 2022). Future research on climatic variables other than degree days will help further elucidate the potential range and seasonality of the exotic T. japonicus in the Intermountain West. Regardless, the capacity to survive and expand its distribution in areas previously considered marginal or unsuitable in geographic models (Avila and Charles 2018) has implications for range expansion of the exotic species in North America.
Halyomorpha halys has demonstrated a notable ability to disperse and thrive outside of predicted ranges (Kriticos et al. 2017, Illán et al. 2022); it follows, therefore, that T. japonicus may demonstrate similar capabilities. Recent analysis of H. halys populations found that proximity to urban areas was one of the most important factors mediating H. halys occurrence and likely reflects human-assisted transport, the use of human structures for overwintering sites, and increased ornamental plant diversity (Illán et al. 2022). Our analysis demonstrates that T. japonicus presence is also highly affected by proximity to urban areas; this may be due to its direct reliance on its host and/or its sustainment from urban area resources.
This study focused on the resources provided by the habitat in a 1 km buffer around each site, and groundcover composition in 2021 surveys of commercial orchard sites. The significant influence of urban habitat in contrast to the nonsignificant factor of groundcover type during peak season may be evidence that T. japonicus populations are utilizing resources from a greater dispersion area than previously hypothesized. Further research utilizing increased buffer sizes around sites may demonstrate an even greater extent of the wasps’ mobility to access floral and other resources. In contrast, this may indicate that urban ornamentals provide resources more appropriate to the exotic T. japonicus as compared to those provided by strip-cropped flowering plants, weeds, or wildflowers naturally occurring in orchard settings.
Overall, orchards with bare soil had comparable captures of T. japonicus to floral sites, while turfgrass sites had few T. japonicus. The interval between cultivation events at bare soil sites may have given rise to forbs that were beneficial to parasitoids. In addition, frequent disruptions of turfgrass from mowing and other management practices may have been a detriment to T. japonicus populations (Horton et al. 2003). Though insecticide applications were assumed to be similar across orchards and groundcover types, application pattern variation may have contributed to biased results as T. japonicus is highly sensitive to certain insecticides and is unlikely to survive in settings with broad-spectrum insecticide applications (Lowenstein et al. 2019).
A large underlying factor in the presence and abundance of T. japonicus is the presence and preferences of its host; the benefits floral understory provide are irrelevant without the insect host to support parasitoid populations. In 2021, we attempted to measure the effect of host presence on parasitoid abundance alongside groundcover by trapping for H. halys; however, the abundance of H. halys was low and these data were condensed to presence absence information. The presence of H. halys adults was a significant factor of T. japonicus abundance and the demonstrated alignment of seasonality between the host and natural parasitoid provides strong support for the continued reliance of T. japonicus on its natural host rather than pentatomids native to North America.
While we did not measure the effect of crop type on T. japonicus abundance, of the orchard crops surveyed in this study (apple, peach, and tart cherry), peach is known to be a host of particular significance to H. halys nymphal development and survivorship (Acebes-Doria et al. 2016). In this study bare groundcover sites more often had peach crop than apple or tart cherry and this may have had a significant effect on H. halys abundance. Regardless of the cause, on average, sites with bare groundcover had higher abundance of H. halys which could have significantly skewed the comparative proportion of T. japonicus at those sites and biased the apparent effect of bare groundcover. Had there been equal abundance of H. halys between all groundcover types, the benefits of floral resources for T. japonicus may have been more distinct.
Trissolcus japonicus presence was significantly affected by groundcover over the course of the season, but captures were not significantly different among groundcover types during the peak season of abundance and our prediction of increases due to floral resources was not supported. In addition to possible host bias, this may be due to overall low populations of T. japonicus in Utah as it is still in the establishment phase (Holthouse et al 2020). Research in Frederick County, VA demonstrated relatively few T. japonicus captures in the first few years of surveys after its initial detection in 2015 followed by subsequent increases in relative abundance in following years (Dyer et al. 2022). Additionally, the suboptimal climatic conditions of the Intermountain West may keep T. japonicus at relatively low densities as compared to locations with more suitable climate and availability of its host, H. halys. Surveys in this study represent T. japonicus still in its early establishment phase, and further investigation of this exotic outside its native range may yield more accurate assessments of its response to varying groundcovers.
Native Trissolcus species made up the majority of Trissolcus detected in agricultural and urban site surveys and provided a much larger sample size to detect true differences due to groundcover. Native species increased in response to floral resources supporting our original hypothesis of parasitoid preference for floral groundcover, though this was only apparent during the peak season of abundance, late in the growing season (>90012℃). Other studies have demonstrated the importance of floral resources for Trissolcus, particularly on fecundity (Hajirajabi et al. 2016, Foti et al. 2017), and foraging may be of increased priority to parasitoids as opportunities to reproduce increase alongside stink bug host abundance.
The results from this study continue to provide essential information on the presence and abundance of Trissolcus parasitoids native to northern Utah. Trissolcus euschisti was the most abundant Trissolcus spp. in northern Utah making up over two thirds of Trissolcus spp. detections on YSC, in line with previous YSC surveys in Utah (Holthouse et al. 2021b). While this wasp does have the ability to attack and successfully parasitize H. halys, rates of wasp emergence are low and have not shown sufficient evidence of effectively suppressing H. halys populations in Utah or elsewhere (Cornelius et al. 2016, Dieckhoff et al. 2017, Schumm et al. 2019, Costi et al. 2020, Holthouse et al. 2020, Konopka et al. 2020). In addition, our results suggest a seasonal misalignment of native Trissolcus abundance with that of H. halys. Conversely, T. japonicus demonstrated similar seasonality to its natural host in our study and has already shown the ability to suppress H. halys populations despite their low abundances (Holthouse et al. 2020).
