Final report for GW23-256
Project Information
The quarantined pest of potato, Globodera pallida, the pale cyst nematode, was first found in Idaho in 2006. Since its’ discovery, the focus has been to contain and eradicate this economically devastating pest of potato. G. pallida can cause up to 80% potato yield loss on susceptible potato varieties, readily spreads in infested soil, and survives for 30+ years in soil. Eradication efforts have relied on soil fumigation. Since many nematicides have been banned due to environmental concerns, development of new sustainable methods for controlling this nematode are essential for success of the eradication program. One alternative eradication measure is the use of Solanum sisymbriifolium, a trap crop commonly called litchi tomato which induces hatch but limits reproduction of G. pallida. However, because S. sisymbriifolium has little economic value as a crop and seeds are largely unavailable, it has not been widely adopted for use by producers in Idaho. Previous research shows butanol extracts of S. sisymbriifolium, containing glycoalkaloids, reduce hatch and viability of G. pallida. The goal of this project is to develop a sustainable nematicide that targets G. pallida but does not affect beneficial nematodes. Further fractionation of the butanol extracts will be tested in field microplots to determine which chemicals are most toxic to G. pallida. Results will be presented to stakeholders through presentations at PAA, IAPP, as well as a published newsletter. Potential discovery of novel sustainable chemistries for nematicide development would be a valuable achievement for Idaho producers, or anyone dealing with G. pallida infestations.
Research Objectives:
- Fractionated 1-butanol extraction of Solanum sisymbriifolium stems and leaves and determine chemical composition and concentration.
- Determined effect of fractions on Globodera pallida hatch and viability in vitro.
- Determined effect of fractions on Globodera pallida reproduction and populations under Idaho field conditions
Education Objectives:
- Utilized professional meetings and conferences to present findings and progress to stakeholders on nematicide development to help eradicate Globodera pallida populations in infested fields.
- Evaluated stakeholder understanding through pre- and post-presentation surveys at professional meetings and conferences.
- Published a newsletter in Potato Growers magazine or Potato Country magazine to thoroughly explain in layman’s terms findings and progress of nematicide development to give stakeholders sustainable options for G. pallida eradication.
Cooperators
- - Producer
- (Researcher)
- - Technical Advisor
Research
Research Objectives:
- Fractionated 1-butanol extraction of Solanum sisymbriifolium stems and leaves and determine chemical composition and concentration.
- Determined effect of fractions on Globodera pallida hatch and viability in vitro.
- Determined effect of fractions on Globodera pallida reproduction and populations under Idaho field conditions.
Objective 1: Fractionated 1-butanol extraction of Solanum sisymbriifolium stems and leaves and determine chemical composition and concentration. High-performance liquid chromatography (HPLC) was used to fractionate the 1-butanol extract of S. sisymbriifolium stems and leaves into four different fractions. Liquid chromatograph and mass spectrometry (LC/MS) was then used to determine the concentration of chemicals in the fractions, as well as the composition.
Objective 2: Determined effect of fractions on Globodera pallida hatch and viability in vitro. To determine which fraction has the toxin that reduces hatch and viability of G. pallida in vitro assays must be accomplished. Following is the methods proposed for this objective.
