Final report for GW21-222
Project Information
Globodera pallida, the pale cyst nematode (PCN) is a quarantined pest of potato in Idaho. With the potential to cause up to 80% yield loss and to remain in the soil for 20 to 30 years, it poses a major threat to the Idaho potato industry. Growers with infested fields are losing profit because they can no longer plant potato until PCN is deemed fully eradicated and they undergo the extensive USDA APHIS deregulation process. This proposal seeks to investigate the efficacy of trap crops and crop rotation as sustainable management strategies for use in eradication efforts. Trap crops must be nonhosts that stimulate PCN hatch but prevent development and reproduction. Previous research has identified litchi tomato and quinoa as crops with PCN trap crop potential. Goals of the project include evaluating the impact and feasibility of litchi tomato and quinoa on populations of PCN over time in both greenhouse and Idaho field conditions. Three-year crop rotations with trap crops and partially resistant potato variety 'Innovator' are also being assessed. Findings will be presented to agricultural stakeholders through various presentations. If litchi tomato and quinoa are successful in significantly reducing PCN populations in the field, they can be recommended to growers with PCN-infested acreage. Quinoa has the added benefit of providing a valuable yield when used in rotation. Ultimately, this project will establish more sustainable strategies for use in an integrated management approach to the eradication of the pale cyst nematode.
Objectives:
- Determine the effect of the quinoa variety ‘Kailey’ and litchi tomato on pale cyst nematode (PCN) populations in both greenhouse and Idaho field conditions.
- Compare the effect of quinoa to that of litchi tomato as trap crops for the pale cyst nematode through evaluating post-treatment hatching effect, viability of eggs, and reproduction on potato.
- Evaluate efficacy of three-year field rotations with resistant potato ‘Innovator’ and litchi tomato on PCN populations under Idaho field conditions.
- Present findings to potato producers, extension specialists, and other stakeholders at field days or meetings and publish a newsletter on implications of the results in eradication of the pale cyst nematode.
Hickman_WSARE updated project timeline
Funding Start: 8/1/21, Funding End: 7/31/23
*Funding will support the next 2 years of research
Research Objectives 1 & 2: Litchi Tomato vs Quinoa as Trap Crops Field Trial
| Maintain 2nd field trial | Aug – Sept 2021 |
| Terminate 2nd field trial at 12 weeks, microplots to cold room for 8 weeks minimum | Sept 2021 |
| Perform evaluations of cyst bag samples collected at 6-weeks and 12-weeks | Sept – Nov 2021 |
| Plant bioassay and grow 12 weeks |
Jan – Apr 2022 |
| Terminate bioassay, dry soil/root samples for cyst extraction | April – May 2022 |
| Extract cysts from soil/root samples, conduct evaluations of recovered cyst bags | May – Sept 2022 |
Research Objectives 1 & 2: Litchi Tomato vs Quinoa as Trap Crops Greenhouse Trial
| Terminate greenhouse trial 1 bioassay, dry soil/root samples for extraction | Oct 2021 |
| Extract cysts. Perform evaluations on recovered cyst bags | Oct – Dec 2021 |
| Plant trial 2, grow for 12 weeks | Jan – Apr 2022 |
| Terminate. Pots into the cold room for 8-week dormancy period. Perform evaluations on the 12-week cyst bag samples | Apr – Jun 2022 |
| Remove pots from cold room, plant bioassay with potato and grow 12 weeks | Jun – Oct 2022 |
| Terminate, dry soil/root samples for cyst extraction. Extract cysts. Conduct evaluations of recovered cyst bags | Oct 2022 – Jan 2023 |
Research Objective 3: Investigation of Crop Rotations for PCN Reduction Over Time
| Maintain trial 1 (year 2) and trial 2 (year 1) in field | Aug– Sept 2021 |
| Terminate trial 1 (year 2) and trial 2 (year 1). Trial 1 (year 2) microplots go to the cold room at U of I. Trial 2 (year 1) microplots go to storage facility. Minimum 8-week cold period. | Sept 2021 |
| Conduct evaluations on end of season cyst bag samples | Oct – Dec 2021 |
| Plant trial 1 (year 3) bioassay with Russet Burbank in greenhouse and grow for 12 weeks. Conduct evaluations on beginning season cyst bag samples. | Jan – May 2022 |
| Plant trial 2 (year 2) in the field for 12 weeks. Conduct evaluations for beginning season cyst bag samples. Extract cysts from trial 1 (year 3) bioassay and conduct evaluations on recovered cyst bags. | May – Sept 2022 |
| Terminate trial 2 (year 2), store microplots in cold room at U of I for 8 weeks. Conduct evaluations on end of season cyst bag samples. | Sept – Dec 2022 |
| Plant trial 2 (year 3) bioassay potato and grow for 12 weeks. Conduct evaluations on beginning season cyst bag samples. | Jan – May 2023 |
| Dry and extract cysts from trial 2 (year 3) bioassay. Conduct evaluations on recovered cyst bags. | May – Jul 2023 |
Objective 4: Outreach & Education
| Present at IAPP meeting | Nov 2021 |
| Present at Idaho Potato Conference | Jan 2022 |
| Present at IAPP meeting | Nov 2022 |
| Present at Idaho Potato Conference | Jan 2023 |
| Publish newsletter on trap crops and crop rotation for PCN eradication | Jul 2023 |
Cooperators
- - Producer
- (Researcher)
- (Researcher)
Research
Research Objectives:
- Determine the effect of the quinoa variety ‘Kailey’ and litchi tomato on pale cyst nematode (PCN) populations in both greenhouse and Idaho field conditions.
