Final report for GW22-239
Project Information
Every year potato producers lose approximately 17% of their yield due to plant pathogenic microorganisms. Potato is the fourth most important staple food crop in the world and accounts for a nearly $2 billion dollar industry in Oregon, Washington, and Idaho alone. To combat this loss, farmers may rely on a combination of control methods such as pesticides application, cultural practices, and planting of disease-free certified seeds and, when available, resistant cultivars. However, for some pathogens, these control methods are either unavailable, inadequate, or unsustainable. Therefore, new control methods are needed. Thiamin, also known as vitamin B1, a compound naturally produced by plants, can prime plants when externally applied to foliage, enabling plants to respond more rapidly and/or more robustly to pathogens and thus annihilating or limiting disease. However, besides one study on potato virus Y, the priming effect of thiamin has never been tested in potato. Therefore, in this project, I propose to test the effectiveness of thiamin priming application on potato against bacterial (Streptomyces and Pectobacterium) and fungal (Alternaria solani) pathogens. The experiments were done in greenhouses as well as in the fields of an Oregon organic producer, Rainshadow Organics, who had recently experienced severe losses due to potato soil bacterial pathogens. I also educated the agricultural community on the priming effects of vitamins through presentations at extension events and publications in extension and scientific journals.
Objective #1: Test the effectiveness of thiamin priming treatments against bacterial pathogens in potato
Sub-objective #1: Evaluation of the effect of thiamin treatment on potato tuber susceptibility to Pectobacterium carotovorum subsp.carotovorum-induced soft rot
Sub-objective #2: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces sp.-induced common scab in the field
Sub-Objective #3: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces scabies-induced common scab in the greenhouse.
Objective #2: Test the effectiveness of thiamin priming treatments against a fungal pathogen in potato
Objective 3: Educate the agricultural industry on vitamin-induced priming through presentations at extension events, publications in extension and scientific journals, and through social media
Sub-objective #1: Present research results to producers/stakeholders and academic and industry scientists through presentations at scientific and extension meetings
Sub-objective #2: Publish papers/articles in scientific peer-reviewed journals, extension newsletters, and trade journals
Sub-objective #3: Post research updates on social media and our lab webpage
Cooperators
- - Producer
- (Researcher)
Research
Objective #1: Test the effectiveness of thiamin priming treatments against bacterial pathogens in potato
Sub-objective #1: Evaluation of the effect of thiamin treatment on potato tuber susceptibility to Pectobacterium carotovorum subsp.carotovorum-induced soft rot
Plant growth – For this sub-objective we grew potato plants Solanum tuberosum cultivar Russet Burbank and Russet Norkotah, major varieties grown in the Pacific Northwest that are susceptible to tuber soft rot, in a greenhouse at the Oregon State University. We first propagated disease-free potato plantlets in tissue culture. Then, after 3-4 weeks on solid Murashige and Skoog (MS) medium (1x MS-modified BC potato salts, 2% sucrose, 100 mg l-1 myo-inositol, 2 mg l-1 glycine, 0.5 mg l-1 nicotinic acid, 0.5 mg l-1 pyridoxine, 0.1 mg l-1 thiamine, pH 5.6), we transferred plants to soil (1 part sand, 4 parts Sunshine Mix 1) in 1-gallon pots containing slow-release fertilizer in a greenhouse at 21℃ day/15℃ night with a 14-hour photoperiod (supplemental day length is provided by sodium lights). Each experiment had at least 5 plants per treatment and had two treatments, mock and thiamin treated. When plants started tuberizing (we monitored swelling at the stolon tips regularly to determine the start of tuberization), we applied 50 mM of thiamin or mock solution either once before harvest, or biweekly during tuberization stage. After 2-3 months, when plants had fully tuberized, we harvested and washed tubers. We then inoculated tubers with a culture of P. carotovorum subsp carotovorum.
Plant treatments – We dissolved thiamin in deionized (DI) water with the addition of the surfactant Tween 20 at 250 µg/L. Surfactants help the product to adhere to the foliar surfaces of the potato plant. We compared the thiamin treated samples to those treated with a mock solution made with only DI water and Tween 20 at 250 µg/L. Foliar application was sprayed until runoff (~30 mL per plant). In experiments where a tuber dip application was used, we first sterilized the tubers in 1% sodium hypochlorite for 3 minutes, then rinsed with DI water 3 times. We then allowed the tubers to dry for at least 2 hours before beginning treatments. We used 3 different treatments for tuber dip assays, thiamin (2.5 g/L), acetylsalicylic acid (ASA) (2.5 g/L), and a mock solution of DI water. ASA was used as a positive control as it shown to reduce the amount of soft rot when tubers were soaked in a solution by a previous study done by (Sallam et al 2010). Tubers were soaked in the solutions for 2 hours, then left to dry for at least 45 minutes, and inoculated with P. carotovorum subsp carotovorum.
Preparation of P. carotovorum subsp carotovorum inoculum and tuber inoculation – A culture of P. carotovorum subsp carotovorum was started from our frozen stock by streaking it out on plates containing LB agar medium. A single isolated colony was used to inoculate liquid LB medium, and the bacteria was grown at 28℃ until an optical density of 0.2-0.3 (as determined with a spectrophotometer). This optical density equals to 108 colony forming units (CFUs) per ml. Tubers were then inoculated using 10 µl of solution at 1 X 108 CFUs/ml into a 5 mm deep hole made using a pipette tip. We then wrapped the tubers in a wet paper towel followed by plastic wrap and incubated them in a chamber at 28℃ and 100% humidity for 72 hours. For tuber dip assays, tubers were incubated at room temperature (~24℃) for six days before being analyzed.
Scoring of the disease – After incubation, the rotten tissue was scooped out and weighed. We used t-tests to determine statistical differences between samples.
Sub-objective #2: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces sp.-induced common scab in the field
For this sub-objective, our experimental trial was done in a field known to produce common scab disease in potato. This field is in central Oregon on the property of an organic producer, Rainshadow Organics (see Letter from Producer). Nearly 50% of their potato production has been affected by common scab in the past growing seasons. After discussion with the farm staff, two fresh market potato varieties, Huckleberry Gold and Viking Purple, were grown on 1200-feet long rows using current practices. During planting the first week of May, we flagged four blocks of ~20 plants for each treatment (two treatments: mock and 50 mM thiamin) for each variety in a completely randomized design (CRD). There were two plots tested, a north plot, which contained only Huckleberry Gold potatoes, and a south plot, which contained a mix of Purple Viking and Huckleberry Gold potatoes. During tuberization, we started applying thiamin or a mock solution containing Safer Soap (0.02%) as a surfactant on a biweekly basis (every other week). Rainshadow Organics currently uses Safer Soap, which has insecticide property in addition to its surfactant property. Buffer rows on the edge of the field and between varieties did not receive any treatment. At harvest in late August-2022, common scab lesion severity and coverage was evaluated as described below. This trial was done in the growing season of 2022.