Effect of fractions on hatch of G. pallida. Cysts were surface sterilized with 0.3% hypochlorous bleach for five minutes then washed in sterile DI water five times. Cysts were then exposed to the fractions and potato root diffusate (PRD) for 7 days at 20°C, one control will be exposed to bare soil diffusate with no potato root diffusate. Another control was exposed to potato root diffusate for the same time and temperature. After one-week cysts were washed in sterile DI water five times. Clean cysts were transferred to fresh 6-week-old Russet Burbank PRD only in 96 well plates and hatched in an incubator at 20°C for three weeks. Hatch was determined by counting the number of second-stage juveniles (J2s) that have emerged using a model no. DMi1 stereomicroscope (Leica Microsystems, Wetzlar, Germany). Eight replicates with individual cysts were used for each treatment and the experiment will be repeated. After J2s are counted cysts will be crushed to count the remaining viable eggs. The equation to calculate hatch percentage is as follows:
Hatch Percentage = Number of J2s hatched/(Number of J2s hatched + Number of viable eggs after cysts are crushed) x 100
Percent Inhibition = ((PRD control % - Treatment %)/PRD control %) x 100
The effect of fractions on viability of G. pallida. Viability will be assessed with an acridine orange staining method (Lejambree et al., 1970; Pillai & Dandurand, 2019). Cysts will be surface sterilized. Eight replicates with individual cysts will be used for each treatment and the experiment will be repeated. The cysts will then be placed individually into a 96 well plate. Cysts will be exposed to the fractions and potato root diffusate for 7 days at 20°C, the control will be exposed to potato root diffusate with no fractions for the same time and temperature. The cysts will be placed into acridine orange staining solution then rinsed (Pillai & Dandurand, 2019). The eggs that fluoresce are not viable and will be counted using a Leica DMi8 fluorescent microscope (Leica microsystems CMS GmbH, Wetzlar, Germany). Then a model no. DMi1 stereomicroscope (Leica Microsystems, Wetzlar, Germany) will be used to count total eggs with J2s (Pillai & Dandurand, 2019). The equation to calculate viability is as follows:
% egg viability = (non-stained eggs/stained eggs + non-stained eggs) x 100
Root diffusate to stimulate hatch. Previous research has demonstrated that glycoalkaloids do not penetrate the egg wall unless a hatching stimulus from potato root diffusate is present (Sivasankara Pillai & Dandurand, 2021). Briefly, root diffusate to obtain this hatching stimulus are obtained as follows. Potato plants cv. Russet Burbank are clonally propagated in sterile tissue culture conditions for 4 weeks, then transferred into 6-inch pots filled with sterile sand and sandy loam soil (3:1) mix growth under greenhouse conditions (18°C±2°C day-time, 14°C±2°C night-time, 16:8 h light:dark period). Diffusate is obtained by soil percolation 6 weeks later by running 200mL of sterile DI water through the soil and collecting the effluent. The liquid collected is then filtered through an 0.45 µm filter (Corning, 430627) and then a 0.22 µm filter (Corning, 430626). The diffusate is stored at -20°C until needed for up to 6 months. Bare soil diffusate (negative control) is made by filling 6-inch pots with sterile sand and sandy loam soil (3:1) mix and allowing them to sit under greenhouse conditions. They are watered along with the other pots. Diffusate is collected by soil percolation 6 weeks later by running 200mL of sterile DI water through the soil and collecting the effluent. This liquid is then filtered and stored as described above.
Objective 3: Determined effect of fractions on Globodera pallida reproduction and populations under Idaho field conditions. The field trials were conducted in an established field research site in Shelley Idaho. Further infestation of G. pallida in the field site is avoided by following established containment protocols (Dandurand et al., 2019; Dandurand et al., 2017). Infestation of microplots occured with mesh cyst bags attached to stakes at a starting infestation rate of 5 eggs per gram of soil. The microplots consisted of susceptible potato variety ‘Russet Burbank’. Each plant had 4 replicate microplots that are exposed to the butanol extract. No extract was the negative control. In total there was 12 microplots. Setup was a randomized complete block design. The potato plants were clonally propagated and grown in media for four weeks then transferred to soil for four weeks then transplanted into the field microplots. There were 6 plants per microplot. Microplots had 3 ft spacing in the field and be embedded into the soil. Planting took place in May and was grown for 12 weeks receiving water as needed and fertilizer biweekly during field maintenance. Field maintenance occurred every two weeks during which microplots were weeded and water in the lower bucket was filtered out. After 12 weeks, plants were terminated and microplots were sealed with lids and brought back to the University of Idaho cold storage. Cysts in the remaining cysts bags were evaluated using hatch and viability assays as described above. Soil and roots were dried for G. pallida cyst extraction in the elutriator. Extracted cysts and number of eggs within cysts were counted to calculate the reproduction factor. The experiment will be repeated. Data analysis for all three objectives was analyzed using analysis of variance and least squares means separations in RStudio. Based on these results the pure chemicals found in this extract can be further tested as a sustainable nematicide.
