Final report for GW22-236
Project Information
Potatoes (Solanum tuberosum L.) are the fourth most important food crop in the world and, in 2020 the total value of all potatoes sold Idaho, Oregon, and Washington was $1.82 billion. This proposal addresses powdery scab a potato blemish disease that results in losses to all market sectors of potato in all major production regions of the U.S. Powdery scab reduces potato aesthetics for directly marketed potatoes and through the transmission of potato mop-top virus (PMTV), a virus that causes tuber necrosis leading to rejection of potatoes used for processing. Complete host resistance to powdery scab is not available in commercially accepted varieties and no management tactics alone or in combination have successfully controlled all phases of the disease. In this project, we plan to use culture-free methods to characterize microbial communities in bulk and rhizosphere soils that are associated with reduced disease and may be responsible for powdery scab suppressive activity. Our research objectives are to 1) characterize the temporal dynamics of the potato rhizosphere microbiome through the growing season and in relation to powdery scab development and 2) assay soils for their ability to suppress powdery scab. Our goal is to identify biotic factors that are associated with disease suppressive activity of soil and identify and management practices that promote disease suppressive activity. Our educational objective is to engage growers in the project and communicate our findings to the stakeholder community, at grower education events, through the dissemination of extension publications, and the research community via peer-reviewed publications.
Objective 1:
Characterize the temporal dynamics of the potato rhizosphere microbiome through the growing season and in relation to powdery scab status.
Objective 2:
Assay soils for their ability to suppress powdery scab by identifying abiotic and biotic factors associated with disease suppression and by identifying systems and/or management practices associated with reduced disease
Objective 3 is to engage growers in project activities and outcomes and communicate research findings about powdery scab to the stakeholder and research community.
Cooperators
- - Producer
Research
Research Plan
The research objectives of this study are to:
- Characterize the temporal dynamics of the potato rhizosphere microbiome through the growing season and in relation to powdery scab status
- Assay soils for their ability to suppress powdery scab
- Identify abiotic and biotic factors associated with disease suppression
- Identify cropping systems and/or management practices associated with reduced disease
Objective 1:
Characterize the temporal dynamics of the potato rhizosphere microbiome through the growing season and in relation to powdery scab status.
Materials and Methods
Field selection and location. An observational field study was conducted in four commercial potato fields selected based on their powdery scab disease history. Within each field, four 0.1-acre grid areas were established for environmental sensor placement and soil and plant sampling. The sensor unit was installed in each plot after potato planting and hilling. Flags were used to mark the sensor location and plot corners. GPS coordinates and elevation for each location were recorded. Hourly soil water and temperature were collected by the sensor unit. The sensors were installed in April and bulk and rhizosphere soils were sampled in April, May, June, July, and August. Previous management history (i.e., previous crops, fumigation events and fungicide applications, soil amendments, etc.) and in-season management practices were documented for each field.
Bulk soil and rhizosphere sampling. We sampled the potato rhizospheres and bulk soils when plots are established and monthly thereafter until plant senescence. To sample bulk soils, 20 standard (1.87 cm diameter) soil cores to a depth of approximately 20 cm were collected within each field plot, 10 m of the sensor. Soil cores were pooled into a collection bag and mixed well by shaking and turning the bag upside-down 10 times. Ten grams of each soil were placed into a 15 ml centrifuge tube. Centrifuge tubes were placed on ice for transport to the laboratory and stored at -20°C until DNA extraction. Based on our experimental design, this resulted in 64 bulk soil samples per year (4 months x 4 fields x 4 locations within field = 64 bulk soil samples per year). At the first sample date, the bag of bulk soil from each plot was used for soil chemical and soil health analyses.
To sample potato rhizospheres, three plants from each location were carefully removed from the soil so that plant roots are left mostly intact. The plants were shaken to remove loose soil from the root system. A 1.0 g sample of root was collected for each plant, and placed in a 15 ml centrifuge tube with 10 ml of phosphate-buffered saline Tween 20 (PBST). Roots were subjected to 3 minutes of vigorous shaking in the field and the cleaned root were removed from the tube. The rhizosphere soil suspended in PBST was stored on ice for transport to the laboratory. In the laboratory, rhizosphere soils were thawed and centrifuged to pellet the soil. The resulting rhizosphere soil pellet was used for DNA extraction. Based on our experimental design, this resulted in 240 rhizosphere samples per year (5 months x 4 fields x 4 locations within field x 3 plants = 240 samples per year).
Objective 2:
Assay soils for their ability to suppress powdery scab by identifying abiotic and biotic factors associated with disease suppression and by identifying systems and/or management practices associated with reduced disease.