Scoring of the disease – The methods are as described by Wanner et al, 2006 [23]. Two components were measured when scoring common scab on tubers: lesion coverage and lesion type. Lesion coverage was measured using percent of tuber covered and rated on a scale of 0 to 5 (0 = no scab lesions; 1 = 1-2%; 2 = 2-5%; 3 = 5-10%; 4 = 10-25%; 5 = >50%). Lesion type was measured on a scale of 0 to 6 (0 = no lesion; 1 = superficial lesions, <10 mm in diameter; 2 = superficial lesions, >10 mm in diameter; 3 = raised lesions, <10 mm in diameter; 4 = raised lesions, >10 mm in diameter; 5 = pitted lesions; 6 = coalescent pitted/raised lesions covering entire tubers). The final disease index was assigned by multiplying the average lesion severity of all scorable tubers (>0.5 cm diameter) in a pot by the average lesion coverage.
Sub-Objective #3: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces scabies-induced common scab in the greenhouse.
We also ran experiments in a greenhouse where we had control of the concentrations of S. scabies inoculum present in soil. This complements our sub-objective #2 where we evaluated thiamin treatment on Streptomyces in a field setting.
Plant growth – We first grew potato plants as described in sub-objective #1. We applied 50 mM of thiamin or mock solution on a biweekly basis using spray bottles on the foliage of the plant, or through a drenching technique, where the thiamin solution was used to water the plant. The mock and thiamin solution contained the surfactant Tween 20 at 250 µg/L.
Preparation of S. scabies inoculum and soil inoculation – To prepare the S. scabies inoculum, several media plates were inoculated with S. scabies from frozen stocks. S. scabies is considered to be the most aggressive species of common scab. We used S. scabies isolates ME01-11h and ND05-01C. The plates were incubated until the culture was uniformly sporulating. Harvesting spores was done by flooding plates with sterile water and rubbing gently with a sterile glass rod to release spores. Water containing the suspended spores was then collected into a sterile test tube. Spores were then counted under a microscope on a haemocytometer slide and solution was adjusted to an optimal concentration of spore solution. Soil was then amended with S. scabies inoculum.
Scoring of the disease – The methods are as described in sub-objective 2. The final disease index was assigned by multiplying the average lesion severity of all scorable tubers (>0.5 cm diameter) in a pot by the average lesion coverage.
Objective #2: Test the effectiveness of thiamin priming treatments against a fungal pathogen in potato, Alternaria solani, the causal agent of potato early blight.
For this objective we grew potato plants Solanum tuberosum cultivar Russet Norkotah, which is susceptible to foliar early blight, as described above in sub-objective 1 for 5-7 weeks. We treated plants with either a mock/control treatment or thiamin treatment at differing concentrations before inoculation with the pathogen. We used the same foliar treatment methods as described in sub-objective #1. We also treated plants with the positive control acibenzolar-S-methyl, a chemical in the benzothiadiazole (BTH) chemical family, which was shown to reduce the incidence of lesions caused by A. solani (Bokshi et al 2003).
Preparation of A. solani inoculum and plant inoculation – We grew A. solani on V8 agar medium (10% clarified V8 juice, 1.5 % CaCO3, and 12.7% Agar) under continuous light for 14-21 days. We then harvested conidia by spreading 5 mL dI water over the mycelium of the plate and dislodged the spores gently using a plastic hockey spreader. Spore solution was then filtered through four layers of cheesecloth to separate the spores from the mycelium. We counted the spores using a hemocytometer and adjusted the concentration to 15,000-30,000 spores/ml. We used at least four plants per treatment and harvested four leaflets per plant from the 3rd and 4th leaves from top, 3 to be inoculated with A. solani, and 1 mock-inoculated using water to ensure symptoms were due to pathogen and not water damage. We arranged the leaflets in garden germination trays according to treatments. We then drop-inoculated the leaflets with 20 µl of the spore solution on the adaxial side with 4 drops per leaf. We covered the trays with clear humidity domes sealed with packing tape to maintain high humidity for optimum infection and placed the trays under lights at the end of the day with photoperiod of 12h at 22℃. A. solani needs darkness to begin its germination. For whole plant assays we first built a chamber made of PVC piping and clear plastic and outfitted the chamber with a small humidifier to run on the lowest setting for 2 hours in both the morning and night. We then treated plants with either a thiamin or mock solution before placing them in the chamber and drop inoculating the leaflets with 10 µl spore solution at concentrations of 15,000-30,000 spores/ml. We covered the chamber with a blackout cloth immediately after inoculation and left it on for the first 16 hours of infection. After 3 days, we detached the infected leaflets from the plant, and took images.
Scoring of the disease – We scored the disease after 3 days by measuring the lesion area of every drop. Susceptibility is correlated to the size of the lesion. We first took images of the infected leaflets, then used the computer program imageJ to measure lesion area.
Data and statistical analyses – Data was analyzed in Excel to calculate means, standard errors, and statistical differences. We used Student's t-test with a p-value cutoff of 0.05. Charts and figures were produced using Excel and Power Point for visualization.
References:
- A.A. Sallam M, M.H. Abd El-Moneem K, A.E. Hassan M, M.M.Khalil, Hadeel: Resistance Induced in Potato Tubers to Soft Rot Caused by Erwinia carotovora Subsp. carotovora by Treatments with Salicylic Acid and Acetylsalicylic Acid.*. Assiut Journal of Agricultural Sciences 41: 1-12 (2010).
