Final report for GW22-231
Project Information
Problem: High-elevation hay meadows are a critical but under-performing component of livestock operations in the Mountain West. Flood irrigation, high elevation, and cool temperatures result in concentration of organic materials near the soil surface, constraining N cycling and forage productivity. Limited forage productivity in meadows is surprising considering meadow soils contain as much as 2400 kg N/ha in the top 10 cm, reflecting a disconnect in the microbial community's ability to mineralize N for plant growth.
Question: How can the resource of stored N in meadow soils be utilized to reduce dependence on N-fertilizer and improve economic and environmental sustainability of western ranches?
Solution: Understanding N mineralization in meadow soils will give ranchers tools to better manage natural N-release and reduce N-fertilizer rates.
Method: We propose an on-farm research trial where soil cores are incubated in-situ and routinely sampled for mineralized N content in order to determine the temporal patterns and magnitude of N release in meadows.
Outreach: Results will be disseminated to stakeholders through producer meetings and publications in extension and peer reviewed articles. We will also focus on training a core group of ranchers to communicate findings within their communities and promote innovation.
Expected outcomes: We expect to find N mineralization in meadows occurs in discrete time periods between flooding events when soil conditions are ideal for N mineralization. This knowledge will give our rancher collaborators tools to innovate novel management strategies to optimize N mineralization and fertility management on their operations.
Research objective 1: Determine the amount of N mineralized in high-elevation hay meadows annually to better predict N availability and reduce dependence on N fertilizer.
Research objective 2: Determine the temporal patterns of N mineralization so meadow management tactics can target critical periods of N mineralization to optimize N cycling in the field.
Research objective 3: Determine relationships between N mineralization and soil health indicators to develop measures for evaluating meadow soils with healthy N cycling.
Education objective 1: Hold producer meetings at three critical stages of the project to relay research findings and updates to local ranchers and stakeholders.
Education objective 2: Develop a core group of 5-10 ranchers who receive regular communication about our research to foster outreach from within local ranching communities.
Education objective 3: Disseminate research findings to producers, industry stakeholders, and academia through written and oral media.
Education objective 4: Hold a producer round-table to develop practical management tactics that implement our research findings.
Cooperators
- - Producer
- - Producer
Research
Research objective 1: Determine the amount of N mineralized in high-elevation hay meadows annually to better predict N availability and reduce dependence on N fertilizer.
Research objective 2: Determine the temporal patterns of N mineralization so meadow management tactics can target critical periods of N mineralization to optimize N cycling in the field.
Research objective 3: Determine relationships between N mineralization and soil health indicators to develop measures for evaluating meadow soils with healthy N cycling.
To achieve our research objectives, we propose an in-field buried soil core mineralization study, which is the most accurate determination of in-situ N mineralization (Sullivan et al., 2020). Our structured experiment, described below, will adhere to the SMART objectives framework, by providing measurable objectives to be met by defined research activities within the scope and timeframe of work to be accomplished by our research team. Please see the additional info section of the project team portion of the narrative for details on project responsibilities among our research team members.
Study Location
The study was initiated in April 2023 at two ranches in southern Wyoming and northern Colorado, producing grass hay on typical flood irrigated meadows. At each ranch we studied two meadows: one that was historically fertilized, and one that was historically unfertilized, both on the same soil series, to observe the effects of long-term fertilizer application. Meadows were managed, irrigated, and harvested normally by our rancher collaborators for the duration of the study.
Our previous research at these sites established three randomly located plots for repeated sampling within each meadow/management location. Randomly located within each plot, we established a completely randomized 1 m2 grid for in-situ incubated soil core determination of N mineralization.
In-Situ Nitrogen Mineralization
In-situ N mineralization studies subject soils to natural edaphic conditions for the determination of net N mineralization. In-situ cores differ from general soil samples in that the sampled core is not exposed to inward and outward fluxes of N for the duration of experiment, controlling for leaching and plant uptake of mineralized N. Our experiment is utilizing the method by Moberg et al. (2013), which combines ion exchange resin (IER) and soil cores to create an incubation environment permeable to gas and water exchange, but impermeable to inorganic N. Briefly, 10-cm diameter polyvinyl chloride (PVC) tubes were inserted into the soil to a depth of 27 cm (representing the active root zone of meadow forage species). In-tact cores encased in their tubing were removed from the soil and the bottom 3 cm of soil was removed from the core to allow room to insert two IER bags. Encased cores with bags inserted in the bottom were re-installed in the hole from which they were sampled.