Materials and Methods
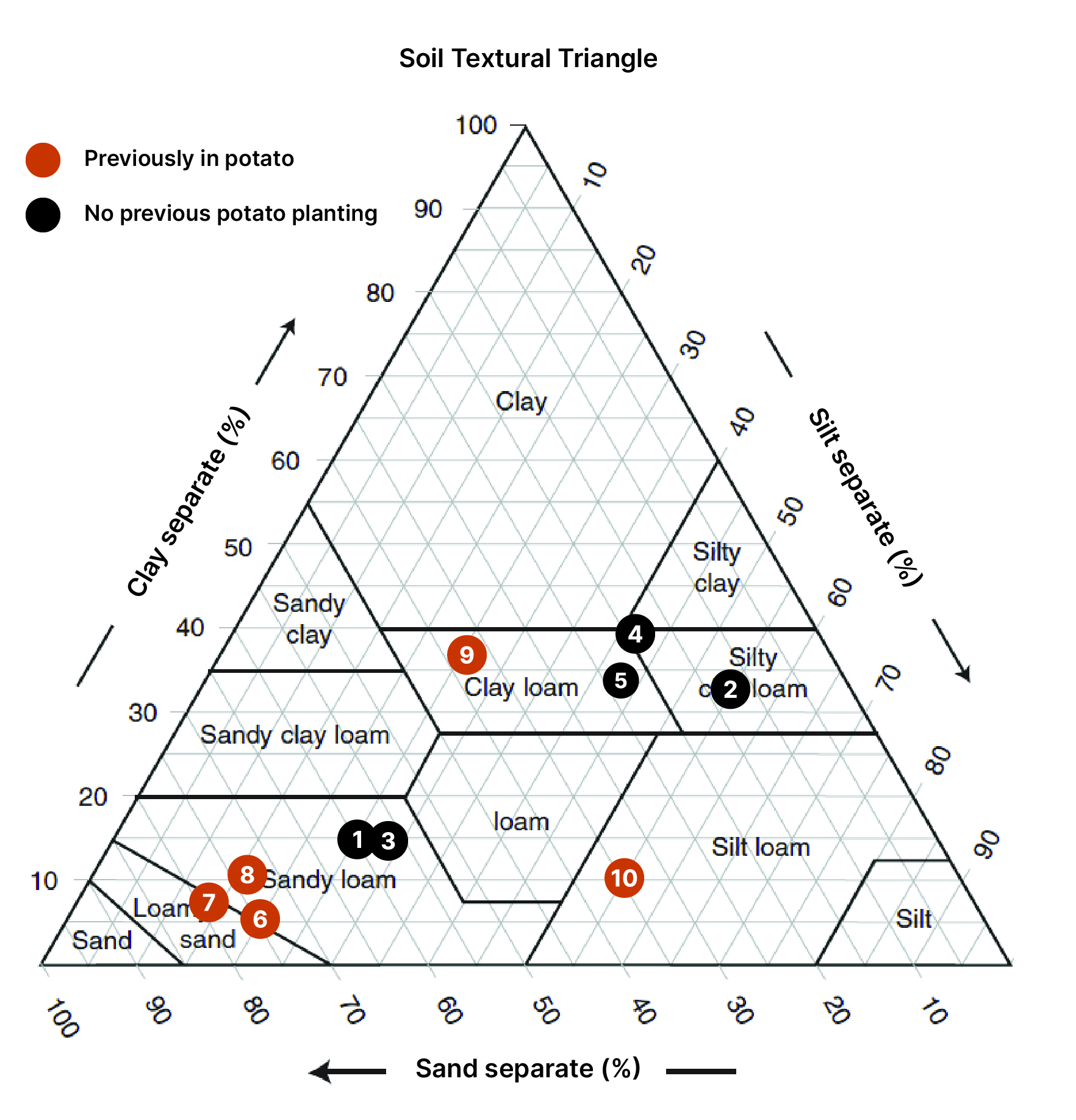
Collection of soils, 2022. A preliminary greenhouse experiment was set up to compare disease suppression from different soils collected in Oregon. Soils with diverse management histories were collected from ten locations. Soils were collected from potato and non-potato agricultural systems in Oregon (e.g., forage crops, long-term pasture, etc.) (Figure 1). Approximately, 12 gallons of each field soil were obtained from each location. For each soil, the chemical and physical properties (i.e., fertility, soil particle sizes, etc.), microbial biomass, and basal respiration were determined.
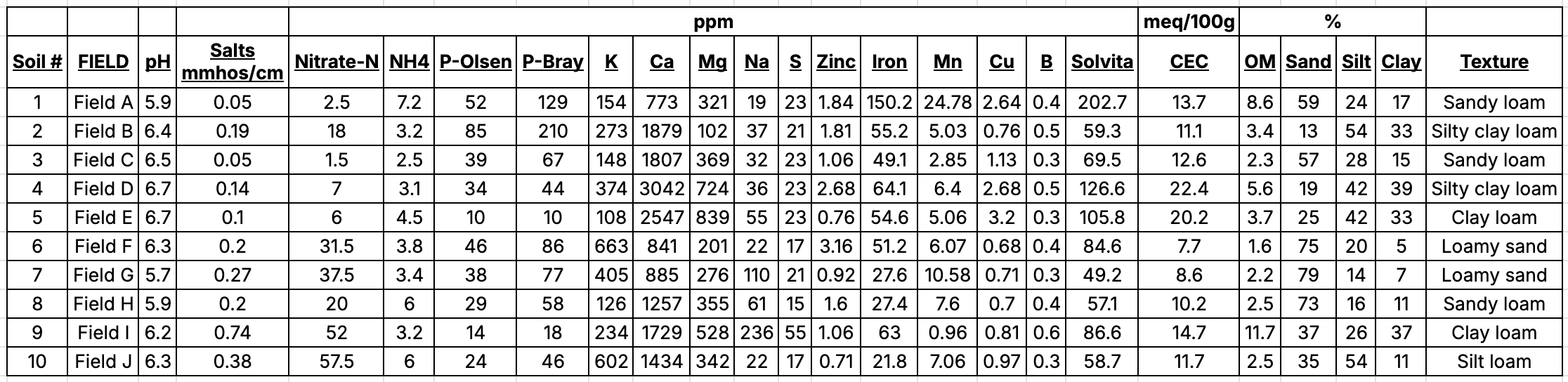
Collection of soils, 2023. A greenhouse experiment was set up to compare disease suppression from different soils collected in Oregon. Soils with diverse management histories were collected from six locations that were informed by the preliminary work from 2022. Soils were collected from potato and non-potato agricultural systems in Oregon (e.g., forage crops, long-term pasture, etc.). Approximately, 20 gallons of each field soil were obtained from each location. For each soil, the chemical and physical properties (i.e., fertility, soil particle sizes, etc.), microbial biomass, and basal respiration were determined.
Greenhouse experiment, 2022. A greenhouse pot experiment was set up to examine disease suppressive activity of the collected field soils. Three different potting mixtures of soil were used in this experiment: potting mixture 1 (PM1) 95% field soil with 5% S. subterranea-infested potting soil, potting mixture 2 (PM2) 10% field soil, 85% potting soil, and 5% S. subterranea-infested potting soil and potting mixture 3 (PM3) 10% autoclaved field soil, 85% potting soil, and 5% S. subterranea-infested potting soil (Figure 2). The three potting mixtures are being used to determine the relative importance of soil edaphic and soil biological factors leading to powdery scab suppressive activity. One treatment consisting of potting soil alone (95% potting soil and 5% S. subterranea-infested potting soil) was included as a control. This experimental design results in a total of 31 treatments. Ten replicate pots, approximately 15 cm diameter, was prepared with each of the potting soil mixtures and one seed tuber of the susceptible cultivar ‘Shepody’ was planted in each of the pots. The experiment was conducted in the plant pathology greenhouse at the HAREC temperatures ranged from 15°C to 44°C, and pots were watered daily to promote S. subterranea tuber infection.