- A.I. Bokshi, S.C. Morris and B. J. Deverall: Effects of benzothiadiazole and acetylsalicylic acid on β-1,3-glucanase activity and disease resistance in potato. Plant pathology 52: 22-27 (2003)
Results
Objective #1: Test the effectiveness of thiamin priming treatments against bacterial pathogens in potato
Sub-objective #1: Evaluation of the effect of thiamin treatment on potato tuber susceptibility to Pectobacterium carotovorum subsp.carotovorum-induced soft rot
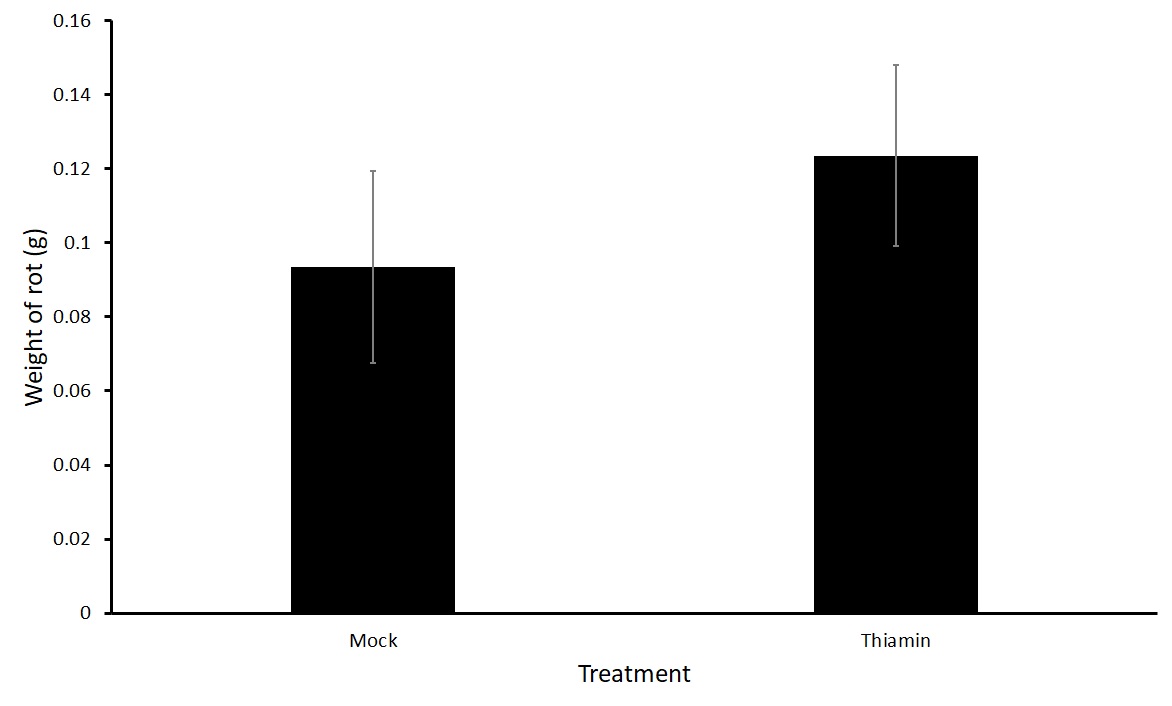
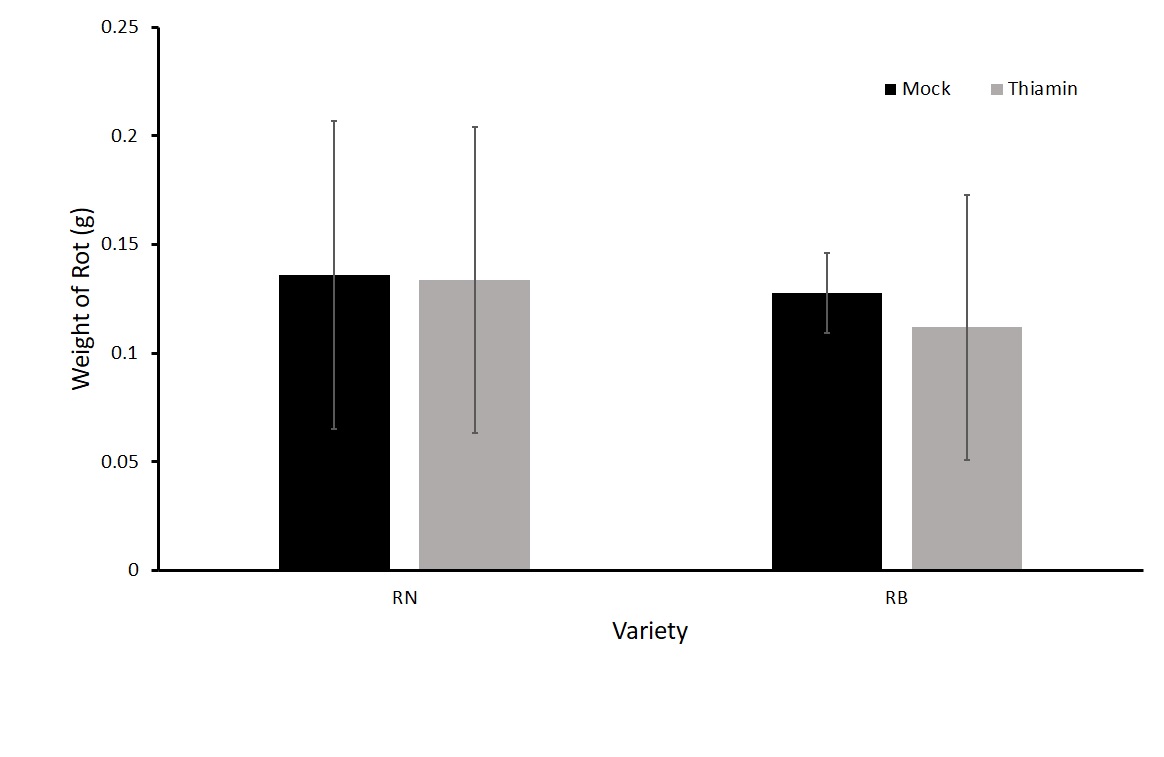
For the first experiment in this objective, we treated plants biweekly three times with either 50 mM thiamin or a mock solution after beginning of tuberization. We then harvested, washed, and inoculated the tubers one day after the final treatment. We saw no difference in the treatment groups (Figure 1). One hypothesis was that the plants were becoming desensitized to the priming effect of thiamin as they were being treated several times before challenged with P. carotovorum subsp carotovorum. Therefore, we tested whether one application with thiamin just before harvest would reduce rot compared to a mock treatment. We treated plants of two different varieties, Russet Norkotah and Russet Burbank with either 50 mM thiamin or a mock solution once 16 hours before inoculation with P. carotovorum subsp carotovorum. No differences were observed in the treatments for either variety (Figure 2). Because there was no difference in treatment groups, we decided to test another form of treatment by dipping tubers in a thiamin solution.
Figures 3 and 4 show average weight of rot on potatoes that were soaked directly in a treatment of thiamin (2.5 g L-1) or ASA (2.5 g L-1) or a mock solution for two hours before being challenged with P. carotovorum subsp carotovorum. No differences were seen in the treatment groups. Because a large variation was present in the data, we are planning on conducting a third trial.