- Compare the effect of quinoa to that of litchi tomato as trap crops for the pale cyst nematode through evaluating post-treatment hatching effect, viability of eggs, and reproduction on potato.
- Evaluate efficacy of three-year field rotations with resistant potato ‘Innovator’ and litchi tomato on PCN populations under Idaho field conditions.
Objectives 1 & 2 Approach: Litchi Tomato vs Quinoa as PCN Trap Crops
Greenhouse Trials
The impact of quinoa and litchi tomato on PCN populations is being assessed in both greenhouse and field trials. In the greenhouse trials, 12 repetitions of quinoa, litchi tomato, barley, and Russet Burbank were grown in a randomized complete block design in 6-inch clay pots inoculated with PCN at a starting rate of 8 eggs per gram of soil. Barley is a known nonhost of PCN with no hatching stimulatory effect that served as a negative control. Russet Burbank is a susceptible potato that served as a positive control. Standard protocols were followed to contain PCN in which the soil was inoculated using cysts contained within 1-in2 mesh cyst bags. Soil was a 2:1 mix of sand to soil sterilized by autoclave. Russet Burbank was grown from disease-free 4-week-old tissue cultures. Barley was grown from seed. Litchi tomato and quinoa were planted as 4-week-old transplants. Plants were grown for 12 weeks while receiving standard amounts of water and fertilizer. At 12 weeks, one cyst bag per pot was sampled to determine egg counts, percent viable eggs, and percent hatch stimulated by potato root diffusate. Egg counts were conducted by breaking open a cyst and using microscopy to count all eggs still containing a juvenile nematode. In order to determine percentage of viability, eggs were stained with a fluorescent dye and later washed. Any eggs that retained the dye have a disrupted membrane and were considered no longer viable. Hatch was determined by applying potato root diffusate to a known number of eggs. Potato root diffusate contains a hatching stimulus so that after eggs were incubated over a 2-week period, percentage of hatched eggs was determined by counting the number of hatched juveniles. Plants were terminated at 12 weeks and pots were be stored in a cold room for 8 weeks to simulate a dormancy period.
After 8 weeks in the cold room, pots were planted with susceptible or resistant potato 4-week-old tissue cultures. Six repetitions were planted with Innovator, a potato variety with partial PCN resistance, and six repetitions were be planted with Russet Burbank as the susceptible variety. After 12 weeks of growth in greenhouse conditions, the experiment will be terminated. Soil and root samples will be dried to undergo extraction. An elutriator will be used to extract PCN cysts from the soil and root samples. Cysts will be counted and average egg counts per sample were calculated in order to determine the reproduction factor. Remaining cysts in the recovered cyst bags will be evaluated for egg counts, percent viable eggs, and percent hatch stimulated by potato root diffusate. The greenhouse trial is being repeated twice.