The data presented have significant implications for T. japonicus as a biological control agent of H. halys in the Intermountain West and other regions. We present evidence of the importance of urban habitat to the presence and establishment of T. japonicus and suggest that the parasitoid is taking advantage of resources outside of resident orchard sites. In addition, the seasonality implications of the research include improved timing precision for mass releases of wasps with optimal ability for successful establishment, avoidance of harmful pesticides, and for provision of beneficial floral resources. This study supports strategies for further research on an effective biological control agent of H. halys and advances our understanding of the establishment of T. japonicus and native Trissolcus spp. in northern Utah.
Objective 2:
Results
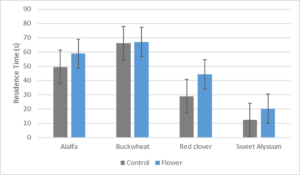
Preliminary results for this experiment were disappointing. When placed in the y-tube apparatus, T. japonicus individuals did not select an odor preference in most experimental runs. Many individuals remained within the insertion tube or in the main stem for the duration of the 5 min trial period without making a choice. After adjusting air flow, the trial wasps would choose to move around the y-tube back and forth between the two arms with no distinct searching behaviors. Results for preliminary trials (Fig. 6) show there was little difference between the control and floral odor arm residence times. ANOVA tests run on the pairs did not show statistical differences. As more trials were run, the average residence time became increasingly similar as seen in the buckwheat trials (n=46).
As part of the wasp longevity and fecundity trials we only ran a few trials to test experimental set up. The few preliminary trials that were run suggest that buckwheat, alyssum, alfalfa, and red clover may be life sustaining, while hairy vetch and birdsfoot trefoil show much less promise as wasps died within a 48 hr period.
Discussion
After reviewing preliminary results, numerous adjustments were made to the experimental equipment and design, including air flow, floral load, and pre-experiment feeding status of wasps. Eventually, the decision was made to not continue this study. Our experimental set-up and/or equipment did not seem to be an appropriate means to measure T. japonicus attraction to floral resources. Research produced after the abandonment of this experiment determined that T. japonicus reaches peak activity 6-7 days post emergence (Paul et al. 2022) and it is possibly that the 24-48 hr old wasps were too young and inactive to produce accurate results.
The results for the y-tube experiment were to determine which flowers should be tested as part of the wasp longevity and fecundity trials. Without the initial results to verify which floral resources may be attractive, we chose to cancel the wasp longevity and reproduction trials. Resources that are life sustaining, but not attractive to T. japonicus, serve little benefit in an agricultural setting without attractive volatiles to guide the wasps to floral resources.
At this point, the study of kairomones as part of objective 3 was showing great promise, but the results were inconclusive due to low parasitism rates. We decided to shift our research focus away from this objective 2 study in favor of being able to perform a more controlled kairomone experiment in a mesocosm arena as part of objective 3.
Objective 3:
Results
Field Trials
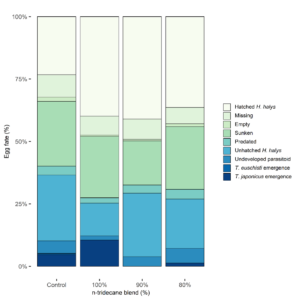
Of 92 total egg masses containing 2,472 eggs deployed in the field, only 13 masses had evidence of parasitism (14.1%; guarding female present or eclosed parasitoids) of which 6 masses supported successful emergence of adult parasitoids (6.5%). The lure treatments in order of highest proportion of eggs with successfully emerged parasitoids were 100% n-tridecane (10.6%), hexane control (5.2%), 80% n-tridecane (2.9%), and 90% n-tridecane (0.0%) (Fig. 7). Though the 100% lure resulted in double the parasitism of the control and more than three times that of the 90% and 80%, the analysis of all egg fate categories did not support statistical differences (p > 0.05; Table 3)
T. japonicus attacked 5.4% of deployed egg masses. This included one egg mass in each of the control and 80% attractant treatments and three in the 100% attractant treatment. Of the 129 H. halys eggs T. japonicus parasitized, there was a high rate of successful emergence (78.3%). Trissolcus euschisti attacked 6.5% of egg masses deployed with two egg masses in each of the control, 100%, and 90% attractant treatments and none in the 80% attractant treatment. Successful emergence of T. eushisti only occurred in one of the control treatment egg masses and the rate of overall emergence from attacked egg masses (136 eggs) was 1.8%. Two egg masses in the control treatment were parasitized by an unknown Trissolcus sp(p) and demonstrated no successful emergence (Table 4). In one instance, a T. euschisti female was found guarding an egg mass which later produced 26 T. japonicus individuals (92.9% emergence rate) and 2 inviable eggs.
Undeveloped parasitoid rates ranged from 1.6-5.9% across treatments, were highest in the 80% attractant treatment, and lowest in the 100% attractant treatment. The rate of unhatched H. halys was also variable across treatments making up the largest percentage of egg fate for the control treatment (26.5%) and was noticeably lower in the 100% attractant treatment compared to others. There was less than 2% difference in the predation between lure treatments. Sunken rates were relatively high (17.7-26.0%), and the combination of missing and empty eggs on average across lure treatments made up <10% of egg fate. Rates of hatched H. halys nymphs were similar across lure treatments containing stink bug kairomones (36.4-41.0%) but were unexpectedly low for the control lure making up approximately half of that seen in non-control lures.
Mesocosm Trials
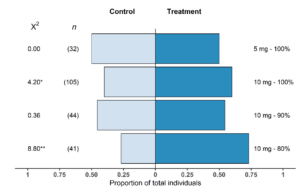
In the mesocosm system, T. japonicus females showed significant preference for the kairomone blend over the control for two of the four treatments tested (Fig. 8). The 10 mg-80% n-tridecane showed the most significant response (p = 0.003) with 73% of individuals choosing the kairomone lure. There was also a significant difference between the control and 10 mg-100% n-tridecane treatment lure (p = 0.04) with 60% of wasps choosing the kairomone-treated side. For the 10 mg-90% n-tridecane treatment, 55% of wasps chose the kairomone lure; however, there was no significant deviation from the expected response (p = 0.55). The choice between the low load rate treatment of 5 mg-100% n-tridecane and the control also showed no significance (p = 1); an even proportion of wasps chose each side. The proportion of no choice wasps per treatment varied from 20%-32% of wasps tested and was not associated with treatments.