Greenhouse experiment, 2023. A greenhouse pot experiment was set up to examine disease suppressive activity of the collected field soils. Inoculum was applied to all pots at a rate of 5 spores per gram of soil. Three different potting mixtures of soil were used in this experiment: potting mixture 1 (PM1) 100% field soil, potting mixture 2 (PM2) 15% field soil and 85% potting soil, and potting mixture 3 (PM3) 15% autoclaved field soil and 85% potting soil. The three potting mixtures are being used to determine the relative importance of soil edaphic and soil biological factors leading to powdery scab suppressive activity. One treatment consisting of potting soil alone and one treatment consisting of autoclaved potting soil was included as a control. This experimental design results in a total of 20 treatments. Six replicate pots, approximately 35 cm diameter, were prepared with each of the potting soil mixtures and one seed tuber of the susceptible cultivar ‘Kennebec’ was planted in each of the pots. The experiment was conducted in the plant pathology greenhouse at the HAREC, temperatures ranged from 15°C to 32°C, and pots were watered daily to promote S. subterranea tuber infection.
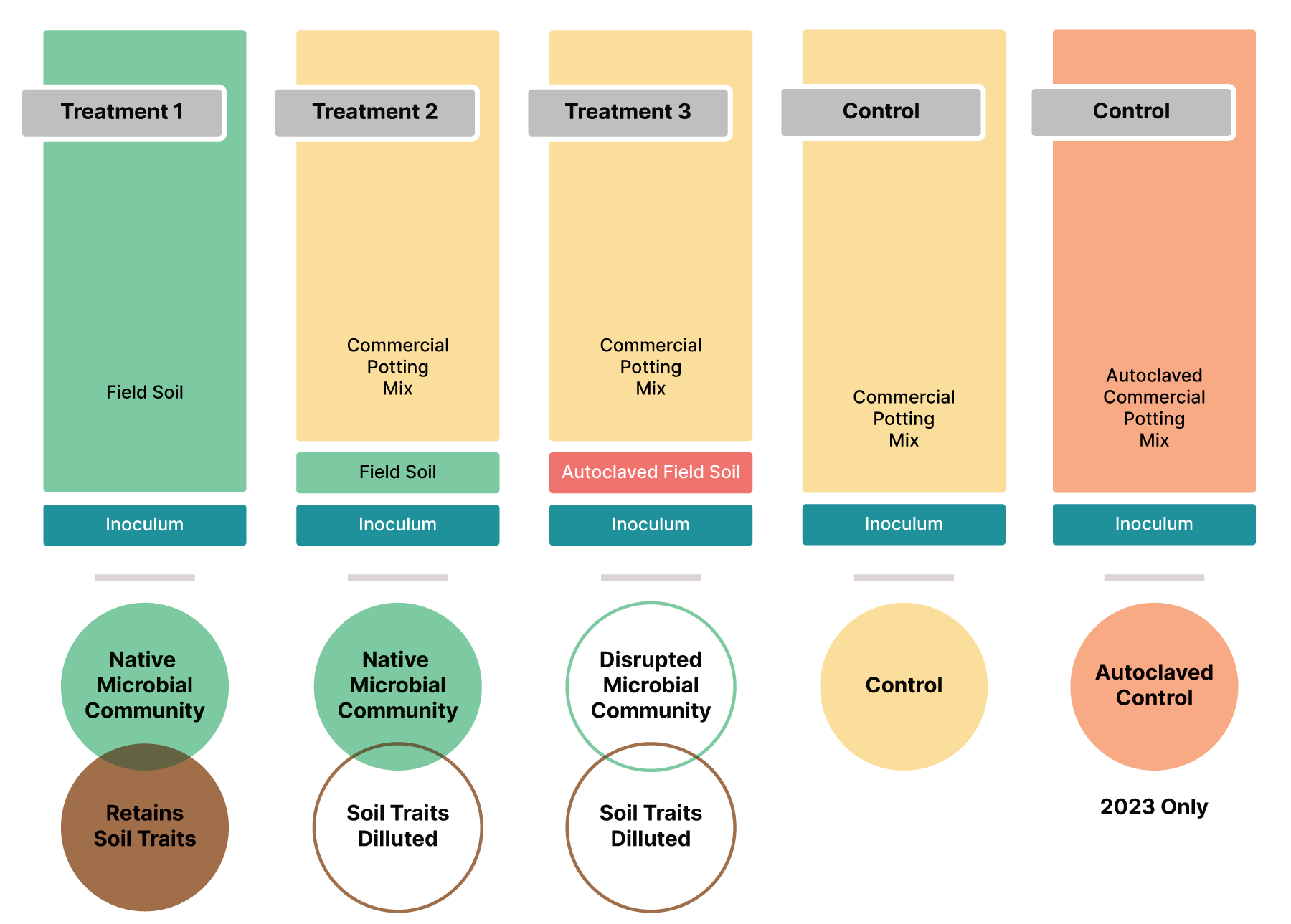
Soil and rhizosphere sampling, 2022. At the start of the experiment, a sample of each potting mixture was collected and stored at -80°C. Bulk and rhizosphere soils were sampled three times throughout the course of the experiment by destructively sampling. Root galling (disease) was assessed on roots during destructive sampling. Bulk soils were sampled directly from each pot. To sample rhizosphere soils, the plant was removed from its pot, then shaken to remove all loose soil from the plant root system. A 0.8 to 1.0 g sample of root was collected for each plant, placed in a 15 ml centrifuge tube with 10 ml of phosphate buffered saline Tween 20 (PBST), and subjected to 3 minutes of vigorous shaking. The cleaned root was removed from the tube and the rhizosphere soil suspended in PBST was centrifuged to pellet the soil. The resulting rhizosphere soil pellet was used for DNA extraction.
Soil and rhizosphere sampling, 2023. At the start of the experiment, a sample of each potting mixture was collected and stored at -80°C. Bulk and rhizosphere soils were sampled once 95 days after planting by destructively sampling. Root galling (disease) was assessed on roots during destructive sampling. Bulk soils were sampled directly from each pot. To sample rhizosphere soils, the plant was removed from its pot, then shaken to remove all loose soil from the plant root system. A 0.8 to 1.0 g sample of root was collected for each plant, placed in a 15 ml centrifuge tube with 10 ml of phosphate buffered saline Tween 20 (PBST), and subjected to 3 minutes of vigorous shaking. The cleaned root was removed from the tube and the rhizosphere soil suspended in PBST was centrifuged to pellet the soil. The resulting rhizosphere soil pellet was used for DNA extraction.