Field Trials:
The field trials are being conducted at a field site located in a PCN-infested field near Idaho Falls. Field trials were contained within microplots to avoid further infestation of field soil with PCN. Microplots consisted of two 5-gallon buckets. The upper bucket was filled with soil and contained holes at the bottom so that excess water could drain through. A lower bucket collected water and soil that drained. During field maintenance trips, the water collected in the lower buckets of the microplots was drained through a filter that collected any escaped cysts. Microplots were infested with mesh cyst bags attached to stakes at a starting infestation rate of 4.3 eggs per gram of soil in hopes of attaining a rate of 2.5 eggs per gram of soil in pre-bioassay control plots. The treatments consisted of quinoa, litchi tomato, and barley as a negative control. There were 12 replications for each treatment and setup was a randomized complete block design. The barley and quinoa were grown from seed while 4-week-old litchi tomato seedlings were transplanted. There were 6 plants per microplot and the soil surface was be covered by layers of mesh and landscape fabric to contain any escaped cysts. Microplots were embedded in the field with 3-ft spacing. Treatments were planted in May and grown for 12 weeks receiving water as needed and fertilizer biweekly. Field maintenance occurred every two weeks in which microplots were inspected and weeded, and water collected in the lower bucket was emptied through a filter. A cyst bag was sampled from each plot at 6-weeks and at 12-weeks to evaluate egg counts, percent viable eggs, and percent hatch stimulated by potato root diffusate. After 12-weeks, plants were terminated and microplots were sealed with lids and brought back to the University of Idaho to be placed in a cold room for 8 weeks to simulate a dormancy period. After 8-weeks, a bioassay with susceptible or resistant potato was planted. Six repetitions were planted with partially resistant variety Innovator while six repetitions were planted with the susceptible variety Russet Burbank. Following 12 weeks of growth in greenhouse conditions, soil and root samples were dried to undergo cyst extraction in the elutriator. Cyst counts and average egg counts were used to determine the reproduction factor. Recovered cyst bags were evaluated for egg counts, percent viable eggs, and percent hatch stimulated by potato root diffusate. This field trial is being repeated three times due to abnormally high temperatures during the second trial in June 2021 which may have impacted the cysts.
The impact of quinoa and litchi tomato are being assessed based on remaining total egg counts, percentage of remaining eggs still viable, and percentage of viable eggs that hatch in comparison to the controls before and after the bioassay with potato. PCN reproduction after treatment with quinoa or litchi tomato is also being evaluated. All data are being analyzed with a SAS analysis of variance for randomized complete block design. Least squares means separation is being used to compare the effects of litchi tomato and quinoa on PCN populations.
Objective 3 Approach: Investigation of Crop Rotations for Reduction of PCN Over Time
The three-year crop rotations are being assessed as field trials. As with the field trials described for Objectives 1 and 2, this field trial is also taking place within microplots at the PCN-infested field site near Idaho Falls. Microplots were inoculated with cyst bags at an initial rate of 7.5 eggs per gram of soil. Sampling cyst bags were included so that there are enough for sampling at the beginning and end of each growing season. These samples were evaluated for egg counts, percent viable eggs, and percent hatch stimulated by potato root diffusate so that the impact of the rotation can be tracked over time. In addition, a 250-cc soil sample approximately 6 inches below the soil line were taken from each microplot at the end of both the first and second years to undergo PCN cyst extraction. This soil sample allowed us to detect reproduction occurring during the rotation.
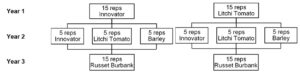
The field trial was planted in May and set up in a randomized complete block design. The 3-year crop rotation scheme is depicted in Figure 1. The first year consisted litchi tomato or the partially resistant potato variety ‘Innovator’. Litchi tomato served as a trap crop to cause PCN hatch but prevented development so it would reduce PCN populations. ‘Innovator’ is a partially resistant variety meaning it allowed some reproduction of PCN and added to the soil infestation level. Litchi tomato and ‘Innovator’ were transplanted as 4-week-old seedlings. There were 6 plants per plot. These plants grew for 12 weeks before termination. Microplots were stored in a storage unit until the following May. In year 2, 5 repetitions were planted as ‘Innovator’, 5 repetitions planted as litchi tomato, and 5 repetitions planted as barley. Barley once again was a nonhost that did not stimulate PCN hatch. These plants were grown in field conditions for 12 weeks. At 12 weeks, plants were terminated and microplot buckets were sealed with a lid and placed into storage for winter. Microplots were returned to the field in May for year 3 in rotation. In year 3, all repetitions were planted with the susceptible potato variety ‘Russet Burbank’. Following 12-weeks of growth, the recovered cyst bags will be analyzed for egg counts, percentage of viable eggs, and percentage of hatch with potato root diffusate. In addition, soil and root samples will be dried for PCN cyst extraction in the elutriator. Any extracted cysts will be counted and have egg counts conducted to calculate the reproduction factor after the 3-year rotation. This rotation field trial is being repeated twice.