Ion exchange resin bags allow water and gas to naturally exchange through the encased soil core, but capture inorganic N by adsorbing it to the ion resin. Because meadows are subject to a high water table, the bottom of the two IER bags at the base of the core eliminates any movement of N into the core with rising water (Moberg et al. 2013), while the upper IER bag captures any N that leaches from the inside of the soil core. A third IER bag was placed at a depth of 5 cm at the transition between the O and A horizons in the soil to distinguish any differences in mineralization rates in the different soil horizons. In this way, an in-situ soil incubation was developed that is exposed to natural edaphic temperature and air/water flow conditions while containing any produced inorganic N. To eliminate the effect of plant uptake of N, sod at the top of the core was killed with non-selective herbicide. Cores were installed immediately following soil thaw, prior to biological soil activity and fertilizer application, in order to capture an entire season of mineralization.
During sampling, cores were removed from the field and taken to the lab for analysis. To avoid any effects of transportation, samples were kept at 4ºC in a cooler and processed in the lab within 24 hours of collection. In the lab, soil was removed from its PVC core and homogenized by thoroughly mixing and passing it through an 8-mm sieve. Two 10-g subsamples were taken for determination of gravimetric water content and extraction of inorganic soil N as ammonium (NH4+) and nitrate (NO3-). The 10 g of soil for N extraction was shaken with 50 ml of 2M KCl in a 120-ml specimen cup for 30 minutes, then decanted and filtered after 12 hours of settling. The supernatant was analyzed for NO3--N and NH4+-N colorimetrically by cadmium reduction (Knepel, 2012) and alkaline phenol/hypochlorite method (Harbridge, 2007), respectively, on a Lachat 8500 QuickChem (Hach Industries, Loveland, CO). Nitrogen leached from the soil core and captured in the IER bag between the O and A horizons, and at the bottom of the core was also extracted. Inorganic N in the IER bag was extracted according to Moberg et al. (2013) by extracting N from the IER bag with a series of three extractions. For each extraction, 25 ml of 2M KCl was added to the IER and shaken for 20 minutes. Following shaking, supernatant was collected. The supernatant from all three extraction rounds was analyzed colorimetrically for NO3- and NH4+. Increases in the measurement of inorganic N as it changed through the season allowed for the quantification of N mineralization in meadows and meet objective 1.
Sampling design
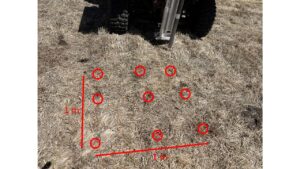
Within sampling plots, we established a 1-m2 grid with nine equidistant points. Each point represents a location where a core was constructed and incubated in the field. The nine points in the grid form a completely randomized block where we assigned samples to be incubated for nine different durations in the field. Duration of incubation was designed to capture periods in the field most likely to promote mineralization, thus reducing sample number to ensure our objective is achievable without sacrificing sensitivity to determine periods of N mineralization, as set by our second objective. Sampling times and corresponding biological significance are listed below.
- Spring, post soil thaw: This marks the first sampling event and represents the baseline N status of the soil prior to the beginning of biological activity for the year.
- One week prior to irrigation: Initiation of irrigation water changes the redox potential of meadow soils. This sampling period will capture N mineralized early season.
- Halfway through irrigation period: Meadows are typically irrigated for 6-8 weeks. A sample taken in the middle of this period will capture mineralization corresponding to the introduction of irrigation water.
- Prior to irrigation termination: Sampling before water recession will capture any changes in mineralization during the irrigation period when soils are warming.
- Two weeks following irrigation termination: The transition of meadow soils from saturated to moist is likely the most optimum combination of temperature and moisture for microbial activity in the whole year. Therefore, a sample taken shortly after this period will capture the potentially active period of N mineralization.