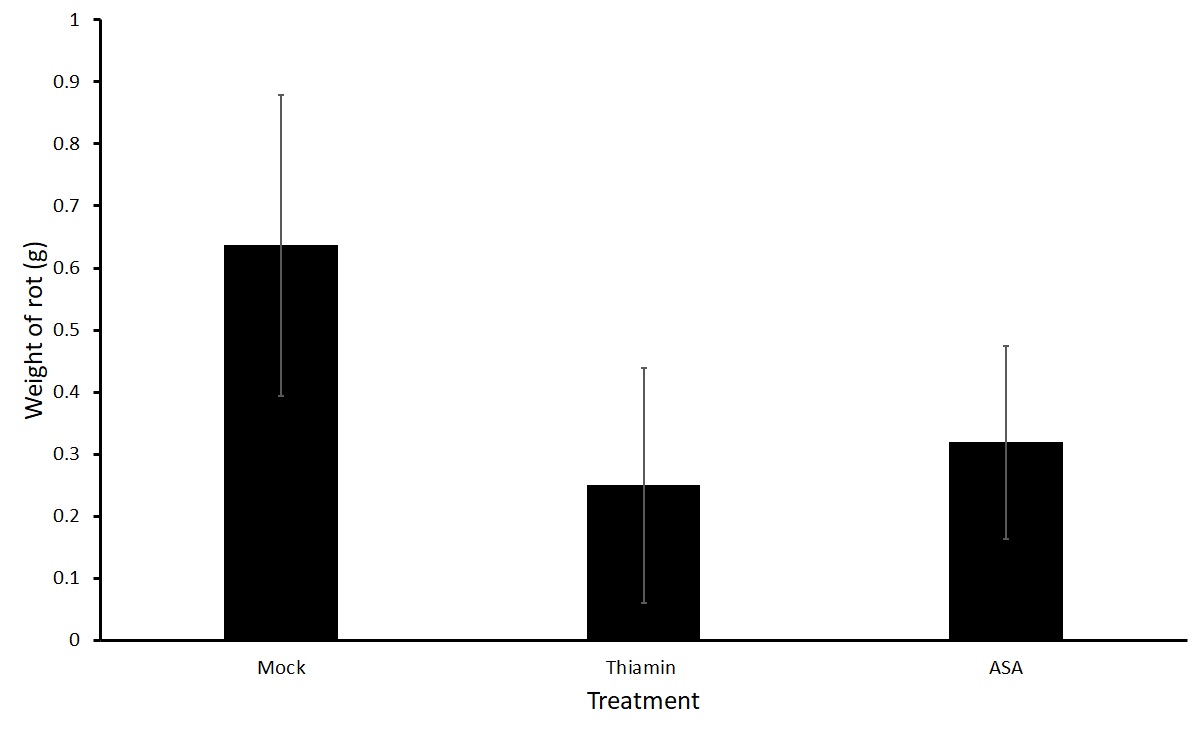
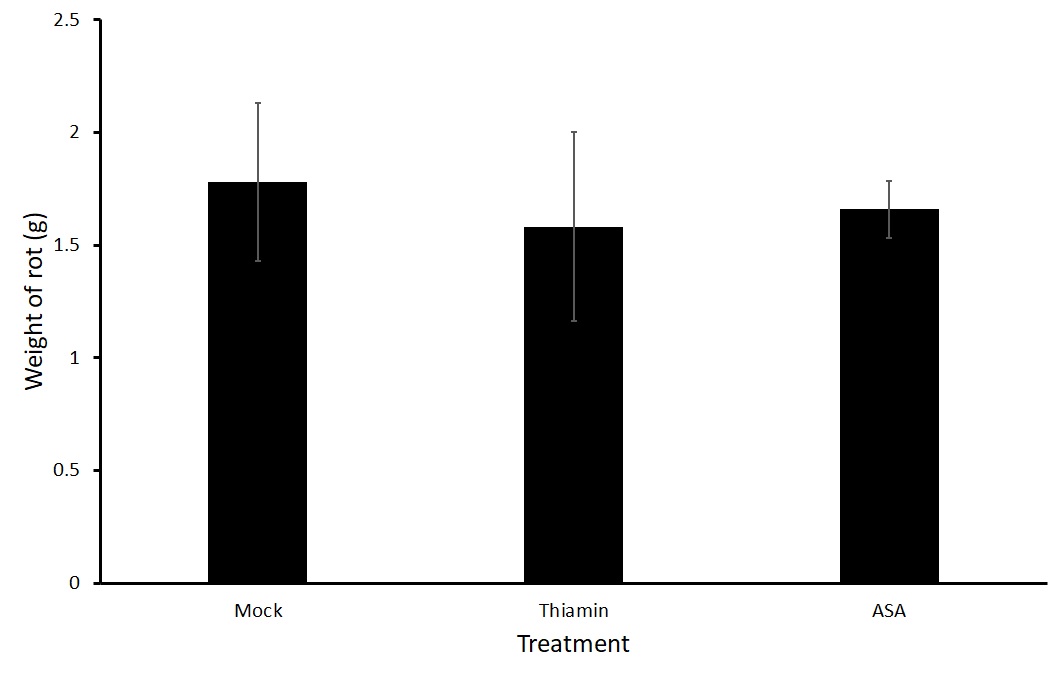
Sub-objective #2: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces sp.-induced common scab in the field
For this sub-objective we treated plants of two varieties susceptible to common scab, Purple Viking and Huckleberry Gold, with either 50 mM thiamin or a mock solution every two weeks beginning at the onset of tuberization.
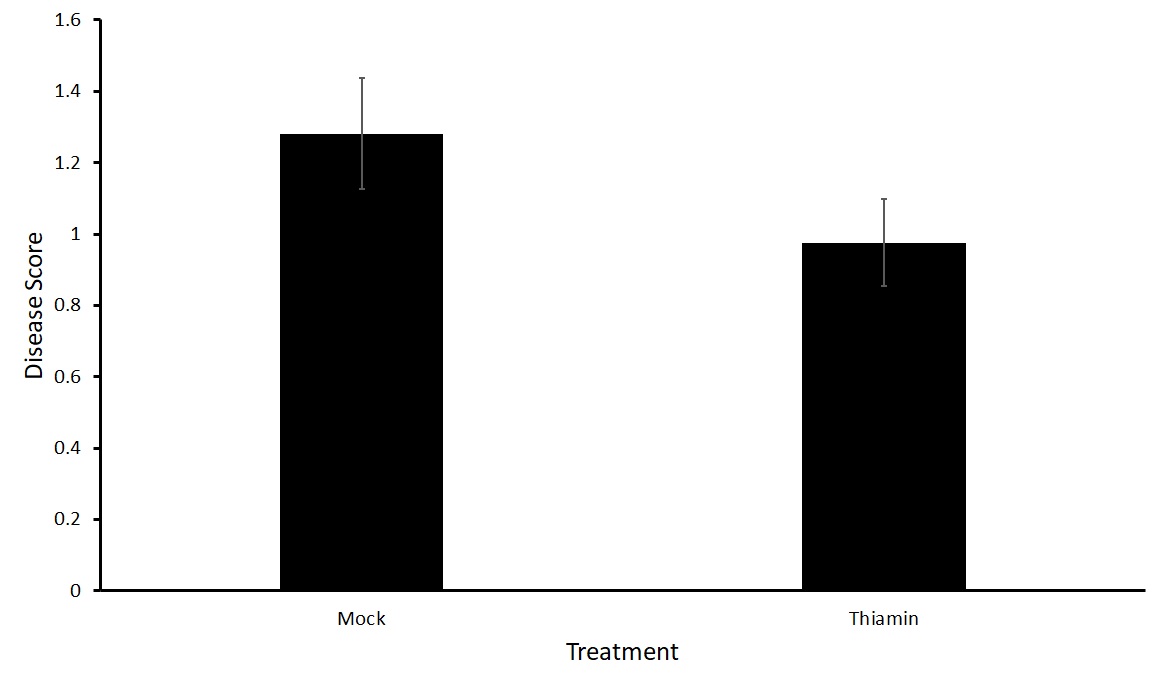
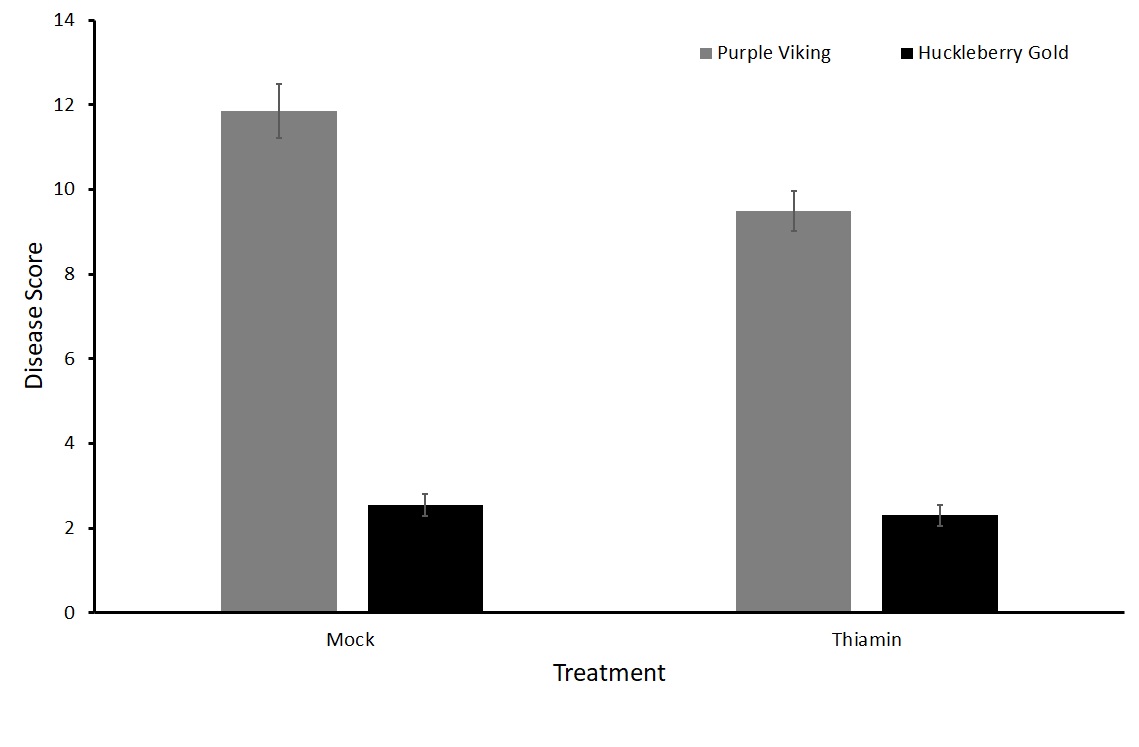
No statistical difference in the treatment groups with either Purple Viking or Huckleberry Gold potato varieties was observed in either the north or south plots (Figures 5 and 6). To verify these results, we decided to run tests in a greenhouse setting where the environment can be more closely controlled.