Disease assessment, 2022 & 2023. Powdery scab was assessed for tubers generated in at the end of the experiment. For each pot, the number of tubers were counted, and powdery scab presence was recorded. Powdery scab incidence and severity were estimated for each pot and averaged for each soil treatment. Roots were surface sterilized and assessed for infection by S. subterranea with PCR. 2023, galls were counted on roots to determine severity.
DNA extraction, soil microbiome assay, and bioinformatics pipeline. DNA was extracted from all the soil samples (control, bulk soil, and rhizosphere). Bacterial and fungal community composition were assessed using amplicon sequencing. For library preparation, DNA samples were used as templates for PCR amplification of the 16S and ITS regions. Amplicon libraries were indexed and sequenced on the Illumina NextSeq 2000 platform (300 bp paired-end) at the Oregon State University (OSU) Center for Quantitative Life Sciences. Taxonomy was assigned from the UNITE and SILVA databases for fungi and bacteria, respectively. Sampling depth was normalized, to account for the difference in sequencing depth, and data may be transformed prior to statistical analysis.

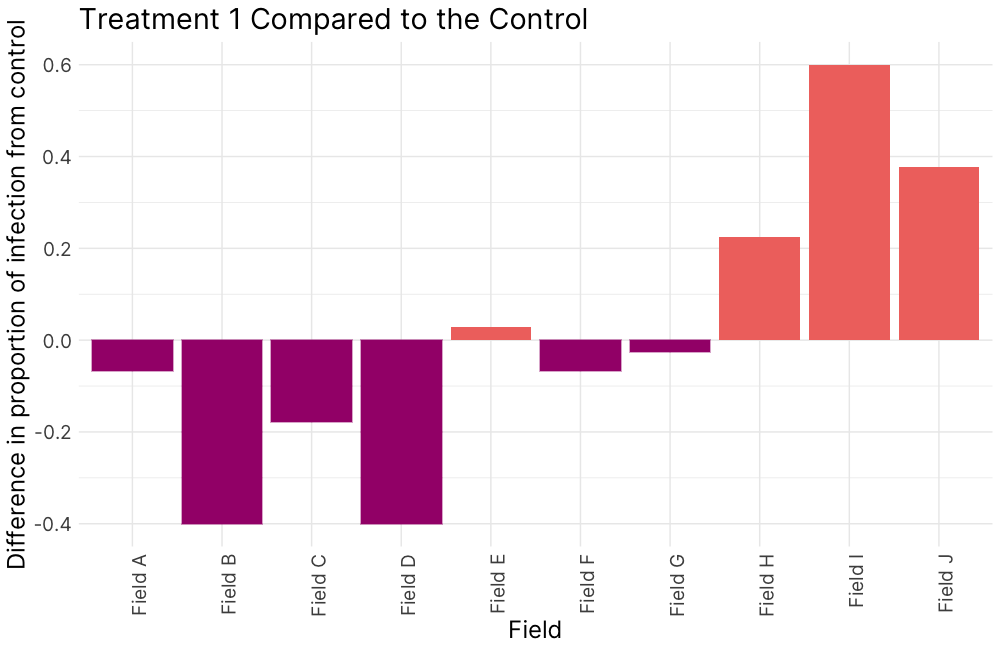
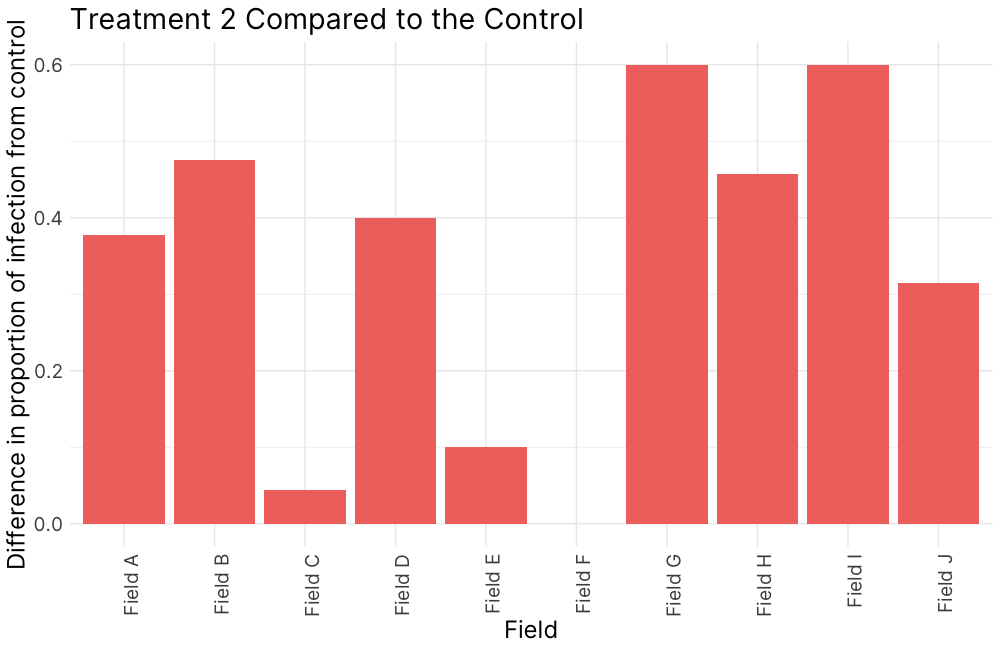
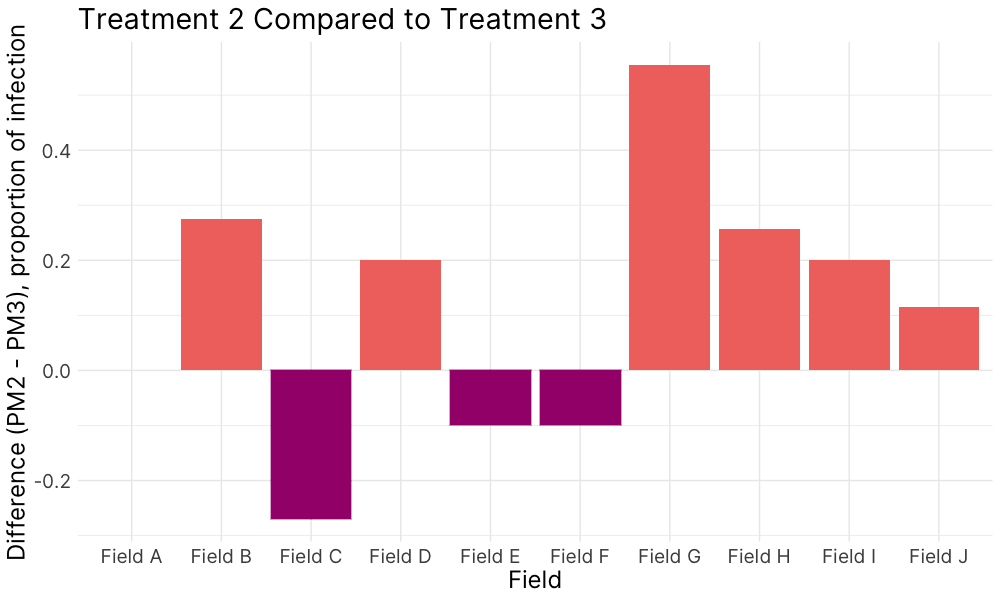
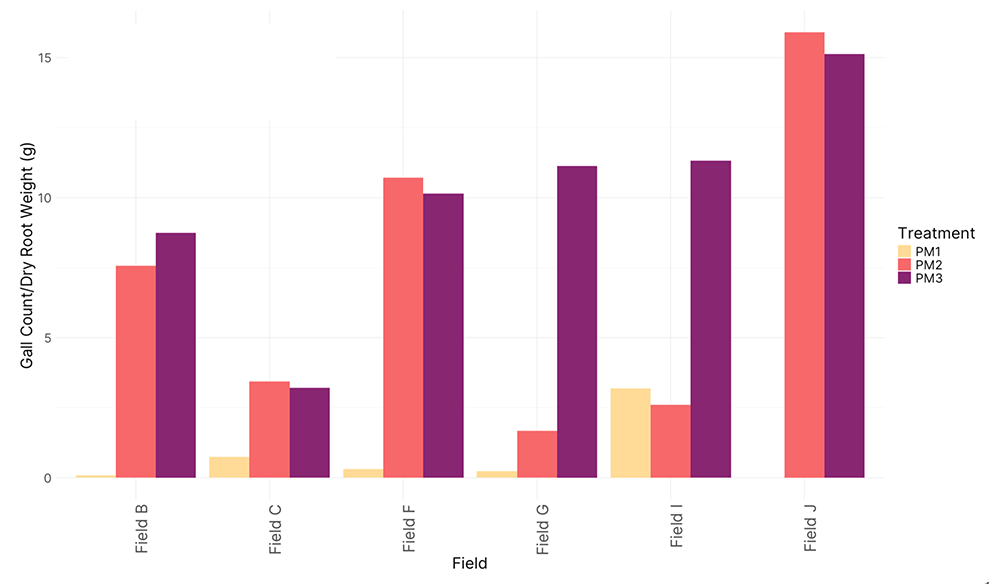
Figure 5. Disease incidence, 2022.