- Four weeks following irrigation termination: Many meadows face a period of drought following irrigation termination. This sampling period will capture the transition from moist to dry soils.
- One-month following sample 6: Meadow productivity tails off in late summer. This sample will capture mineralization occurring late season.
- One-month following sample 7: This sample will capture mineralization that occurs in the transition to fall and cooler soil temperatures.
- Prior to soil freeze: This sample will capture total net N mineralization through a whole season.
The sampling grid was replicated three times for a total of n = 3 at each site. However, each meadow system is replicated in two locations for a total of n = 6, which is the minimum replication suggested by Kolberg et al. (1997). The study was repeated in 2024, to allow for a total of 4 site-years for both unfertilized and fertilized meadows (8 site-years in total). Each year, a total of 108 samples will be analyzed. Although more replication through space and time would be ideal to better represent meadows in a variety of conditions, we feel our labor-intensive method must be scaled to a level that can be achieved with a team of 2-3 researchers within the 2-year duration of our study while still allowing us to meet our research objectives for this novel approach in meadows.
Mineralization/Soil Health Relationships
Although mineralization studies offer the best method for determining N release from soils, they are retroactive and time consuming, rendering them unsuitable for predicting future soil health and performance (Sullivan et al., 2020). Therefore, other methods have been proposed to assess soil N cycling. Our team has experience in lab incubations for N mineralization. Anaerobic potentially mineralizable N (PMN) tests have been shown to be the most useful soil health indicator related to field mineralization (Sullivan et al., 2020), and have been successfully used to adjust early-season N applications (Anderson et al., 2010).
Early season samples allow producers an opportunity to evaluate soil N status before making seasonal management decisions (Anderson et al., 2010), therefore, we also took a composited soil sample from the perimeter of the plot at the initiation of the study, following soil thaw, for analysis of PMN (Waring and Bremner, 1964, Anderson et al., 2010). This initial PMN status of the soil will then be correlated to net N mineralization at the end of the season to determine if a relationship exists between lab and field N mineralization and if PMN measured in the lab is a useful soil health indicator to evaluate meadow N cycling (objective 3).
Data Analysis
All data analysis was performed using R. Cumulative N mineralization will be determined from cores incubated the entire duration of the season as net inorganic N content compared with samples taken at the initiation of the study (Moberg et al., 2010). Temporal patterns in N mineralization were determined by plotting cumulative N mineralization through time to observe periods of the season where N mineralization occurs most rapidly. Regression analysis was also be performed to define relationships between lab-derived PMN and in-situ net N mineralization. Analysis of Variance was performed to detect differences in meadow management and sites, with +/- fertilizer application as the fixed effect and location as the random effect. In order to determine differences among sampling points, a response-feature analysis was performed by calculating the rate of N mineralization for each plot between two discrete sampling events. Analysis of variance was then performed on the rate of N mineralization with fertilizer application as a fixed effect, sampling period as a repeated measure, and location as a random effect.
References
Anderson, N.P., J.M. Hart, N.W. Christensen, M.E. Mellbye, M.D. Flowers, et al. 2010. Using the Nitrogen Mineralization Soil Test to Predict Spring Fertilizer N Rate. Oregon State Univ. Ext. Serv. EM 9020(November): 1–5.
Harbridge, J. 2007. Determination of ammonia (salicylate) in 2M Kcl soil extracts by flow injection analysis (high throughput). Lachat Instruments QuikChem Method 12-107–06(2): F.
Knepel, K. 2012. Determination of nitrate in 2M KCl extracts by flow injection analysis. Lachat Instruments QuikChem Method 12-107-04-(1-): B.
Kolberg, R.L., B. Rouppet, D.G. Westfall, and G.A. Peterson. 1997. Evaluation of an In Situ Net Soil Nitrogen Mineralization Method in Dryland Agroecosystems. Soil Sci. Soc. Am. J. 61(2): 504. doi: 10.2136/sssaj1997.03615995006100020019x.