Discussion
This study is the first to assess and provide proof-of-concept for kairomone-infused rubber septa lures to attract T. japonicus and other potential H. halys parasitoids in field and mesocosm settings. Though the kairomone chemicals tested herein have been previously evaluated in a small-scale lab setting (Y-tube olfactometer) (Zhong et al. 2017) implementation in the field may provide different results due to the influence of external factors on the shape, concentration, longevity, and spatial extent of the kairomone odor plume. In addition, interaction of the lure plume with nearby plant surfaces and mixing with other volatiles may give rise to plume masking or plume amplification, of which little is known in a parasitoid searching context (Dicke et al. 2003).
Our field results contrast those of Malek et al. (Malek et al. 2021) where the combination of the attractant and repellent was preferred over the attractant alone. In our study, not only did the 100% n-tridecane attractant lure have a numerically (not statistically) higher parasitism rate than the control, the lures containing the previously identified repellent (E)-2-decenal had less parasitism than those without the repellent, including the hexane control. These results may suggest that under field conditions a combination of the attractant and repellent is less effective at increasing parasitism by Trissolcus spp., at least with the specific load rate tested and placement in near proximity to the target host egg masses.
The lures used in the field study contained a load rate of 10 mg per lure, high in comparison to standard pheromone lures (1 mg), in the hopes of ensuring the attraction of parasitoids (Danielle Kirkpatrick Trécé, Inc., Adair, OK); however, this relatively high load rate may have been counterproductive to our objective. In studies of pheromone lure load rates, it has been found that attraction often plateaus and can even become repellent to the insect at high release rates (Knight and Light 2005, Stelinski et al. 2005, Charles et al. 2015). In addition, our lures were placed directly adjacent to the H. halys egg masses (Fig. 5- Methods section), which may deter parasitoid attraction as the host female stink bug does not typically remain near the eggs following oviposition.
Effect of lure load rate on parasitoid attraction was explored by examining both a 5 and 10 mg treatment of only the attractant n-tridecane in the mesocosm trials. The results of these trials demonstrated that the higher load rate of 10 mg was a more viable option for attracting T. japonicus and did not have a repellent effect. In contrast, the 5 mg treatment demonstrated no difference in attraction as compared with the hexane control lure. Based on these findings, the load rate may not have been detrimentally high in the field trials; however, we did not assess if a load rate greater than 10 mg could increase parasitoid attraction in a field setting.
Given the low number of egg masses tested in field trials and lack of statistically significant results, the parameters of this field study may have been unsuitable to discern differences in attractiveness of the different kairomone lure treatments. We observed low parasitism rates across all egg mass deployment periods in this study. In previous surveys, the site selected for the field study had the highest abundance of T. japonicus observed in northern Utah with concomitant high parasitism rates of H. halys (Holthouse et al. 2020). However, compared to T. japonicus populations in its native geographic regions and other adventive populations in the U.S., the abundance of T. japonicus in Utah is relatively low (Talamas, Herlihy, et al. 2015, Quinn et al. 2021). In addition, Utah suffered from significant drought over the time frame of this study (summer 2021) with 99.94% of the state in “extreme” or “exceptional” drought categories (Utah Climate Center) which may have had a negative effect on host abundance, parasitoid wasp populations, and H. halys egg parasitism rates (Zhu et al. 2012).
Other researchers have observed that lab-reared egg masses perform inferiorly to wild egg masses in terms of their attractiveness to parasitoids (Jones et al. 2014, Abram et al. 2017, Holthouse et al. 2020). The lures tested here were an initial attempt to solve this issue and increase the accuracy of parasitism rates detected in deployed egg mass surveys. Parasitism was observed in wild H. halys egg masses near deployed, unparasitized egg masses during the field study. Without an in-depth analysis of the kairomone plume release from lures, it is unknown if the plumes for each lure treatment may have overlapped within the tree block (lures were separated by approximately 3 m of tree canopy), and potentially interfered with one another. In addition, this may suggest the lure dispersed kairomones had unknown interactions with the surrounding environment or may have lacked the necessary kairomone load rate and/or ratio necessary to match the high attractiveness of wild egg masses with their natural kairomones intact.
Interestingly, the results of the mesocosm environment did not fully align with the field trial results, suggesting lures containing (E)-2-decenel may be repellent, but were better aligned with those of Malek et al. (Malek et al. 2021) where the 80% n-tridecane ratio was more attractive than n-tridecane alone. The result of wasp attraction to the 100% and 80% lures and unexpected lack of attraction to the 90% treatment occurred both in the field and in the mesocosm trials. Research has suggested that the variable secretions by different sexes and life stages of H. halys may be linked to their specific functions at said life stages and physiological states (Harris et al. 2015, Nixon et al. 2018, Malek et al. 2021). Perhaps the 100% and 80% n-tridecane treatments in this study are more closely associated with the gravid/ovipositing adult female or egg life stages that can be exploited by T. japonicus, while the 90% treatment is associated with other life stages. Further investigation is necessary to understand these counterintuitive results.
The mesocosm trials presented the opportunity to test the response of a much larger sample size and demonstrate significant differences between the control and treatments verifying the validity of these lures. While these trials supported the attractiveness of certain lures in a controlled environment, they also didn’t include many of the external factors at play in an authentic environment such as plant volatiles and competing parasitoids and predators. In addition, our mesocosm trials did not include the presence of host eggs which may have altered T. japonicus response.