The three-year rotations will be evaluated based on the reduction viable eggs in the recovered cyst bags over time. At the end of the rotation, the overall impact on PCN infestation level can be assessed. All data are being analyzed using analyses of variance and least squares means separation conducted in SAS. Based on these results, the rotation most effective in reducing PCN populations can be further investigated as a possible strategy for growers.
Figure 1. 3-year crop rotation scheme
Figure 1. 3-year crop rotation scheme
Litchi Tomato Compared to Quinoa as PCN Trap Crops
Trap crops for the pale cyst nematode (PCN) in Idaho must be nonhosts that stimulate hatch of the nematode so that it hatches but is unable to develop and reproduce. Litchi tomato is known to be a successful PCN trap crop because when followed by susceptible potato, it can cause up to 99% reduction in PCN reproduction (Dandurand et al., 2019; Dandurand & Knudsen, 2016). There is evidence that quinoa is a nonhost that causes hatch of PCN (Franco et al., 1999). However, prior to this study, quinoa had yet to be evaluated as a PCN trap crop in Idaho. In order to assess the potential of quinoa as a trap crop in Idaho field conditions and to compare its efficacy to litchi tomato, a field experiment including litchi tomato, quinoa, and the nonhost barley was set up and inoculated with PCN in microplots in the field. At the end of the growing season, a cyst bag was retrieved from each plot. Cysts were broken open in order to enumerate remaining encysted eggs, determine percent viability of these eggs, and to calculate percent hatch of remaining eggs after application of potato root exudates. As expected, the field results showed barley to have the greatest number of remaining encysted eggs and highest hatch because it is a nonhost that did not stimulate hatch of the nematode (Table 1). Remaining eggs and hatch were significantly lower after quinoa, but hatch and egg count was lowest after the litchi tomato (Table 1). Compared to the control, quinoa reduced eggs by 51% while litchi tomato reduced eggs by 83%. Litchi tomato also significantly reduced viability more than quinoa and barley (Table 1). There is evidence that litchi tomato has toxicity to the nematode in addition to causing hatch. Next, susceptible or resistant potato was grown in microplots to determine PCN reproduction following the nonhosts quinoa, litchi tomato, and barley. Cysts were extracted from the microplot soil. As expected, the nonhost barley which is not a PCN trap crop followed by susceptible potato had the highest PCN reproduction with an average of 868 cysts (Table 2). Reproduction on susceptible potato after planting quinoa was significantly lower than with the barley with an average of 507 cysts (Table 2). Litchi tomato followed by susceptible potato was significantly lower than the quinoa but statistically the same as barley and quinoa followed by resistant potato (Table 2). Compared to barley, quinoa reduced PCN reproduction on susceptible potato by approximately 40% while litchi tomato reduced reproduction on susceptible potato by about 97%. This shows the value of a resistant potato in rotation. However, litchi tomato followed by resistant potato was significantly lowest with zero PCN reproduction (Table 2).
The greenhouse trials showed similar trends to the field trials. Compared to the barley control, quinoa reduced eggs by 23% while litchi tomato and Russet Burbank reduced eggs by 52% (Table 3). Remaining egg hatch was greatest after barley and lowest after Russet Burbank and litchi tomato (Table 3). Litchi tomato also significantly reduced viability similar to the field trials (Table 3). Compared to barley, litchi tomato followed by susceptible potato reduced progeny cysts by 98% while quinoa followed by susceptible potato reduced progeny cysts 54% (Table 4). In the greenhouse, progeny cysts resulting from barley, quinoa, and litchi tomato followed by resistant potato were statistically the same (Table 4). Susceptible potato followed by susceptible potato resulted in the greatest cysts by far as expected (Table 4).
In all field and greenhouse trials, quinoa reduced cyst production on susceptible potato by about 30 to 50% while litchi tomato reduced cyst production by about 97 to 99%. Although quinoa is not as effective as litchi tomato in reducing PCN, it does reduce populations more so than a nonhost control like barley and is has potential to be more effective if followed by a resistant potato.