Sub-Objective #3: Evaluation of the effect of thiamin treatment on potato susceptibility to Streptomyces scabies-induced common scab in the greenhouse.
The effect of thiamin treatment on the severity and incidence of potato common scab was tested in a greenhouse setting by collaborators at the USDA-ARS. Plants were grown in pots of soil amended with Streptomyces bacteria to induce potato common scab symptoms. We tested two different bacterial strains, ME01-11h and ND05-01C.
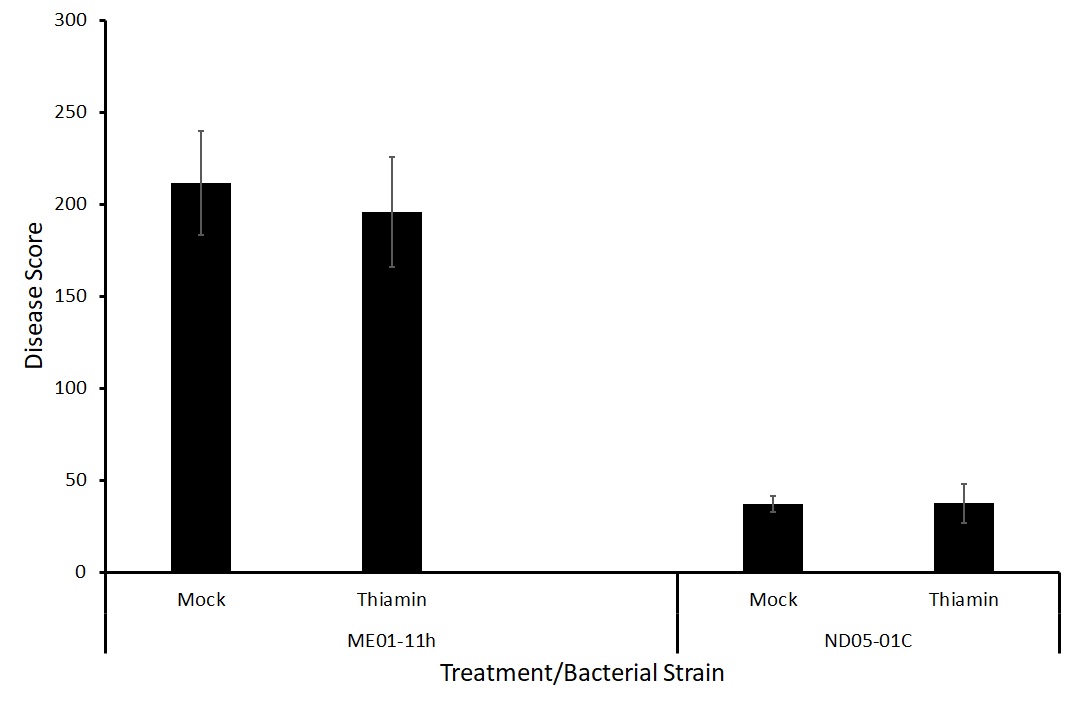
Because there was no difference between treatments with either strain of the bacteria (Figure 7), we decided to test two different forms of application, foliar spray and soil drenching. We hypothesize that treatment via soil drenching may be more effective at priming the potatoes against potato common scab as the treatment is reaching the site of pathogen challenge directly.