In contrast, the field trials provide essential information about the interaction of host and parasitoid in a novel geographic and climatic environment. The results further verify the preliminary research in Utah on the effectiveness of the exotic T. japonicus and the common native T. euschisti. In this study, as in Holthouse et al. (Holthouse et al. 2020), T. euschisti demonstrated the ability to parasitize H. halys in a similar proportion to the natural parasitoid of H. halys, T. japonicus. However, T. euschisti seems an inviable option for successful biological control due to very low adult wasp emergence and successful stink bug nymph development likely associated with the failure of T. euschisti eggs to hatch or early death of larvae within H. halys eggs (Konopka et al. 2020). Additionally, this poses an evolutionary trap for T. euschisti and other native Trissolcus species that accept H. halys eggs as ovipositional sites despite their poor reproductive investment (Peri et al. 2021), although there is evidence that the recent arrival of T. japonicus may have implications for success of native Trissolcus on H. halys egg masses.
Research has demonstrated that Trissolcus spp. are able to parasitize host eggs that have previously been parasitized. In the case of T. japonicus and T. mitsukurri (Ashmead), each species outperformed the other when it was the first to oviposit, though T. mitsukurri was more aggressive in chasing off T. japonicus when present concurrently (Costi et al. 2022). Similar timing of competition and outperformance may have been the case in our observation of T. euschisti guarding an egg mass (presumably parasitized by T. japonicus prior to T. euschisti oviposition) that only produced T. japonicus offspring. Conversely, research by Konopka et al. (Konopka et al. 2016) demonstrated that parasitism by the exotic T. japonicus can provide facultative parasitism opportunities for native Trissolcus such as T. cultratus (Mayr) to successfully develop in H. halys eggs when they would otherwise fail.
Regardless of their reproductive success, native Trissolcus species can reduce the developmental success of H. halys embryos providing low levels of control for the pest (Abram et al. 2014). The attraction of T. eushisti and other native Trissolcus species to kairomone lures containing n-tridecane and (E)-2-decenal were not explored in a mesocosm environment in this study. However, the use of these kairomone lures in the field did result in attacks from T. euschisti in addition to T. japonicus. Consequently, further research into the physiological and behavioral interactions between the exotic T. japonicus and North American native Trissolcus requires investigation to fully evaluate the potential efficacy of biological control programs against H. halys.
While much investigation is needed into the complex system of parasitoids using semiochemicals to locate their host, the mesocosm results presented support the validity of using rubber septa as a release device for kairomones of stink bugs, and this research provides not only preliminary results but also a baseline for future work with experimental lures for H. halys parasitoids in a field environment.
Tables and Figures
Table 1. The number of H. halys adults and Trissolcus spp. captured in orchards with bare, turfgrass, and floral understories. Proportional abundance of species captured in each groundcover type is provided in parentheses. Data were collected 19 May through 5 October 2021 at 24 commercial orchard sites in northern Utah.
|
|
Trissolcus |
|||||||
|
Groundcover |
H. halys adults |
japonicus |
euschisti |
utahensis |
hullensis |
ruidus |
spp.* |
Total |
|
Bare |
63 (0.72) |
31 (0.48) |
294 (0.22) |
18 (0.44) |
10 (0.19) |
1 (0.50) |
40 (0.29) |
394 (0.23) |
|
Turfgrass |
20 (0.23) |
9 (0.14) |
301 (0.23) |
22 (0.12) |
24 (0.46) |
1 (0.50) |
29 (0.21) |
386 (0.23) |
|
Floral |
5 (0.06) |
24 (0.38) |
720 (0.55) |
78 (0.44) |
18 (0.35) |
0 (0.00) |
68 (0.50) |
908 (0.54) |
|
Total |
88 |
64 |
1315 |
118 |
52 |
2 |
137 |
1688 |
*Trissolcus wasps that were unidentifiable below the genus level due to damage are presented as Trissolcus spp.
Table 2. The number of Trissolcus spp. identified on yellow sticky cards in northern Utah, 2019-2021. Proportional abundance of each species by year is provided in parentheses.
|
|
Trissolcus |
|||||||||
|
Year |
Sites |
japonicus |
euschisti |
utahensis |
strabus |
hullensis |
ruidus |
parma |
spp.* |
Total |
|
2019 |
24 |
37 (0.03) |
690 (0.61) |
117 (0.10) |
0 (0.00) |
47 (0.04) |
0 (0.00) |
0 (0.00) |
247 (0.22) |
1138 |
|
2020 |
31 |
69 (0.07) |
691 (0.69) |
98 (0.10) |
15 (0.02) |
51 (0.05) |
0 (0.00) |
0 (0.00) |
76 (0.08) |
1000 |
|
2021 |
36 |
123 (0.04) |
2462 (0.79) |
206 (0.07) |
4 (0.00) |
50 (0.02) |
5 (0.00) |
1 (0.00) |
261 (0.08) |
3112 |
*Trissolcus wasps that were unidentifiable below the genus level due to damage are presented as Trissolcus spp.
Table 3. Statistical results from Kruskal-Wallis tests to compare each egg fate category differences across treatment lure types.
|
Egg Fate |
X2 |
df |
p |
|
Hatched H. halys |
2.79 |
3 |
0.426 |
|
Missing |
2.06 |
3 |
0.561 |
|
Empty |
0.98 |
3 |
0.806 |
|
Sunken |
1.21 |
3 |
0.751 |
|
Predated |
0.22 |
3 |
0.974 |
|
Unhatched H. halys |
6.62 |
3 |
0.085 |
|
Undeveloped parasitoid |
5.68 |
3 |
0.128 |
|
T. euschisti emergence |
3.00 |
3 |
0.392 |
|
T. japonicus emergence |
4.01 |
3 |
0.260 |
Table 4. Number of Halyomorpha halys egg masses parasitized (and percent of eggs with wasp emergence) in kairomone lure treatments for observed parasitoid species. Lure treatments are labeled based on percentage of n-tridecane attractant to (E)-2-decenal repellent; the control contained only hexane. Ninety-two H. halys egg masses were deployed containing 2,472 eggs on Catalpa speciosa leaves in Salt Lake City, UT, from 24 June through 27 August 2021.
|
|
Parasitized Egg Masses (% Emergence) |
|||
|
Treatment |
T. japonicus |
T. euschisti |
Unknown |
Total |
|
Control |
1 (100.0%) |
2 (5.5%) |
2 (0.0%) |
5 (23.0%) |
|
100% |
3 (89.0%)* |
2 (0.0%) |
0 (0.0%) |
5 (51.6%) |
|
90% |
0 (0.0%) |
2 (0.0%) |
0 (0.0%) |
2 (0.0%) |
|
80% |
1 (28.6%) |
0 (0.0%) |
0 (0.0%) |
1 (28.6%) |
|
Total |
5 (78.3%) |
6 (1.8%) |
2 (0.0%) |
13 (30.2%) |
*A T. euschisti female was found guarding an egg mass that resulted in 0% T. euschisti and 92.9% T. japonicus emergence. It was therefore only counted in the T. japonicus column.