Table 1. Litchi Tomato vs quinoa field trial end of season cyst evaluations with average remaining eggs per cyst, average egg hatch, and average egg viability.
|
Field Treatment |
Average Eggs Per Cyst |
Average % Hatch |
Average % Egg Viability |
|
Barley |
120.3 a |
14.3% a |
53.7% a |
|
Quinoa |
69.4 b |
9.7% b |
45.5% a |
|
Litchi Tomato |
45.5 c |
3.2% c |
24.3% b |
*Significant differences denoted by different letters beside means.
Table 2. Litchi tomato vs quinoa field trial reproduction on susceptible or resistant potato.
|
Field Treatment |
Bioassay Cultivar |
Average Cysts Per Plot |
|
Barley |
Russet Burbank |
868 a |
|
Quinoa |
Russet Burbank |
507 b |
|
Litchi Tomato |
Russet Burbank |
27 cd |
|
Barley |
Innovator |
48 c |
|
Quinoa |
Innovator |
41 cd |
|
Litchi Tomato |
Innovator |
0 d |
*Significant differences denoted by different letters beside means.
Table 3. Litchi tomato vs quinoa greenhouse trial end of season cyst evaluations with average remaining eggs per cyst, average egg hatch, and average egg viability.
| Average Eggs per Cyst | Average % Viability | Average % Egg Hatch | |
| Barley | 192.4 a | 85.3% a | 35% a |
| Quinoa | 148.3 b | 79.6% a | 11.3% b |
| Russet Burbank | 92.6 c | 57.8% ab | 4.3% c |
| Litchi Tomato | 93.7 c | 39.1% b | 4.5% c |
*Significant differences denoted by different letters beside means.
Table 4. Litchi tomato vs quinoa greenhouse trial reproduction on susceptible or resistant potato.
| Treatment | Bioassay Cultivar | Average Cysts |
| Russet Burbank | Russet Burbank | 1590 a |
| Barley | Russet Burbank | 498 c |
| Quinoa | Russet Burbank | 231 d |
| Litchi Tomato | Russet Burbank | 10 e |
| Russet Burbank | Innovator | 891 b |
| Barley | Innovator | 6.5 e |
| Quinoa | Innovator | 3.2 e |
| Litchi Tomato | Innovator | 0.7 e |
*Significant differences denoted by different letters beside means.
Evaluation of Crop Rotations to Reduce PCN Over Time
The impact of three-year crop rotations on potato cyst nematode has been evaluated in Idaho field conditions. In year 1, microplots were grown with either litchi tomato or resistant potato 'Innovator'. Innovator is a European variety with moderate PCN resistance that serves as a model for a resistant russet variety that may one day be developed for Idaho. Cysts used in the original soil inoculation were evaluated for remaining encysted eggs, egg viability, and egg hatch when potato exudates are applied at the end of the growing seasons. In the first year for both trials, we see that cysts treated with litchi tomato and resistant potato have statistically the same average remaining encysted eggs and percent egg hatch (Table 5). However, litchi tomato significantly reduced egg viability compared to the resistant potato (Table 5). This was expected because litchi tomato is suspected to have additional toxicity to the nematode. Samples of approximately 500 cc of soil were also taken at the end of the first and second growing seasons to assess reproduction of PCN. Litchi tomato is a nonhost and as expected had no reproduction (Table 6; Table 8). Innovator is not fully resistant to PCN and thus can have some reproduction. After year 1, no progeny cysts were detected in Innovator plots (Table 6). After year 2, plots that had two years of Innovator had significantly greater progeny cysts with approximately 1 cyst per plot (Table 8). Although this reproduction on resistant potato is low, it reveals that rotations with the nonhost trap crop litchi tomato may be more effective in reducing PCN populations over time.
In year 2, plots received litchi tomato, resistant potato 'Innovator', or the nonhost barley. Originally inoculated cysts were sampled and evaluated at the beginning of year 2 and end of year 2. Based on remaining encysted eggs in these samples, we can see that PCN is greatly reduced in all treatments because initial population reduction was so great after year 1 (Figure 1). At the end of year 2, there is no difference in average remaining eggs in originally inoculated cysts for all treatments (Table 7) but eggs have been reduced to about 10% of the original eggs per cyst. Ultimately, we see the impact of these rotations after year 3, in which all plots were planted with susceptible potato Russet Burbank. After year 3, originally inoculated cysts have no remaining viable eggs. Additionally, 2-kg samples of soil per plot was extracted to extrapolate average progeny cysts per plot. Rotations that included litchi tomato had significantly less progeny cysts after susceptible potato than rotations including resistant potato (Table 9). Interestingly, rotations that had two years of litchi tomato had significantly less potato yield in year 3 (Table 10). It may be worth investigating whether multiple seasons of litchi tomato has a negative impact on potato yield. In conclusion, litchi tomato is more effective in reducing potato cyst nematode in rotation than resistant potato. However, a resistant potato also reduces populations over time and would be more profitable in rotation.