Figure 5. (A) Treatment 1 (PM1, 95% field soil with 5% S. subterranea-infested potting soil) was differentially compared to the control for infection of root tissue (B) Treatment 2 (PM2, 10% field soil, 85% potting soil, and 5% S. subterranea-infested potting soil) was differentially compared to the control for infection of root tissue (C) and Treatment 2 was differentially compared to Treatment 3 (PM3, 10% autoclaved field soil, 85% potting soil, and 5% S. subterranea-infested potting soil).
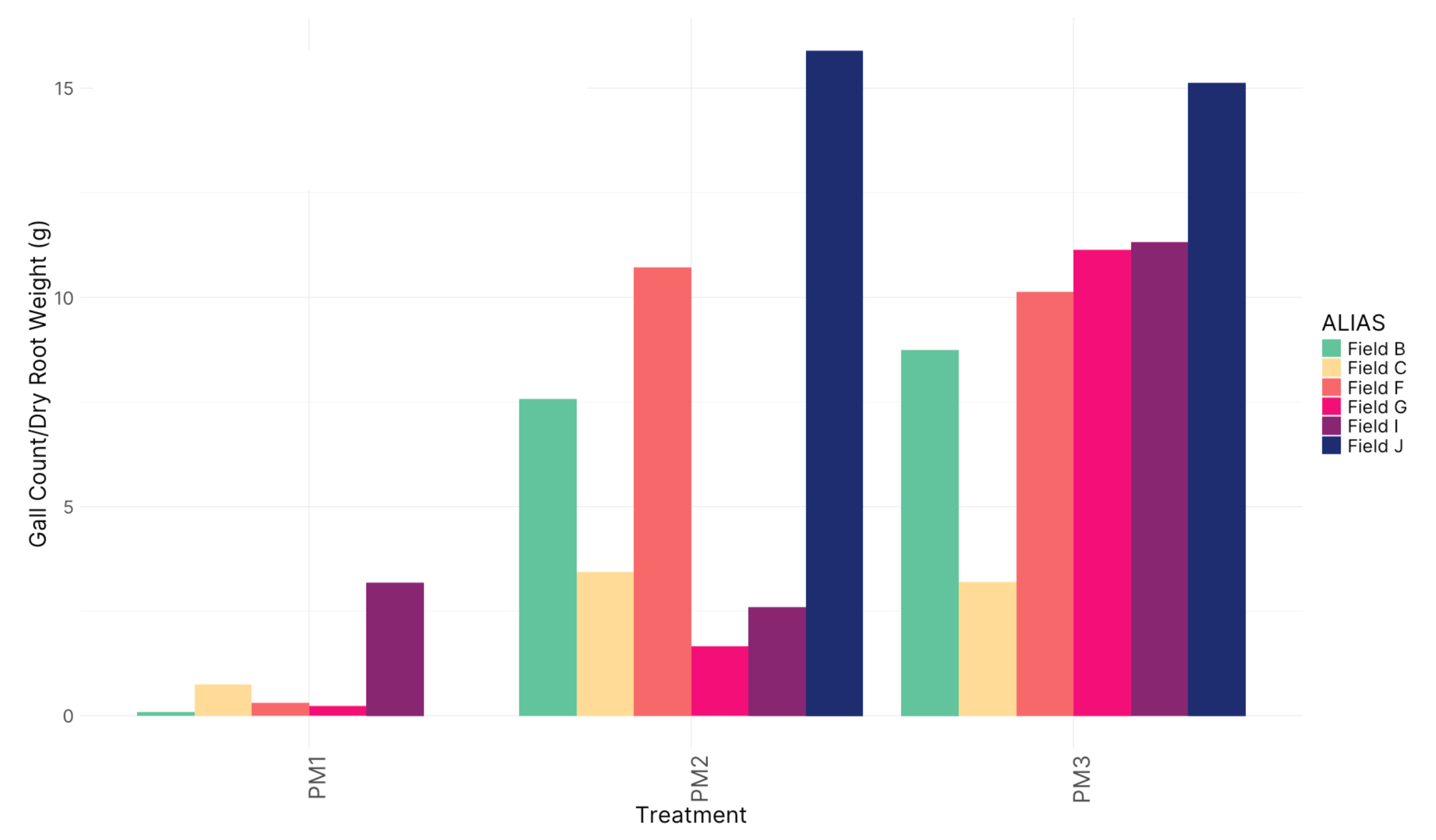
Figure 6. Disease incidence and severity, 2023
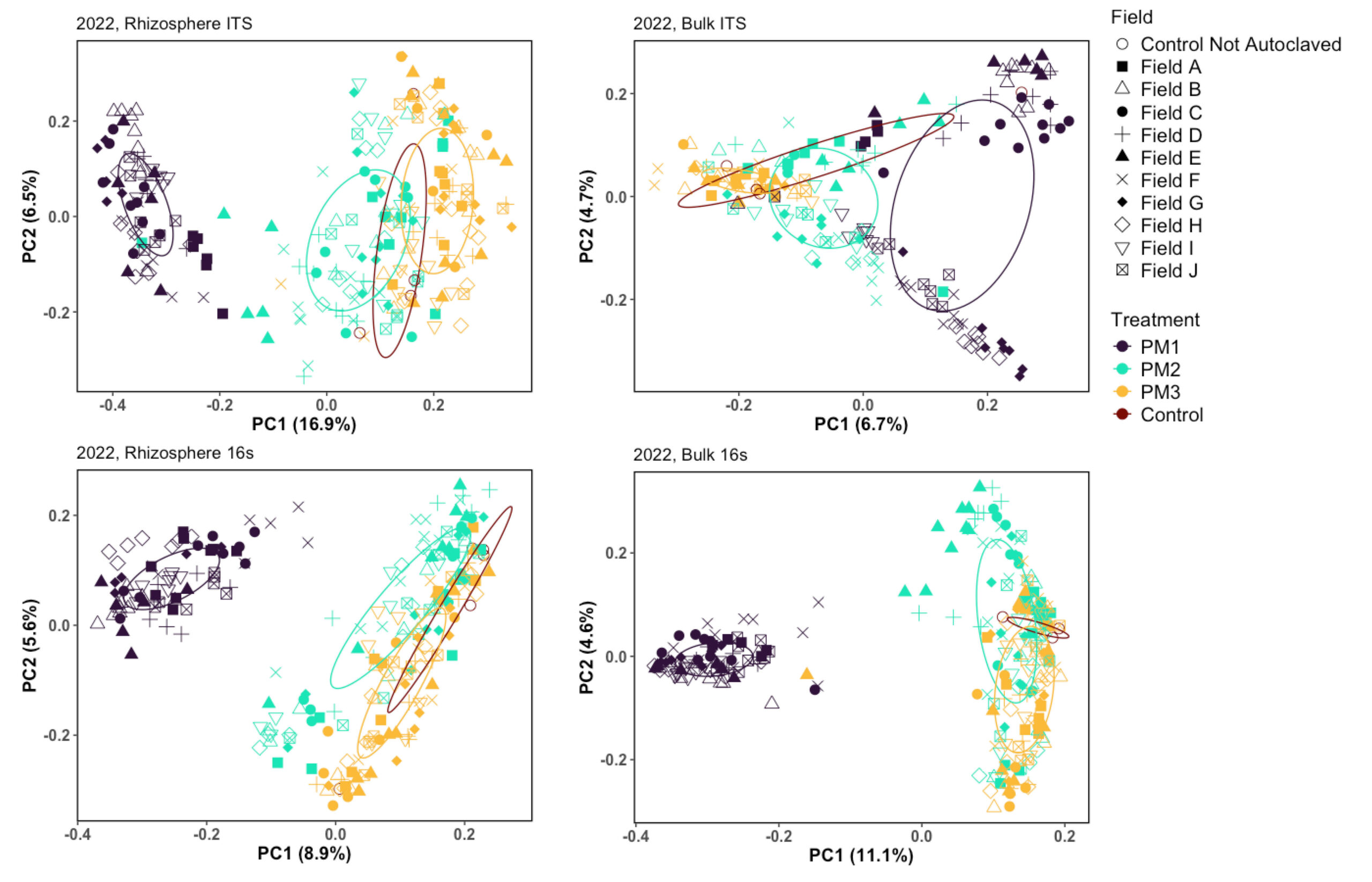
Figure 7. PCoA plots of 2022 microbial communities
Principal Coordinates Analysis, showing the results of Bray-Curtis distance for samples in 2022, illustrating a shift in the microbial communities after treatment.