Fig. 1. Trissolcus japonicus captures from late May through early October in northern Utah 2019-2021. Number of sites surveyed per year is shown as n at the top of each map. The map insets by year depict T. japonicus captures at and near the original detection site in Salt Lake City.
Fig. 2. Mean captures of Trissolcus japonicus on yellow sticky cards from late May through early October 2019-2021. Results are plotted by average degree days accumulated (DD12℃) at the end date of each 14 d (+1 d) deployment period.
Fig. 3. Mean biweekly captures of Trissolcus japonicus (A) and native Trissolcus spp. (B) in sites with low, medium, and high proportions of urban land in the surrounding 1 km boundary of survey sites. Lowercase letters represent significant differences among urbanness categories within a year. Uppercase letters represent significant differences among years within urbanness categories (Kruskal-Wallis test and Bonferroni corrected Dunn’s test, p > .05).
Fig. 4. Mean captures of Halyomorpha halys (A) on clear dual panel sticky strap, and Trissolcus japonicus (B) and Trissolcus spp. (C) on yellow sticky cards by average accumulated degree days of each deployment end date. Counts were averaged by groundcover designations of bare, turfgrass, and floral with eight sites of each designation.
Fig. 5. Mean captures of Trissolcus species per biweekly yellow sticky card deployment from May-September 2021. Cards were deployed at eight sites of each groundcover designation (bare, turfgrass, and floral). “Unidentifiable spp.” represents wasps that were unidentifiable beyond genus due to physical damage during capture.
Fig. 6. Residence time in the treatment vs. control arm of the Y-tube olfactometer out of 5 minutes.
Fig. 7. Fate of field-deployed Halyomorpha halys eggs (n=2,472) in each kairomone lure treatment, shown as percentage of total eggs. Lure treatments are labeled based on percentage of n-tridecane attractant to (E)-2-decenal repellent: hexane (control, n=598), 100% (n=615), 90% (n=617), and 80% (n=597). A total of 6 egg mass deployments were made in Salt Lake City, UT, from 24 June through 27 August 2021.
Fig. 8. Proportional response of Trissolcus japonicus female adults that chose a kairomone lure treatment or control (n = 32–105) within the 30 min experimental period. Chi-squared values are presented and numbers in parentheses represent sample size; * p < 0.05; ** p < 0.01.
Abram, P. K., T. D. Gariepy, G. Boivin, and J. Brodeur. 2014. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biol. Invasions. 16: 1387–1395.
Abram, P. K., K. A. Hoelmer, A. Acebes-Doria, H. Andrews, E. H. Beers, J. C. Bergh, R. Bessin, D. Biddinger, P. Botch, M. L. Buffington, M. L. Cornelius, E. Costi, E. S. Delfosse, C. Dieckhoff, R. Dobson, Z. Donais, M. Grieshop, G. Hamilton, T. Haye, C. Hedstrom, M. V. Herlihy, M. S. Hoddle, C. R. R. Hooks, P. Jentsch, N. K. Joshi, T. P. Kuhar, J. Lara, J. C. Lee, A. Legrand, T. C. Leskey, D. Lowenstein, L. Maistrello, C. R. Mathews, J. M. Milnes, W. R. Morrison, A. L. Nielsen, E. C. Ogburn, C. H. Pickett, K. Poley, J. Pote, J. Radl, P. M. Shrewsbury, E. Talamas, L. Tavella, J. F. Walgenbach, R. Waterworth, D. C. Weber, C. Welty, and N. G. Wiman. 2017. Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. J. Pest Sci. 90: 1009–1020.
Acebes-Doria, A. L., T. C. Leskey, and J. C. Bergh. 2016. Host plant effects on Halyomorpha halys (Hemiptera: Pentatomidae) nymphal development and survivorship. Environ. Entomol. 45: 663–670.
Avila, G. A., and J. G. Charles. 2018. Modelling the potential geographic distribution of Trissolcus japonicus: a biological control agent of the brown marmorated stink bug, Halyomorpha halys. BioControl. 63: 505–518.
Baggen, L. R., and G. M. Gurr. 1998. The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Biol. Control. 11: 9–17.
Bertoldi, V., G. Rondoni, J. Brodeur, and E. Conti. 2019. An egg parasitoid efficiently exploits cues from a coevolved host but not those from a novel host. Front. Physiol. 10: 746.
Charles, J. G., V. A. Bell, A. J. Hall, D. M. Suckling, J. T. S. Walker, L. M. Cole, P. W. Shaw, D. R. Wallis, and J. G. Millar. 2015. Evaluation of the synthetic sex pheromone of the obscure mealybug, Pseudococcus viburni, as an attractant to conspecific males, and to females of the parasitoid Acerophagus maculipennis. Entomol. Exp. Appl. 157: 188–197.
Christman, M. E., L. R. Spears, J. P. Strange, W. D. Pearse, E. K. Burchfield, and R. A. Ramirez. 2022. Land cover and climate drive shifts in Bombus assemblage composition. Agric. Ecosyst. Environ. 339: 108113.