Table 5. Crop rotation end of year 1 remaining eggs, egg hatch, and egg viability of originally inoculated cysts.
|
Year 1 Treatment |
Average Eggs Per Cyst |
Average % Hatch |
Average % Egg Viability |
|
Litchi Tomato |
99.2 |
4.1% |
61.7% b |
|
Innovator |
102.6 |
5.5% |
88.8% a |
*Significant differences denoted by different letters beside means.
Table 6. Crop rotation end of year 1 progeny cysts in 250g soil sample.
|
Y1 Treatment |
Average Progeny Cysts in 500-cc soil |
|
Litchi Tomato |
0 |
|
Innovator |
0 |
Table 7. Crop rotation end of year 2 average remaining viable eggs from originally inoculated cysts.
|
Year 1 Treatment |
Year 2 Treatment |
Average Eggs/Cyst |
|
Litchi Tomato |
Barley |
16.9 |
|
Litchi Tomato |
Innovator |
13.9 |
|
Litchi Tomato |
Litchi Tomato |
20.5 |
|
Innovator |
Barley |
11.6 |
|
Innovator |
Innovator |
20.8 |
|
Innovator |
Litchi Tomato |
20.3 |
*No significant difference between treatments.
Table 8. Crop rotation end of year 2 progeny cysts in 250-g soil sample.
|
Year 1 Treatment |
Year 2 Treatment |
Average Progeny Cysts in 500-cc soil |
|
Litchi Tomato |
Barley |
0 b |
|
Litchi Tomato |
Innovator |
0 b |
|
Litchi Tomato |
Litchi Tomato |
0 b |
|
Innovator |
Barley |
0.4 b |
|
Innovator |
Innovator |
1.0 a |
|
Innovator |
Litchi Tomato |
0 b |
*Significant differences denoted by different letters beside means.
Table 9. End of 3-year rotation progeny cysts per plot. Average progeny cysts per plot was extrapolated based on cysts in 2-kg sub-sample.
|
Year 1 Treatment |
Year 2 Treatment |
Year 3 Treatment |
Final Progeny Cysts/Plot |
|
Innovator |
Barley |
Russet Burbank |
25 a |
|
Innovator |
Innovator |
Russet Burbank |
25 a |
|
Innovator |
Litchi Tomato |
Russet Burbank |
13 ab |
|
Litchi Tomato |
Barley |
Russet Burbank |
5 b |
|
Litchi Tomato |
Innovator |
Russet Burbank |
2 b |
|
Litchi Tomato |
Litchi Tomato |
Russet Burbank |
0 b |
*Significant differences denoted by different letters beside means.
Table 10. End of 3-year rotation potato yield mass and number of tubers.
|
Year 1 Treatment |
Year 2 Treatment |
Year 3 Treatment |
Average Yield Mass (g) |
Average Number of Tubers |
|
Innovator |
Barley |
Russet Burbank |
392.8 ± 56.4 a |
16.8 ± 2.2 a |
|
Innovator |
Innovator |
Russet Burbank |
449.2 ± 24.6 a |
27.4 ± 2.7 ab |
|
Innovator |
Litchi Tomato |
Russet Burbank |
338.0 ± 31.5 ab |
16.8 ± 0.8 b |
|
Litchi Tomato |
Barley |
Russet Burbank |
420.3 ± 16.8 ab |
18.4 ± 1.6 b |
|
Litchi Tomato |
Innovator |
Russet Burbank |
428.3 ± 48.0 ab |
22.2 ± 1.4 b |
|
Litchi Tomato |
Litchi Tomato |
Russet Burbank |
309.5 ± 40.7 b |
17.4 ± 2.1 b |
*Significant differences denoted by different letters beside means.
Conclusions:
Based on the results of these trials, quinoa can moderately reduce PCN populations as a trap crop in Idaho field conditions. However, this study has provided further evidence that litchi tomato is a more effective trap crop with much greater impact on PCN populations. In rotation, both litchi tomato and a highly resistant potato variety can substantially reduce PCN over time.