Moberg, D.P., R.L. Johnson, and D.M. Sullivan. 2013. Comparison of Disturbed and Undisturbed Soil Core Methods to Estimate Nitrogen-Mineralization Rates in Manured Agricultural Soils. Commun. Soil Sci. Plant Anal. 44(11): 1722–1732. doi: 10.1080/00103624.2013.783060.
Sullivan, D.M., A.D. Moore, E. Verhoeven, and L.J. Brewer. 2020. Baseline soil nitrogen mineralization : measurement and interpretation. Oregon State Univ. Ext. Publ. (March): 10–11.
Waring, S.A., and J.M. Bremner. 1964. Ammonium Production in Soil under Waterlogged Conditions as an Index of Nitrogen Availability. Nature 201: 951–952.

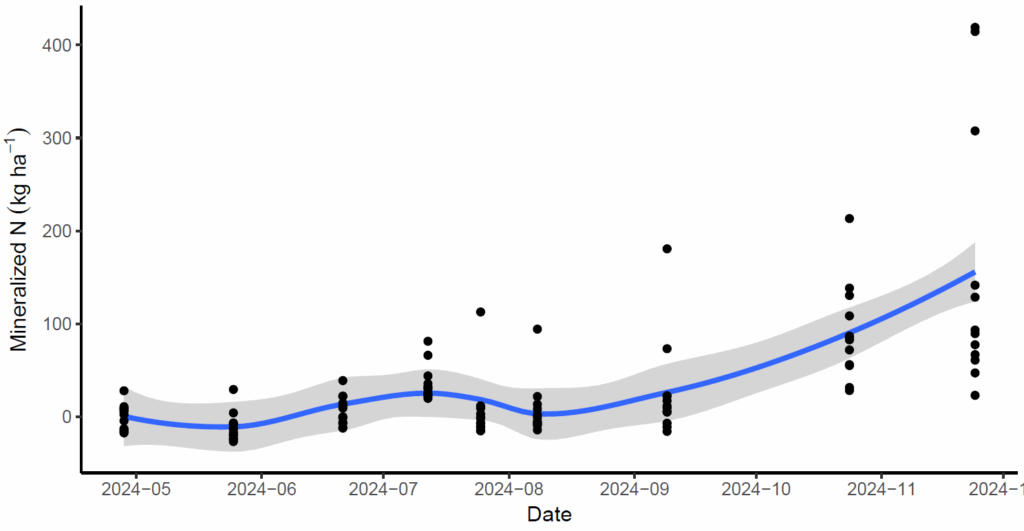
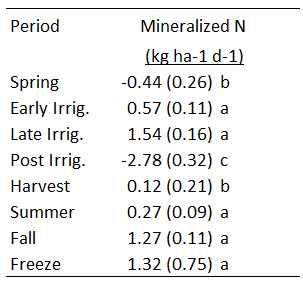
Figure 4: Mineralization rate (kg N/ha/d) during unique sampling periods for four meadows in 2023. Error bars represent the 95% confidence interval for the mean. Pictures represent typical field conditions for the unique sampling period different letters denote significant differences between periods at p = 0.05.
For research objectives 1 and 2 we successfully completed the study as proposed in 2023 and 2024. For research objective 1, we determined N mineralization was not affected by historic fertilizer management in the field, and that average N mineralization among all research sites in 2023 was approximately 40 kg N/ha/yr (Figure 2). This result was encouraging, as it confirmed the robustness of our study because it represents the baseline amount of N required to achieve typical unfertilized yields in the field. In 2024 this result changed dramatically, as we were able to measure an average of 186 kg N/ha/yr mineralized (Figure 3). Large increases in N mineralization occurred late-season in 2024, possible as a result of freeze-thaw that occurred earlier in the season than 2023 which may have stimulated some N release from the microbial pool.