(Climate at a Glance | National Centers for Environmental Information (NCEI)) . 2022. Climate at a Glance | National Centers for Environmental Information (NCEI). (https://www.ncei.noaa.gov/access/monitoring/climate-at-a-glance/).
Cornelius, M. L., C. Dieckhoff, K. A. Hoelmer, R. T. Olsen, D. C. Weber, M. V. Herlihy, E. J. Talamas, B. T. Vinyard, and M. H. Greenstone. 2016. Biological control of sentinel egg masses of the exotic invasive stink bug Halyomorpha halys (Stål) in Mid-Atlantic USA ornamental landscapes. Biol. Control. 103: 11–20.
Costi, E., E. Di Bella, D. Iotti, and L. Maistrello. 2022. Biocontrol implications of multiparasitism by Trissolcus mitsukurii and Trissolcus japonicus on the invasive brown marmorated stink bug. Entomol. Exp. Appl. 170: 772–781.
Costi, E., W. H. L. Wong, J. Cossentine, S. Acheampong, L. Maistrello, T. Haye, E. J. Talamas, and P. K. Abram. 2020. Variation in levels of acceptance, developmental success, and abortion of Halyomorpha halys eggs by native North American parasitoids. Biol. Control. 151: 104396.
Dicke, M., J. Boer, M. Höfte, and C. Rocha Granados. 2003. Mixed blends of herbivore-induced plant volatiles and foraging success of carnivorous arthropods. Oikos. 101: 38–48.
Dieckhoff, C., K. M. Tatman, and K. A. Hoelmer. 2017. Natural biological control of Halyomorpha halys by native egg parasitoids: a multi-year survey in northern Delaware. J. Pest Sci. 90: 1143–1158.
Utah Climate Center. 2020. Utah, USA - Climate data and average monthly weather. Weather Atlas. (https://www.weather-us.com/en/utah-usa-climate).
Dyer, J. E., E. J. Talamas, T. C. Leskey, and J. C. Bergh. 2022. Influence of trap location in the tree canopy on captures of adventive Trissolcus japonicus (Hymenoptera: Scelionidae). J. Econ. Entomol. 115: 904–908.
Foti, M. C., M. Rostás, E. Peri, K. C. Park, T. Slimani, S. D. Wratten, and S. Colazza. 2017. Chemical ecology meets conservation biological control: identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest Sci. 90: 299–310.
Gutiérrez Illán, J., E. H. Bloom, C. H. Wohleb, E. J. Wenninger, S. I. Rondon, A. S. Jensen, W. E. Snyder, and D. W. Crowder. 2020. Landscape structure and climate drive population dynamics of an insect vector within intensely managed agroecosystems. Ecol. Appl. 30: e02109.
Hajirajabi, N., M. M. Fazel, J. A. Harvey, A. Arbab, and S. Asgari. 2016. Dietary sugars and proline influence biological parameters of adult Trissolcus grandis, an egg parasitoid of Sunn pest, Eurygaster integriceps. Biol. Control. 96: 21–27.
Harris, C., S. Abubeker, M. Yu, T. Leskey, and A. Zhang. 2015. Semiochemical Production and Laboratory Behavior Response of the Brown Marmorated Stink Bug, Halyomorpha Halys. PLOS ONE. 10: e0140876.
Hickman, J. M., and S. D. Wratten. 1996. Use of Phelia tanacetifolia strips to enhance biological control of aphids by hoverfly larvae in cereal fields. J. Econ. Entomol. 89: 832–840.
Holthouse, M. C., Z. R. Schumm, E. J. Talamas, L. R. Spears, and D. G. Alston. 2020. Surveys in northern Utah for egg parasitoids of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) detect Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae). Biodivers. Data J. 8.
Holthouse, M. C., L. R. Spears, and D. G. Alston. 2021a. Comparison of yellow and blue sticky cards for detection and monitoring parasitoid wasps of the invasive Halyomorpha halys (Hemiptera: Pentatomidae). J. Insect Sci. 21: 1.
Holthouse, M. C., L. R. Spears, and D. G. Alston. 2021b. Urban host plant utilisation by the invasive Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in northern Utah. NeoBiota. 64: 87–101.
Horton, D. R., D. A. Broers, R. R. Lewis, D. Granatstein, R. S. Zack, T. R. Unruh, A. R. Moldenke, and J. J. Brown. 2003. Effects of mowing frequency on densities of natural enemies in three Pacific Northwest pear orchards. Entomol. Exp. Appl. 106: 135–145.
Illán, J. G., G. Zhu, J. F. Walgenbach, A. Acebes-Doria, A. M. Agnello, D. G. Alston, H. Andrews, E. H. Beers, J. C. Bergh, R. T. Bessin, B. R. Blaauw, G. D. Buntin, E. C. Burkness, J. P. Cullum, K. M. Daane, L. E. Fann, J. Fisher, P. Girod, L. J. Gut, G. C. Hamilton, J. R. Hepler, R. Hilton, K. A. Hoelmer, W. D. Hutchison, P. J. Jentsch, S. V. Joseph, G. G. Kennedy, G. Krawczyk, T. P. Kuhar, J. C. Lee, T. C. Leskey, A. T. Marshal, J. M. Milnes, A. L. Nielsen, D. K. Patel, H. D. Peterson, D. D. Reisig, J. P. Rijal, A. A. Sial, L. R. Spears, J. M. Stahl, K. M. Tatman, S. V. Taylor, G. Tillman, M. D. Toews, R. T. Villanueva, C. Welty, N. G. Wiman, J. K. Wilson, F. G. Zalom, and D. W. Crowder. 2022. Evaluating invasion risk and population dynamics of the brown marmorated stink bug across the contiguous United States. Pest Manag. Sci. n/a.
Jervis, M. A., N. A. C. Kidd, and M. Walton. 1992. A review of methods for determining dietary range in adult parasitoids. Entomophaga. 37: 565–574.
Jones, A. L., D. E. Jennings, C. R. R. Hooks, and P. M. Shrewsbury. 2014. Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug, Halyomorpha halys. Biol. Control. 78: 61–66.