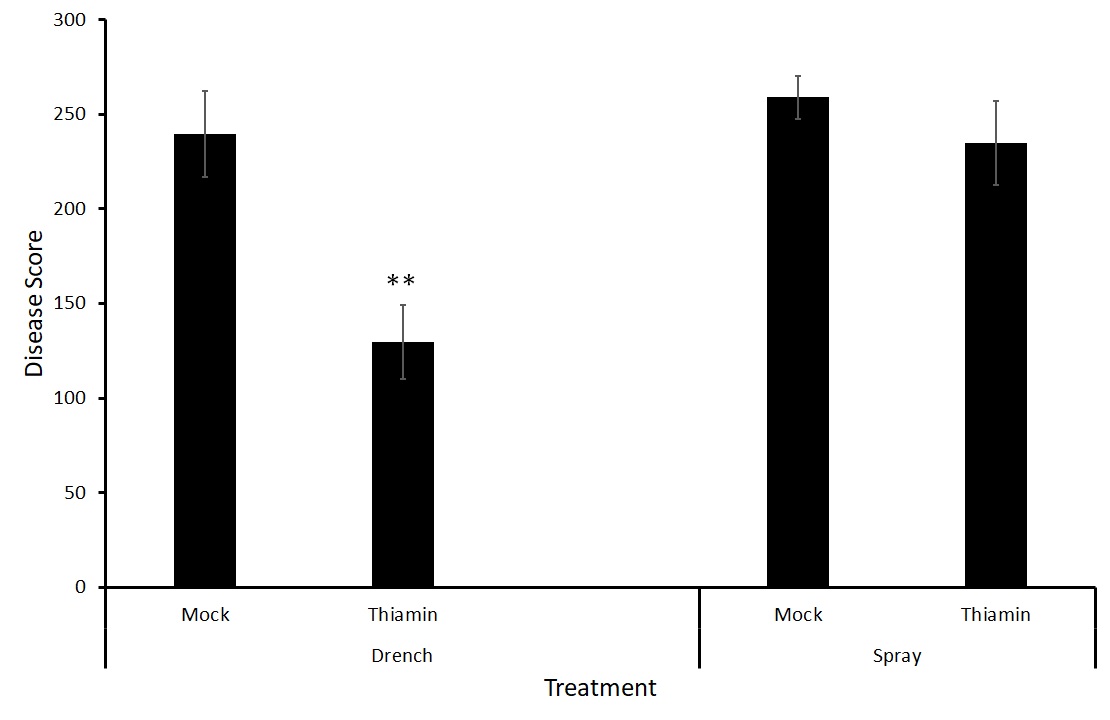
Interestingly, there was a significant difference between the mock and thiamin treatments when thiamin was applied via soil drenching but not when the treatment was applied to the foliage of the plants (Figure 8). Soil drenching thiamin treatments may be effective in controlling potato common scab caused by S. scabiei. This experiment is being repeated to ensure consistent results.
Objective #2: Test the effectiveness of thiamin priming treatments against a fungal pathogen in potato, Alternaria solani, the causal agent of potato early blight.
To test the effectiveness of thiamin priming in potato against fungal pathogens we used the potato variety Russet Norkotah grown in a greenhouse. Russet Norkotah is susceptible to early blight disease. The pathogen used for these tests was A. solani strain-Arizona 0813. We first decided to test a range of concentrations of thiamin from 1 to 50 mM to see if there is any reduction in lesion area of A. solani compared with a mock solution and challenged with the pathogen four hours after treatment.
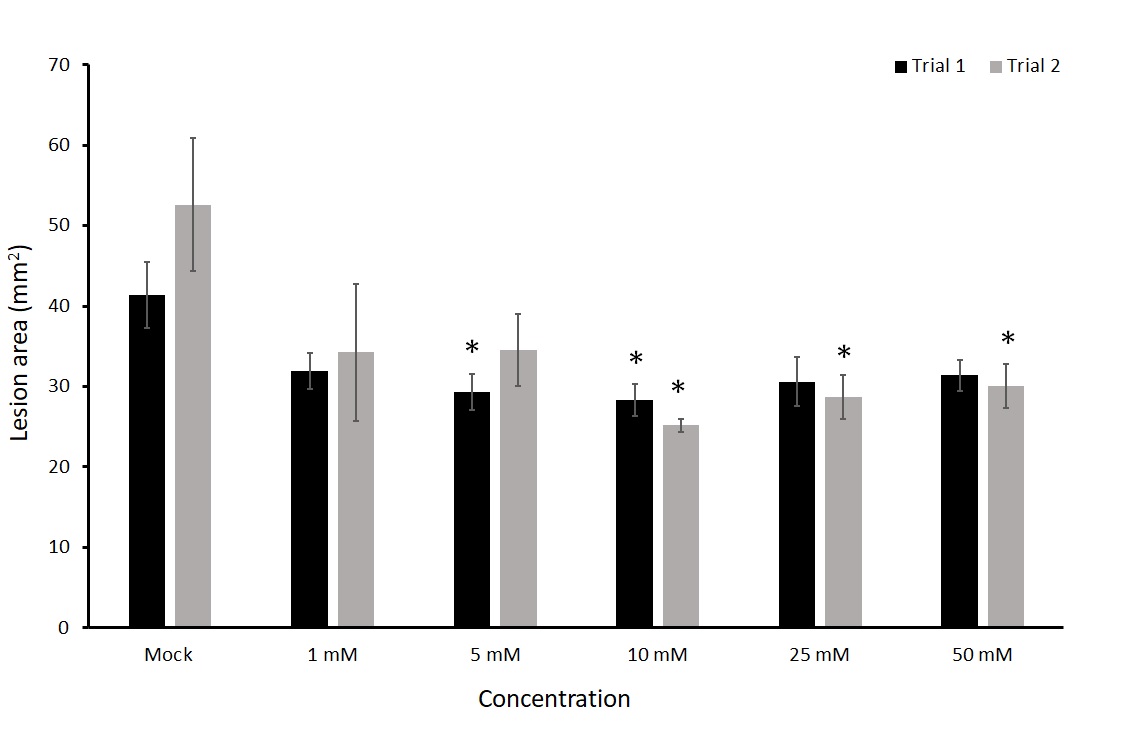
We tested the effect of thiamin as a foliar application at five different concentrations in detached leaf assays. This experiment was repeated twice. In both trials there was a significant decrease in early blight lesion area at 10 mM of thiamin when compared to a mock treatment (Figure 9). Concentrations of 5 mM, 25 mM, and 50 mM also showed significant differences, but only in one of the two trials. Mock treated plants had an average of 41.37 and 52.62 mm2 in each trial compared to 28.26 and 25.16 mm2 in plants treated with 10 mM thiamin. This equates to 32% and 52% decrease in the 10 mM treated leaves compared to mock treatments. The 10 mM treatment group was most consistent at exhibiting smaller lesion area and was therefore used as the concentration for further experiments. We then wanted to know for how long the treatment would be effective in reducing lesion size of A. solani.