Figure 8. PCoA plots of 2023 microbial communities
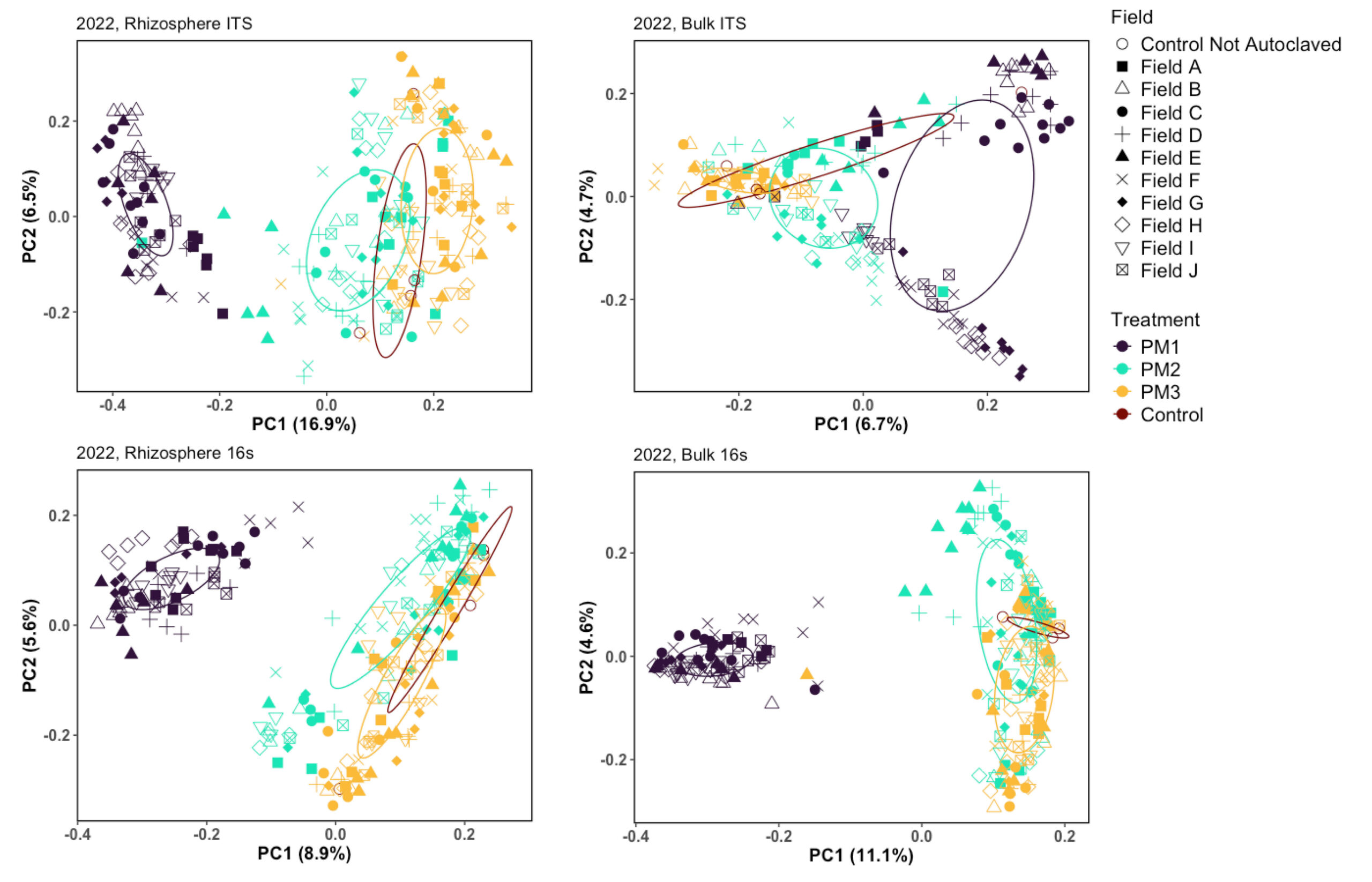 Principal Coordinates Analysis, showing the results of Bray-Curtis distance for samples in 2023, illustrating a shift in the microbial communities after treatment.
Principal Coordinates Analysis, showing the results of Bray-Curtis distance for samples in 2023, illustrating a shift in the microbial communities after treatment.
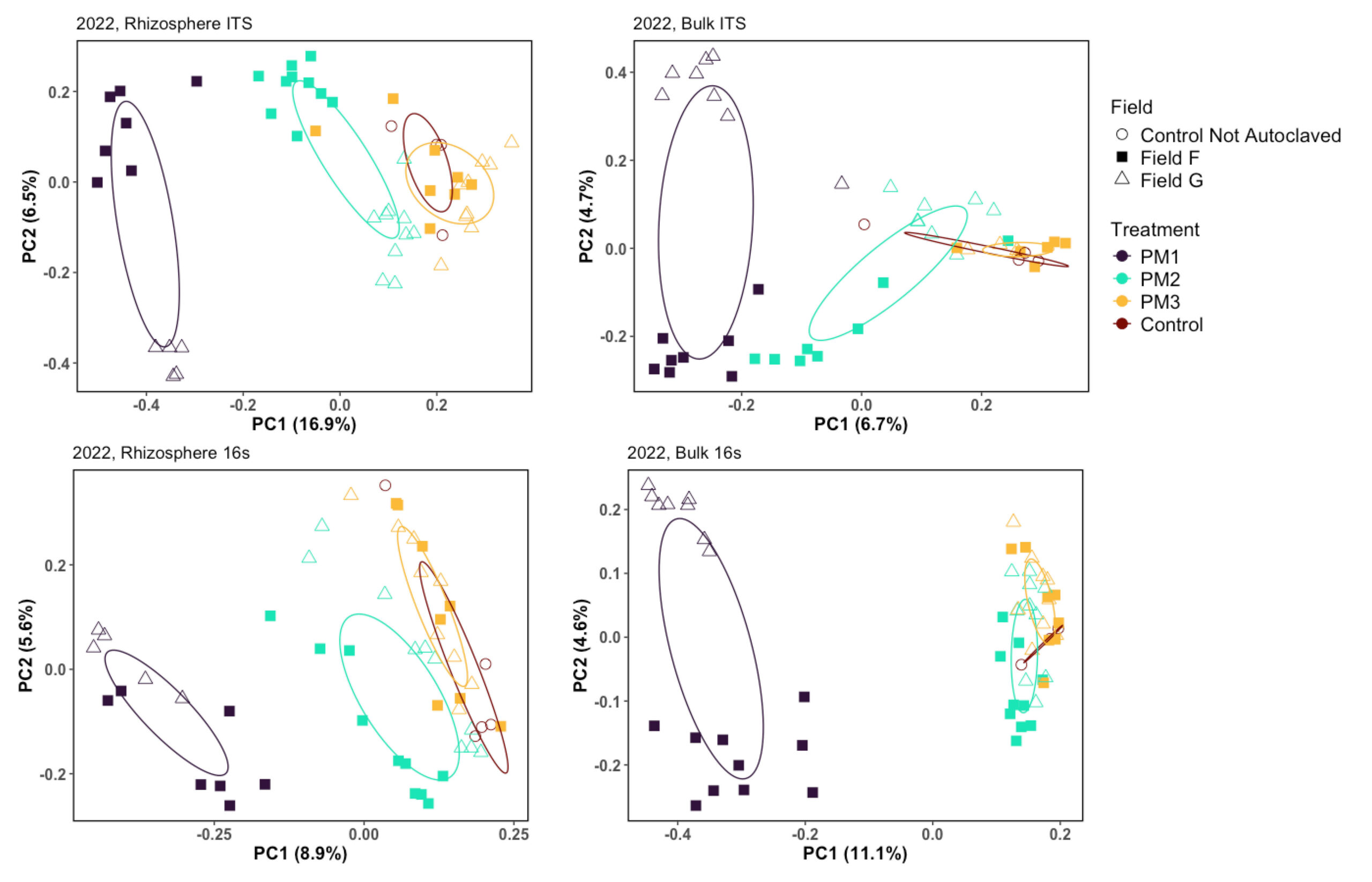
Figure 9. PCoA plots of 2023 microbial communities, Field F and G
Principal Coordinates Analysis, showing the results of Bray-Curtis distance for samples in 2022 of Field F and G, illustrating an increase of similarities between communities after treatments in a susceptible(Field F) and a suppressive field (Field G).