Knight, A. L., and D. M. Light. 2005. Dose–response of codling moth (Lepidoptera: Tortricidae) to ethyl (E, Z)-2,4-decadienoate in apple orchards treated with sex pheromone dispensers. Environ. Entomol. 34: 604–609.
Konopka, J. K., T. Haye, T. Gariepy, P. Mason, D. Gillespie, and J. N. McNeil. 2016. An exotic parasitoid provides an invasional lifeline for native parasitoids. Ecol. Evol. 7: 277–284.
Konopka, J. K., D. Poinapen, T. Gariepy, D. W. Holdsworth, and J. N. McNeil. 2020. Timing of failed parasitoid development in Halyomorpha halys eggs. Biol. Control. 141: 104124.
Kriticos, D. J., J. M. Kean, C. B. Phillips, S. D. Senay, H. Acosta, and T. Haye. 2017. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 90: 1033–1043.
Lee, J. C., G. E. Heimpel, and G. L. Leibee. 2004. Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Appl. 111: 189–199.
Lowenstein, D. M., H. Andrews, A. Mugica, and N. G. Wiman. 2019. Sensitivity of the Egg Parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to Field and Laboratory-Applied Insecticide Residue. J. Econ. Entomol. 112: 2077–2084.
Malek, R., J. M. Kaser, G. Anfora, M. Ciolli, A. Khrimian, D. C. Weber, and K. A. Hoelmer. 2021. Trissolcus japonicus foraging behavior: Implications for host preference and classical biological control. Biol. Control. 161: 104700.
McIntosh, H. R., V. P. Skillman, G. Galindo, and J. C. Lee. 2020. Floral resources for Trissolcus japonicus, a parasitoid of Halyomorpha halys. Insects. 11.
Nixon, L. J., W. R. Morrison, K. B. Rice, E. G. Brockerhoff, T. C. Leskey, F. Guzman, A. Khrimian, S. Goldson, and M. Rostás. 2018. Identification of volatiles released by diapausing brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). PLOS ONE. 13: e0191223.
Peri, E., M. C. Foti, L. Martorana, A. Cusumano, and S. Colazza. 2021. The invasive stink bug Halyomorpha halys affects the reproductive success and the experience-mediated behavioural responses of the egg parasitoid Trissolcus basalis. BioControl. 66: 329–342.
Qiu, L., Y. Zhongqi, and T. Wanqiang. 2007. Biology and population dynamics of Trissolcus halyomorphae. Sci. Silvae Sin. 43: 62–65.
Quinn, N. F., E. J. Talamas, T. C. Leskey, and J. C. Bergh. 2021. Seasonal captures of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) and the effects of habitat type and tree species on detection frequency. Insects. 12: 118.
(R version 4.1.1; R Core Team 2021) . n.d. R version 4.1.1; R Core Team 2021.
Salerno, G., E. Conti, S. Colazza, and E. Peri. n.d. Volatile chemicals released by pentatomid bugs having a kairomonal effect on Trissolcus basalis: their role on host specificity and prospects for IPM. 8.
Schumm, Z. R., M. C. Holthouse, Y. Mizuno, D. G. Alston, and L. R. Spears. 2019. Parasitoid wasps of the invasive brown marmorated stink bug in Utah. Utah State Univ. Ext. Fact Sheet. ENT-198-19: 6.
Stelinski, L. L., J. R. Miller, and L. J. Gut. 2005. Captures of two leafroller moth species (Lepidoptera: Tortricidae) in traps baited with varying dosages of pheromone lures or commercial mating-disruption dispensers in untreated and pheromone-treated orchard plots. Can. Entomol. 137: 98–109.
Talamas, E. J., M. V. Herlihy, C. Dieckhoff, K. A. Hoelmer, M. Buffington, M.-C. Bon, and D. C. Weber. 2015. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 43: 119–128.
Talamas, E. J., N. F. Johnson, and M. Buffington. 2015. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). J. Hymenopt. Res. 43: 45–110.
(Utah Climate Center - Utah State University) . 2022. Utah Climate Center - Utah State University. (https://climate.usu.edu/mchd/index.php).
Zhong, Y.-Z., J.-P. Zhang, L.-L. Ren, R. Tang, H.-X. Zhan, G.-H. Chen, and F. Zhang. 2017. Behavioral responses of the egg parasitoid Trissolcus japonicus to volatiles from adults of its stink bug host, Halyomorpha halys. J. Pest Sci. 90: 1097–1105.
Zhu, G., W. Bu, Y. Gao, and G. Liu. 2012. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLOS ONE. 7: e31246.
Research outcomes
Objective 1:
- Continue phenological work on samurai wasp in the Intermountain West. It is still not understood well how and where these parasitoids overwinter. Supporting successful overwintering would continue to increase this wasps prevalence.
- Study the effects of other climatic variables other than degree days, for example, precipitation.
- Further research the extent of the wasps' mobility to access sites in increased buffer sizes.
- Continue monitoring for the expansion of the samurai wasp in Utah and other Western states.
Objective 2:
- Study the attraction of samurai wasps to floral resources using a range of ages of wasps.
- Adapt y-tube apparatus or utilize a different approach to test wasp attraction.
- Investigate nutritional quality and accessibility of common cover crops to samurai wasp.
Objective 3:
- Research the plume and range of attraction experimental lures provide.
- Research the effect of temperature on the kairomone lures.
- Investigate higher load rates and different chemical make-ups.
- Run mesocosm trials with the addition of egg masses adjacent to lures.
- Evaluate lures in more field trials, potentially with supplied wasps.
- Explore the potential for facultative parasitism and the competition between native wasps and the samurai wasp.
Education and Outreach
Participation summary:
Fact Sheets:
- Schumm, Zachary R.; Richardson, Kate V.; Holthouse, Mark Cody; Mizuno, Yota; Alston, Diane G.; and Spears, Lori R. (2022). "Parasitoid Wasps of the Invasive Brown Marmorated Stink Bug in Utah" Utah State University Extension – Fact Sheet ENT-198-19.