For objective 2, we successfully determined the temporal pattern of N mineralization. As hypothesized, N mineralization slowed during the irrigation season and sped up following irrigation termination when soils re-aerated and microbial activity increased (Figure 2). A response-feature analysis allowed us to clearly distinguish differences in N mineralization rate among sampling periods (Figure 4). N mineralization rate was slowest during the second half of the irrigation season, when soil anoxia is at its peak. Mineralization rate was fastest from 2-4 weeks following irrigation termination, when soils re-aerated and warmed following harvest of the grass canopy which shades the soil. Other sampling periods of note were late summer, and late fall, prior to soil freeze, when soils are either too dry or too cold to mineralize N.
In 2024, unfortunately, we observed the opposite pattern. N mineralization increased during the irrigation season and declined rapidly following irrigation termination (Figure 3). Instead, a majority of mineralization occurred in summer through fall (Figure 3, Table 1). It is possible denitrification occurred following aeration of the soil that nitrified a large portion of ammonium to nitrate at irrigation termination.
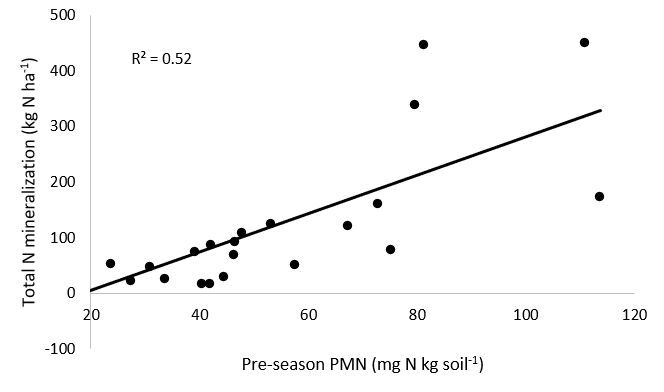
For research objective 3, we performed a preseason N mineralization test using soil samples taken at the initiation of the study in 2023 and 2024. There is a weak, positive correlation between lab and field measures of N mineralization (R2 = 0.52) Although the slope is significant (p=0.02), the correlation between lab and field measured N mineralization indicated there is little power in pre-season soil sampling to predict actual field N mineralization (Figure 5).
Research outcomes
Results supported our hypothesis that a majority of N mineralization occurs outside of the growing season when soils are well aerated and warm, allowing for increased microbial activity. These results support early recommendations from University Extension, which encouraged producers to alter their irrigation methods to promote "pulsing" of irrigation water instead of continuous irrigation application (Lorimer 1975; Rumburg & Sawyer, 1965). Pulses of flood water allow soils to re-aerate between irrigation events, promoting greater N mineralization and less need for supplemental N. Pulsing irrigation water may also extend the irrigation season to make use of forage production in the late summer, when mineralization potential is also high.
References
Lorimer, J. (1975). "Irrigation", in Alternative Meadow Livestock Management Systems. Colorado State University Experiment Station Bulletin 565S.
Rumburg, C., and Sawyer, W. (1965). Response of wet-meadow vegetation to length and depth of surface water from wild-flood irrigation. Agronomy Journal, 57:245-247.
Education and Outreach
Participation summary:
Education objective 1: Hold producer meetings at three critical stages of the project to relay research findings and updates to local ranchers and stakeholders.
Education objective 2: Develop a core group of 5-10 ranchers who receive regular communication about our research to foster outreach from within local ranching communities.
Education objective 3: Disseminate research findings to producers, industry stakeholders, and academia through written and oral media.
Education objective 4: Hold a producer round-table to develop practical management tactics that implement our research findings.
Rationale
Research has shown that communication of innovation is not a linear process, but is most successful when users are involved in the development of innovation and also active communicators of its results (Leeuwis and Aarts, 2011). Our group has found this to be true, as ranchers respond well to disseminating knowledge within their local communities and are quick to offer practical suggestions that guide our research through all stages of a project. To take advantage of this, we propose educational objectives that promote rancher interaction from the initiation of the project.
We realize formal meetings only achieve so much, and that only a few hours of information do not always correlate to lasting change. To overcome the challenges of formal extension meetings, we have focused our initial education and communication energy on objective 2 by developing a core group of local ranchers who are passionate about our project and capable of disseminating information and educating fellow ranchers in the community. Our team is fortunate to have established connections with ranches in southern Wyoming and northern Colorado through our ongoing meadow research. Our core group of ranchers was composed of 8 individual producers. Each of these ranchers received regular outreach from our team in the form of monthly phone calls with project status updates and to make them aware of research being conducted on collaborating ranches. We also personally met with the ranchers when on their operations for field research. We confirmed that our regular personal contact maintained trust and open lines of communication for the ongoing work of this project.