Objective 1. Characterize the temporal dynamics of the potato rhizosphere microbiome through the growing season and in relation to powdery scab status.
This study investigated the impact of various soil treatments on powdery scab incidence and severity, along with the role of microbial communities in suppressing infection by Sss. Due to limited germination in some treatments we were unable to determine temporal dynamics in disease incidence and severity, but the available data still provided insights into treatment effects.
In 2022, soils from fields A-G demonstrated greater suppressive activity against powdery scab compared to the control soil, highlighting the potential of native soil characteristics or microbiota to reduce disease severity (Fig. 5 A). When soil texture was modified through dilution, the suppressive activity became more variable. (Fig. 5 B). Three specific soils showed reduced infection rates with native microbial communities compared to when the community was disrupted which suggests that soil microbial communities play a role in disease expression (Fig. 5 C).
In 2023, we did increase pot size and decrease temperatures in the greenhouse and were able to encourage gall formation. Preliminary results show that Potting Mix 3 exhibited a significant increase in gall formation per gram of dry root weight compared to other treatments (p = 0.00917) (Fig. 6 D). This finding highlights the potential susceptibility of this potting mix to infection or its inability to support beneficial suppressive microbiota. When the field soil was autoclaved, disrupting the microbial community, it led to increased gall counts, further emphasizing the importance of the native microbial community mitigating disease severity. No significant differences in disease suppressiveness were observed across the tested fields in 2023 (Fig 6 E).
Objective 2: Assay soils for their ability to suppress powdery scab by identifying abiotic and biotic factors associated with disease suppression and by identifying systems and/or management practices associated with reduced disease.
Principal coordinate analyses were conducted using Bray-Curtis distance matrices to observe the differences between samples caused by the fields and treatments (Figures 7 and 8). This approach allowed for the visualization of patterns and the identification of distinct shifts in microbial community composition associated with each treatment. We expected to see a shift in the community as we disrupt the soil physical properties and native microbial community.
Field F is an example of one of the susceptible fields and Field G is suppressive (Figure 5C, 9). Field F and G both have a history of potato and both have a loamy sand soil texture. The native microbial communities are different in treatment 1 and become more similar in treatment 2 and 3 (Figure 9).
Overall, the results highlight the dynamic interactions between soil properties, microbial communities, and plant disease. These findings have implications for developing sustainable agricultural practices to minimize powdery scab impact in potato production.
Research Outcomes
Currently, our research results do not change powdery scab management recommendations.
This study highlighted the challenges of working with Spongospora subterranea because it’s an obligate biotroph, making it a challenging organism to study. Achieving consistent disease development in the greenhouse remains challenging. We will continue to develop standardized methods to create a consistent and reliable disease response in future studies.
Education and Outreach
Participation Summary:
Objective 3 is to engage growers in project activities and outcomes and communicate research findings about powdery scab to the stakeholder and research community. Our analysis of the data took longer than we anticipated and have not been able to communicate our results to our stakeholders. Ongoing outreach, including the dissemination of a pre/post survey, will include meetings with stakeholders about research data at Hermiston Potato Field Day in June 2025 and at the Hermiston Farm Fair December 2025.
Daniella Echeverria and Dr. Ken Frost have presented research results from this project at the following events:
- Echeverria, D. M. Graduate Student Celebration Thesis Proposal Seminar. Corvallis, OR. May 5, 2023 (~150) Invited
- Echeverria, D. M. Identifying biotic characteristics of soils that suppress powdery scab of potato (Solanum tuberosum). American Phytopathological Society National meeting, Denver, CO, August 16, 2023 (~100) Attended.
- Echeverria, D., and Frost, K.E. Identifying soils and soil properties suppressive to powdery scab. OSU-HAREC Potato Field Day, Hermiston, OR, June 21, 2023 (~100) Invited
- Frost, K.E. Powdery scab, the environment, and implications for disease management. Hermiston Farm Fair, Hermiston, OR, November 29, 2023 (~90). Invited
- Frost, K.E. The soil environment and its effect on powdery scab of potato. 2023 Southern Rocky Mountain Ag Conference, Monta Vista, CO, February 7, 2023 (~75). Invited
- Echeverria, D. M. American Phytopathological Society Pacific Division meeting 2024 (poster). Corvallis, OR, March 27, 2024 (~100) Invited.
- Frost, K. Changes to soil health indicators, microbial communities, and potato yield following biofumigation and application of composted manure. UC Davis Department of Plant Pathology Seminar Series, October 7, 2024, Davis, CA. (~50) Invited
- Echeverria, D., Skillman, V., Rivedal, H., Temple, T., and Frost, K., 2024. Characterizing the microbiome of soil that suppresses powdery scab. In The Potato Virus Initiative, ed. by Hendricks, Olsen, and Karasev, 2024 Issue, 50 pp.
This project has reached a diverse audience, including growers, extension specialists, academic and industry researchers, as well as the public. We have shared our findings nationally at professional society meetings and locally presented at grower meetings and field days. Participants were engaged and showed an interest in learning about our results and recommendations.
The results of this research will be submitted to peer-reviewed journals such as Phytopathology as we are developing a greenhouse assay to research this difficult to study obligate plant pathogen. The results in this manuscript will discuss the methodology of the assay and a comparison of soil factors involved in disease incidence and severity for researchers who are interested in novel approaches to studying Sss.