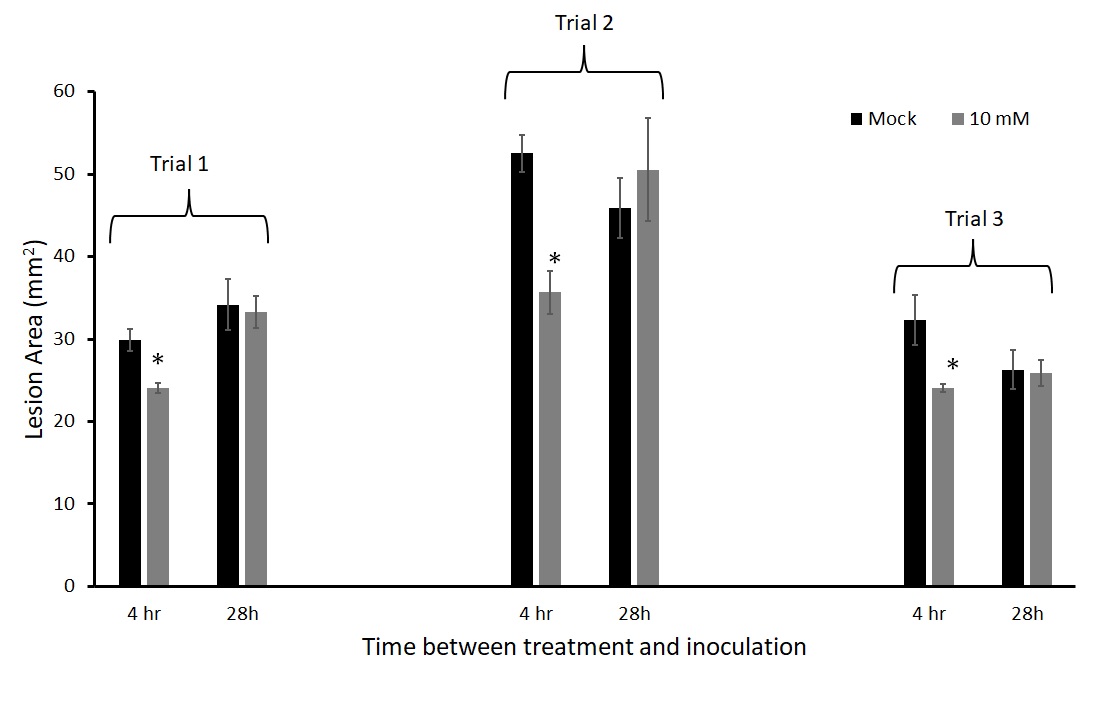
We inoculated leaves with A. solani 4 hours and 28 hours after thiamin application. This experiment was repeated three times. When the plants were treated and challenged with pathogen 28 hours after thiamin application, there was no protection with thiamin in all three trials (Figure 10), while there was consistent protection when leaves were inoculated 4 hours after thiamin treatment.
We then wanted to compare the effects of thiamin priming with another known plant immunity inducer, BTH, which was shown to be effective in reducing lesion incidence of A. solani in a study done by (Bokshi et al 2003).
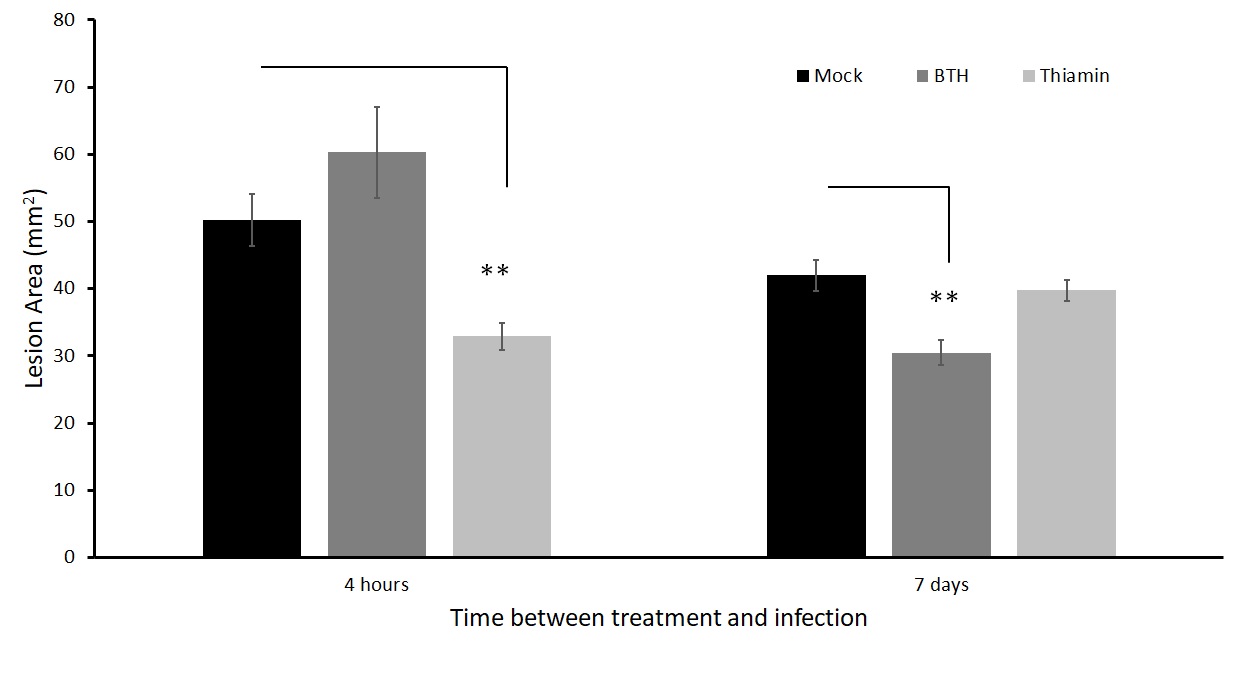
We inoculated leaves 4 hours and 7 days after treatment. There was a significant reduction in lesion size when leaves were inoculated with A. solani 4 hours after thiamin application, but not when they were inoculated 7 days after thiamin application (Figure 11). Conversely, there was no significant reduction in lesion size when leaves were inoculated with A. solani 4 hours after BTH application, while there was a significant reduction in lesion size when leaves were inoculated 7 days after BTH application (Figure 11). To ensure that thiamin priming treatments also work on whole plants (not just detached leaves), we set up an experiment where we infected the leaflets on the plants in a humidity chamber.