Published press articles, newsletters:
- Richardson, K. V., Alston, and L. Spears. 2023. Efficacy of Kairomone Lures to Attract Parasitoids of Halyomorpha halys. Insects, 14: 125.
- Richardson, K. V., C. Holthouse, D. Alston, and L. Spears. Landscape Effects on Native and Exotic Trissolcus spp. (Hymenoptera: Scelionidae) in Northern Utah. (manuscript in prep).
- Richardson, K. V., Alston, and L. Spears. 2023. Adventive population of Trissolcus japonicus (Hymenoptera: Scelionidae), parasitoid of Halyomorpha halys (Hemiptera: Pentatomidae), discovered in southwestern Idaho. Journal of Integrated Pest Management. (in press).
- Richardson, K. V., Z. Ross, D. Alston, and L. Spears. 2023. Status of the Samurai Wasp (Trissolcus japonicus) in Utah. Utah Pests News, Utah State University Extension. Vol 16: Winter edition. https://extension.usu.edu/pests/files/up-newsletter/2023/UtahPestsNews-winter23.pdf
- 14,000+ subscribers
- Richardson, K.V. Bee Assassin Bug Predating on BMSB. Utah Pests News, Utah State University Extension. Vol 15: Summer edition. https://extension.usu.edu/pests/files/up-newsletter/2021/UtahPestsNews-summer21.pdf
- 14,000+ subscribers
Webinars, talks and presentations:
- Richardson, K. V., M. C. Holthouse, D. Alston, and L. Spears. 2022. Urban Landscape and Floral Resource Effects on Trissolcus japonicus, Parasitoid of Halyomorpha halys, in Utah. Entomological Society of America, November 13-16 ESA, ESC, ESBC Joint Annual Meeting- Student 10 min paper competition.
- Richardson, K.V., D. Alston, and L. Spears. 2022. Biological Control of Brown Marmorated Stink Bug. 10th Annual Utah Urban and Small Farms Conference, February 24.
- 82 live attendees
- 674 YouTube views
- Richardson, K.V., D. Alston, and L. Spears. 2022. Brown Marmorated Stink Bug Biological Control. Utah State Horticultural Association Annual Convention, January 20.
- 28 live attendees
- Richardson, K.V., D. Alston, and L. Spears. 2021. Kairomone Lures to attract parasitoids of the invasive Halyomorpha halys (Stål). Entomological Society of America, October 31- November 3 National Annual Meeting– Student 10 min paper competition.
- Richardson, K.V. Beneficial Insects: Wasps and Flies. Utah Pests Extension, June 29 Vegetable IPM Twilight Meetings.
- 61 live attendees
- 783 YouTube views
- 232 Facebook views
Workshop/field days:
- Richardson, K.V. Insect Basics Class. Freckle Farm, June 17 Little Farmers Camp.
- ~20 live attendees
- Richardson, K.V. Biological control of BMSB by samurai wasp (Trissolcus japonicus). Utah Pests Extension, September 30 First Detector Workshop.
- 22 live attendees
- Richardson, K.V. Research on Samurai Wasp (Trissolcus japonicus) in Utah. Utah Tree Fruit Field Day, July 5.
- ~25 live attendees
Other:
- Richardson, K.V. Samurai Wasp (Trissolcus japonicus Ashmead) Discovered in Idaho by Utah State University. Idaho State record announcement.
Objective 1: Promote T. japonicus for the sustainable management of BMSB to approximately 10,000 specialty crop producers and other relevant stakeholders.
This objective was met through the creation and distribution of research materials. This included the revision of a parasitoid fact sheet published through USU Extension available online as well as two newsletter articles published in the Utah Pests Quarterly Newsletter. This newsletter currently has over 14,000 subscribers, many of which are specialty crop producers and other relevant stakeholders. In addition to these informational products published through USU extension, a peer-reviewed article is currently in press with the Journal of Integrated Pest Management which is an extension-focused journal with the objective to target a non-technical audience of anyone engaged in any aspect of integrated pest management, including, but not limited to farmers and ranchers, consultants, extension professionals, retailers, manufacturers and suppliers of pest management products, educators, foresters, animal and human health professionals, and pest control operators. This journal has an impact factor of 4.107 and the article published there is likely to reach a broad audience.
In addition to written materials, presentations, webinars, workshops, and field days were given at six different stakeholder conferences and meetings. These events provided an opportunity to update stakeholders and producers on sustainable integrated pest management programs and gather information on future needs.
Objective 2: Add to the national H. halys research knowledge base and share project results with professional colleagues through two journal publications and three scientific conference presentations.
The results from this project contributed to the national knowledge base of H. halys research. Collaborations were made with other organization for the completion of projects including Oregon Department of Agriculture and Trece, Inc. One published research article is currently available to professional colleagues in the journal Insects which has an impact factor of 3.141. This article is being published in a special issue "Biological Control in Temperate Orchards" and should reach many researchers in the agricultural field. Another journal manuscript is currently in prep to be submitted to Environmental Entomology which has an impact factor of 2.387 and reaches many members of the Entomological Society of America.
The results from the project were also disseminated to national researchers of H. halys through three presentations at Entomological Society of America meetings, one of which was a joint meeting with the Entomological Society of Canada and the Entomological Society of British Columbia. Networking with colleagues was also conducted at these conferences. Open channels were kept with researchers at other organizations, and important discoveries and results were relayed via emails.
Objective 3: Disseminate information on invasive species and sustainable pest management to the general public via varied outreach venues and formats.
The results from the project were widely disseminated to the general public. This was done through extension outreach channels. A large portion of the 14,000 subscribers to the Utah Pest Newsletter are master gardeners or interested members of the general public. In addition, presentations given at grower conferences and meetings are accessible to the public on youtube and facebook. These videos are clearly being taken advantage of as evidenced by the number of views they have garnered.