Literature Cited:
Dandurand, L. M., & Knudsen, G. R. (2016). Effect of the trap crop Solanum sisymbriifolium and two biocontrol fungi on reproduction of the potato cyst nematode, Globodera pallida. Annals of Applied Biology, 169(2), 180-189.
Dandurand, L. M., Zasada, I. A., & LaMondia, J. A. (2019). Effect of the trap crop, Solanum sisymbriifolium, on Globodera pallida, Globodera tabacum, and Globodera ellingtonae. Journal of Nematology, 51(1), 1-11.
Franco, J., Main, G., & Oros, R. (1999). Investigation-Research: Trap Crops as a Component for the Integrated Management of Globodera spp.(Potato Cyst Nematodes) in Bolivia. Nematropica: 51-60.
Research Outcomes
In lab and greenhouse studies, quinoa has been shown to be moderately effective in reducing populations of Globodera pallida, the potato cyst nematode (PCN). Quinoa production is becoming more popular in southern Idaho where there are growers with PCN-infested fields. Quinoa also provides profitable yield. It could benefit growers with PCN-infested fields to grow quinoa. However, there is no evidence that quinoa is effective in controlling other nematodes. Quinoa can help reduce G. pallida because it produces a hatching stimulus in its root exudates that causes some PCN hatch, but quinoa is not a host of G. pallida and thus prevents development in the roots, leaving the hatched nematode to die.
Litchi tomato is far more effective in reducing PCN than quinoa but production is not as practical. Access to litchi tomato seed is limited and there is concern it could become an invasive weed in Idaho. While litchi tomato produces an edible fruit, it will likely not be as valuable commercially as other crops in Idaho.
A resistant potato has been shown to be a helpful strategy for reducing PCN in infested fields. This project used the European variety 'Innovator' with moderate PCN resistance as a model to determine how a resistant variety in rotation could reduce PCN over time. However, 'Innovator' is a yellow-flesh potato variety and would not be grown in Idaho because the market wants white-flesh Russet varieties. There is currently no commercially-accepted resistant Russet potato for Globodera pallida in Idaho. If a resistant variety can be developed for Idaho, it may be possible to use it in rotation to keep PCN below detectable levels.
Ultimately, using trap crops and resistant potato varieties for control of potato cyst nematode are more sustainable options compared to the conventional control strategy of soil fumigation with nematicide. In addition to being very costly, fumigant nematicides often have non-target effects on beneficial soil organisms and can have other damaging environmental effects. If affected growers can apply the control strategies from this project to PCN-infested acreage, perhaps it would help in eradicating PCN so they can pass through the deregulation process and reduce the need for fumigation.
Recommendations for Future Studies:
There is interest in growing quinoa in southern Idaho and new varieties are under development. Future research projects should test these newly developed varieties for their effect on G. pallida. It may be possible that there is another variety that could be more effective as a trap crop causing higher percentages of the nematode to hatch and die. It would also be worthwhile to identify the compounds produced by quinoa roots that cause the nematode to hatch. Typically, G. pallida hatch is only stimulated by plants in the Solanaceae family which includes its main host, potato. Chenopodium quinoa is not a member of this family yet still causes some nematode hatch, which is why it can serve as a trap crop. If this compound can be identified, then quinoa breeding programs could aim to develop varieties that produce more of it.
While quinoa has some use as a trap crop for potato cyst nematode, it is not as effective as litchi tomato. However, there is concern that litchi tomato could become a weed and access to seed is limited. Future research projects should seek to identify the compounds in litchi tomato root exudate that cause the nematode to hatch and become non-viable. If this compound is isolated, it may be possible to use it to develop a nematicide or soil amendment that would give the effect of litchi tomato without growers having to plant it in the field. This would be more sustainable because growers would not need to expend resources growing litchi tomato and could grow a more valuable crop in its place.
This research has also indicated that a moderately resistant potato variety in rotation can help reduce potato cyst nematode in infested fields. There is currently no potato variety with G. pallida resistance that is commercially accepted in Idaho. There are breeding programs that are working to develop resistant Russet varieties for Idaho. Once these varieties are developed, future studies should test them in different rotations to determine the best rotation for reducing potato cyst nematode and keeping populations undetectable.