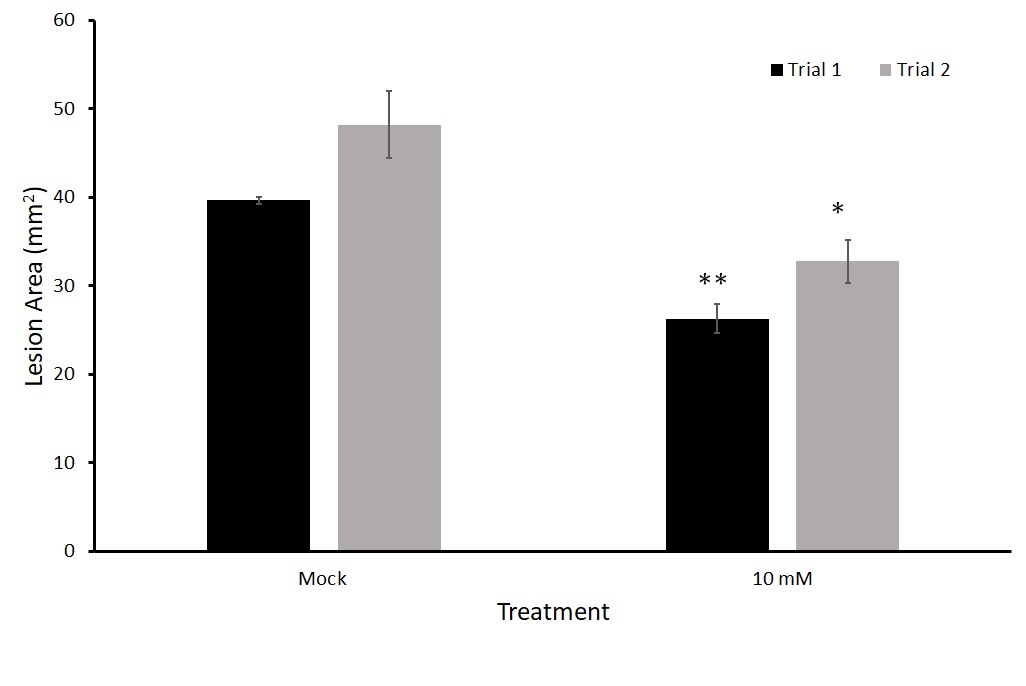
We tested this twice (Figure 12). In both trials, thiamin-treated-plants had a statistically significant lower average lesion area than those treated with a mock solution comparable to those found in the detached leaf trials.
Objective 3: Educate the agricultural industry on vitamin-induced priming through presentations at extension events, publications in extension and scientific journals, and through social media
Sub-objective #1: Present research results to producers/stakeholders and academic and industry scientists through presentations at scientific and extension meetings:
I have given two oral presentations and presented two posters at outreach events, the Potato Association of America’s annual meetings, Hermiston Agricultural Research and Extension Center (HAREC) Potato Field Day - an event attended by producers, scientists, and stakeholders, and the AgSci Career Fair and Student Showcase at Oregon State University.
Berrian T, Clarke C, Goyer A (2022) Testing thiamin as an immunity inducer against diseases of potato. 107th Annual Meeting of the Potato Association of America. Charlottetown, PEI, Canada. 23-27 July. Oral Presentation.
Berrian T, Goyer A (2022) Thiamin (vitamin B1) priming to control for potato common scab. Potato Field Day at the Oregon State University Hermiston Agricultural Research and Extension Center. 21 June. Hermiston, OR. Poster Presentation.
Berrian T, Clarke C, Goyer A (2022) Testing thiamin as an immunity inducer against common scab in potato. 106th Annual Meeting of the Potato Association of America. Missoula, MT. 17-21 July. Oral Presentation.
Flagg C, Berrian T, Goyer A (2022) Disease management via thiamin priming in potatoes. AgSci Career Fair and Student Showcase, Oregon State University. Corvallis, OR. 19 Oct. Poster Presentation.
Sub-objective #2: Publish papers/articles in scientific peer-reviewed journals, extension newsletters, and trade journals:
A paper was published in the peer-reviewed journal Phytopathology. https://apsjournals.apsnet.org/doi/10.1094/PHYTO-09-24-0277-R.
Sub-objective #3: Post research updates on social media and our lab webpage
Updates and research posts have been made on the twitter account (@berriansbotany) including a video of our field trial harvest. https://twitter.com/berriansbotany
More posts will also be added to the Goyer Lab website during this ongoing study.
Conclusions
- Foliar application of thiamin had no effect on the incidence and severity of potato common scab. However, we found a significant reduction of symptoms via drench application.
- Thiamin foliar application or direct soaking of tubers in a thiamin solution did not reduce the amount of rotted tissue caused by carotovorum subsp carotovorum present in tubers. However, we will repeat the tuber soaking experiment because there was a large variance in data.
- Foliar application of thiamin reduced lesion size caused by solani by ~33%, and the concentration of 10 mM was the most effective and consistent across repeated trials. This concentration was also effective at controlling early blight in whole plant assays.
- Thiamin priming was effective within the first few hours after treatment, i.e., 4 hours, after which its effectiveness faded away.
- Conversely, BTH, another known plant defense activator, reduced lesion area by ~27% when leaves were challenged 7 days after application, while it had no effect when leaves were inoculated 4 hours after treatment. These results suggest that applying a mixture of thiamin and BTH might have an additive effect on reducing lesion size.
Research outcomes
Our findings demonstrate that foliar applications of thiamin decrease the size of lesions caused by the necrotrophic fungal pathogen A. solani in potato. However, immune priming was limited both temporally and to locally infected tissue, thereby presenting a challenge to immediate use in managing early blight disease in potato. Future experiments could investigate pathways to enhance potato immune response of potato to thiamin, such as combinations of thiamin with other chemical elicitors of pathogen defense with different modes of action.
Our results also showed that soil drenching with thiamin may be effective in reducing symptoms of potato common scab caused by S. scabiei in greenhouse experiments. We are continuing to investigate this potential mode of management.
Education and Outreach
Participation summary:
Presentations, both oral and poster, were done to engage the grower and scientific communities. A poster presentation was done at the Potato Field Day in Hermiston, Oregon in 2022. Two oral presentations on this research projected were also done at the annual meetings of the Potato Association of America for years 2022 and 2023. Training of undergraduate students was done to educate the next generation of agriculture professionals. So far, three undergraduate students have had an opportunity to work hands-on with this project. One student presented a poster at the AgSci Career Fair and Student Showcase, disseminating some of the preliminary results of this research project. Posting on social media was done to engage and educate the online community. Several posts have been made on the twitter account @berriansbotany regarding this research project. More posts are planned for the future. Working with a producer in this project has also given us the opportunity to demonstrate techniques related to this project to the farm staff. Sub-objective 2 under Objective 3 of our proposal states we will publish papers/articles in scientific peer-reviewed journals, extension newsletters, and trade journals. Now that data has been collected, publications are in preparation. An extension video regarding this research is also in progress to extend our educational reach.
Up to date, several education events have been conducted including a presentation at the Potato Field Day 2022 at the Hermiston Agricultural Research and Extension Station. This event is typically attended by ~150 growers and industry representatives. This event was a good opportunity to engage the growers interest in our research project. Undergraduate students have been trained in agricultural research through helping with research objectives. Through the student's training, they have learned lab, greenhouse, and field techniques critical to conducting agricultural research. Presentations that were done for 2022 and 2023 at the annual Potato Association of America meetings were critical for feedback from other scientists as well as industry professionals. Engagement with the project was greater in 2023 than in 2022 at these meetings, showing that the communication has been successful. Moderate engagement with the posts made on @BerriansBotany twitter page were seen, although ways to increase engagement are being considered. A post was also made on the departmental website's Good News section regarding funding acquired from the Drs. James and Stella Melugin Coakley endowment award to assist with travel for this project. Citations for all presentations conducted so far can be found in the research section of this report.