Outside of our core group of ranchers, we were able to cohost and participate in 4 extension events (see results).
References
Leeuwis, C., and N. Aarts. 2011. Rethinking communication in innovation processes: Creating space for change in complex systems. J. Agric. Educ. Ext. 17(1): 21–36. doi: 10.1080/1389224X.2011.536344.
For objective 2, we focused our initial efforts in early 2023 by successfully developing our core group of ranchers who learned about our project and received regular communication about its progress. Of these eight ranchers, four were directly present in the field at project initiation. Of the four who were present, two directly participated in creating incubation cores, installing them in the field, and collecting samples.
Communicaiton frequency among the core ranchers varied. Three were highly involved and interested in the study and communicated with us multiple times a month. Two were interested and communicated monthly. The remaining three were harder to reach out to and received communication and education about our study design and justification at the initiation of the study and one follow-up communication 6-8 weeks later.
One rancher shared our research and objectives with a local agronomic professional who in turn met with us to discuss our project in fall 2023.
For objective 1 and 3, we were able to cohost two producer meetings and presented at a third event within the last year. In December 2023 we co-hosted a meadow workshop with Nutrien Ag Solutions and presented research findings to 20 ranchers and 2 industry representatives from southern Wyoming and northern Colorado. In March 2024, we cohosted a meeting with the Albany County Cattlewomen. In this meeting we were able to cater lunch for the local cattlewomen using WSARE funds and present and discuss research findings. At the end of the meeting, 15 women participated in a short survey about our research and outreach techniques. In April 2024 we were invited to present our research findings at the quarterly meeting for the Upper Laramie River Irrigation District. Ten attendees participated in the same survey as above. In July 2024 we met with 4 industry representatives from Nutrien Ag Solutions for a short field day to show them results of our field studies. Finally, in March 2025 we were able to host our final extension meeting where 11 ranchers were in attendance including 2 new participants.
Results from the surveys indicated that 52% of participants believed word-of-mouth interactions were highly effective for disseminating scientific information within ranching communities. 92% of participants believed university talks and extension presentations were highly effective for disseminating scientific information within ranching communities. 20% of participants believed written resources were highly effective for disseminating scientific information within ranching communities. Among these three questions, there was a significantly different response between the effectiveness of extension presentations and written resources (p = 0.01).
Results from this study were published in Wyoming Livestock Roundup on March 15, 2025. Other proposed publications for objective 2 (extension bulletin and peer-reviewed publication) are currently in production.
For Objective 4, we hosted a producer roundtable at the end of our final extension meeting. Eleven ranchers participated in a discussion regarding meadow management. The following prompts were discussed:
- What practices are currently important for your meadow production?
- What practices would you consider implementing on your operation?
- What practices presented in this research to you think are unfeasible?
- What other ideas do you have for improved meadow management?
For prompt 1, producers mentioned the importance of dragging their meadows in the spring to homogenize manure. Others mentioned ditch maintenance for irrigation management.
For prompt 2, producers mentioned that our results indicate that fertilizer availability is low in the growing season, so they are considering alternative application strategies to overcome this, including fertilizer additives like urease inhibitors to limit urea volatilization and provide some slow-release activity. Others discussed the importance of cutting timing on forage quality as opposed to simply chasing yield.
For prompt 3, producers mentioned how large investments in infrastructure to improve irrigation pattern and efficiency are economically impractical. Leveling or purchasing of a pivot for improved irrigation require excessive financial and engineering challenges that cannot be practically overcome for the average rancher.
For prompt 4, one producer mentioned burning to potentially release nutrients from the O-horizon. This prompted a discussion of the feasibility of burning in the meadow system. Producers felt it might be possible in the spring, but predominately dry conditions in summer and fall make this appear to be a high-risk management tactic.