Education and Outreach
Participation Summary:
In year 1 of this project, data was insufficient to present to agricultural professionals and stakeholders. In year 2, a presentation was given to fellow researchers at the Society of Nematologists annual meeting in September 2022. Also in year 2, a poster was presented at the University of Idaho Center for Human Health in the Human Ecosystem research symposium in April 2023. Attendees included University of Idaho students, professors, and research faculty. Additionally, a presentation was given at the University of Idaho Snake River Weed Management Tour and Field Day on June 27, 2023. The audience included industry professionals, growers, researchers, and extension agents. A factsheet was distributed at this field day and a poster was used as a visual. The SARE outreach survey and an additional survey question regarding participants' thoughts on the practicality of the PCN control strategies were also distributed. Surveys were collected to determine the number of participants with intent to employ or share the research findings presented.
This project was extended to allow for another presentation to stakeholders at the Idaho Association of Plant Protection meeting in Fall 2023. The SARE outreach survey was distributed and received a good response rate. Participants included growers, extension agents, university faculty, researchers, agricultural company representatives, and other stakeholders. Over 60 people were in attendance. The findings of the research were presented in terms of how the strategies could be used to help growers with PCN-infested acreage. The objectives of the presentation were to educate affected growers who can benefit from these strategies and to reach educators who can pass on this information to affected growers. Another goal was to increase awareness of the potato cyst nematode infestation in Idaho and how the problem can be controlled. Outreach surveys were collected to determine the number of participants and their responses to the presentation.
In the near future, this work will be submitted to a scientific journal for publication. The findings will also be included in a newsletter to distribute to growers and other stakeholders.
Outreach event presentations and resulting findings are described below. An overview of attendance and outreach survey responses are also provided (Table 11).
University of Idaho Snake River Weed Management Tour and Field Day:
Based on the audience's engagement and responses during the field day presentation, posters as visuals and factsheets in layman's terms seem to be effective tools in engaging stakeholders and communicating project results. Extension specialists, industry professionals, and growers were able to read over the factsheet and poster prior to the field day presentation. These materials were kept fairly brief to highlight the main points of this research within the limited allotted time. The presentation was used to explain methods and results in more detail. Several audience members then asked relevant questions regarding the feasibility of the control strategies for potato cyst nematode, as well as whether they would be effective for other nematode issues. Following the presentation, several audience members approached with more questions. One person was an industry professional developing quinoa varieties who wanted to know if variety would affect the results and whether their newly developed varieties could be tested. This was noted as a potential area of future research. At the conclusion of the field day, several attendees returned the SARE survey. 100% of survey participants agreed that the presentation improved their awareness and provided new knowledge on potato cyst nematode in Idaho and control strategies. One extension professional responded that they would like to make this resource available to producers. One producer responded that they would like to utilize quinoa in rotation as a control strategy for potato cyst nematode.
University of Idaho Research Symposium:
At the research symposium, a poster was used that included detailed methods and results that would be of interest to fellow researchers. The audience primarily consisted of other researchers in various fields who wanted more context on this research. During the symposium, attendees were free to look at the poster for a longer duration and ask for more detail as needed. Several attendees had never heard of the potato cyst nematode and became educated on the issue, its threat to the Idaho potato industry, and how this research can help eradication of the pest.
2023 Idaho Association of Plant Protection Meeting:
The audience at this outreach event included growers, extension professionals, university faculty/staff, and representatives from agricultural companies, including a private research company. Based on the survey responses, the primary impact was on extension professionals and other educators who intend to share these findings with other professionals and growers with infested acreage who could benefit from the control strategies being researched. The research findings are most applicable to potato growers afflicted by the potato cyst nematode infestation in Idaho. Extension professionals in the area of PCN infestation are particularly interested. 75% of survey responses indicated that respondents intend to share or implement what they learned from the presentation. The Idaho Association of Plant Protection meeting typically consists of many extension agents and university faculty/staff, along with growers and other agricultural stakeholders. Based on this feedback, presenting the research findings at an event where there is a mix of such professions is beneficial because the educators are likely to pass on the information to other stakeholders not in attendance.
Table 11. Overview of outreach event attendance and survey results
| Outreach Event | Attendance | Outreach Survey Responses Received | Number of Those Likely to Use or Share the Project Findings |
| University of Idaho Snake River Weed Management Tour and Field Day |
25 Estimated to be 10% research/extension faculty and staff, 30% growers, 60% agricultural professionals |
6 | 4 |
| University of Idaho Research Symposium |
50+ Estimated to be 95% research faculty/staff and students |
n/a | n/a |
| 2023 Idaho Association of Plant Protection Meeting |
60 Estimated to be 20% research/extension faculty and staff, 30% growers, 50% agricultural professionals |
20 | 15 |