References
Research Results Final:
Final Project Report: Research Results and Discussion:
Objective 1: Fractionate 1-butanol extraction of Solanum sisymbriifolium stems and leaves and determine chemical composition and concentration.
Extracts from 8-week-old Solanum sisymbriifolium were made using solvents of increasing polarity including: hexane, dichloromethane, ethyl acetate, and 1-butanol. These were tested for the glycoalkaloid solamargine and concentration was determined (Fig. 1). As figure 1 shows, the glycoalkaloid solamargine is found in the highest concentrations in the stems and leaves of plants extracted with 1-butanol at 4mg/L. The second highest concentration is contained in stem/leaf material extracted with dichloromethane at 1.25mg/L.
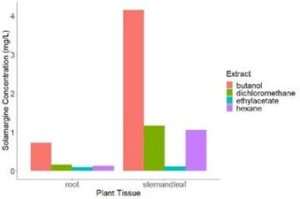
Figure 1. Solamargine concentration in extracts of 8-week-old Solanum sisymbriifolium plant tissues (root or stem and leaf).
Objective 2: Determine effect of fractions on Globodera pallida hatch and viability in vitro.
For the extract hatch assays, the most toxic extract was found to be from the stem/leaf tissue extracted with 1-butanol. The stem/leaf 1-butanol extract was then fractionated using high pressure liquid chromatography. Extracts were collected for one minute each on a methanol-water gradient. Hatch and viability assays were conducted on the 55 collected fractions.
Fifty-five fractions were tested via in vitro hatch assays. Fraction 47 was the only fraction that was significantly toxic to G. pallida. Fraction 47 reduced hatch by 59% compared to the potato root diffusate control (Fig. 2). No other fractions significantly reduced hatch.
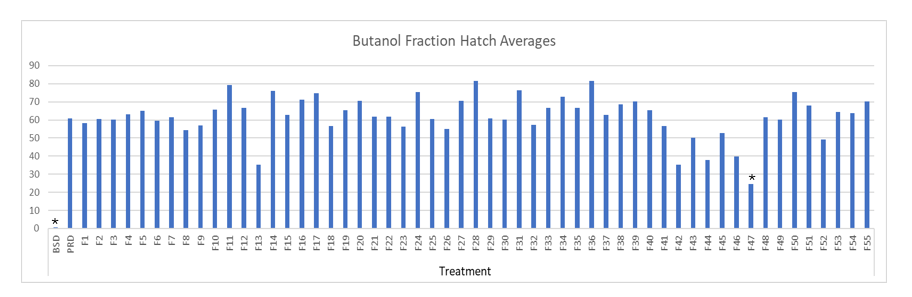
Figure 2: Globodera pallida hatch assay with 1-butanol S. sisybriifolium stem/leaf fractions as treatments (1-55). Data is hatch percentage. BSD = Bare Soil Control; PRD = Potato Root Diffusate Control. The stars represent significance levels, with no significant difference at level P > 0.05 for treatments with no star (N = 16). N represents the number of replications used for statistical analysis.
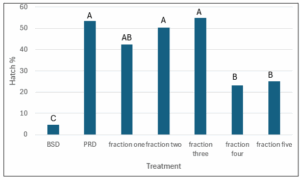
The hatch assays for the 55 fractions (figure 2) were not reproducible due to small concentrations of chemicals producing inconsistent results, so the number of fractions was reduced to five fractions from the butanol extract to better see the toxic effect. This was also done using high pressure liquid chromatography using a methanol gradient from 0% to 100% methanol. The five fractions represent all the chemicals found in the 1-butanol extract but may be not as separated as in the 55 fraction tests. Figure 3 shows the results of the five fractions on hatch of G. pallida. Fractions 4 and 5 significantly reduce hatch of G. pallida compared to the potato root diffusate control (figure 3). It is speculated that fraction four and five would have the highest concentration on glycoalkaloids based on the polarity (Dinan et al., 2001; Sánchez-Maldonado et al., 2014). This shows that there are significant toxic effects in the fourth and fifth fractions of the 1-butanol extract on G. pallida.
Figure 3: Globodera pallida hatch assay with 1-butanol S. sisybriifolium stem/leaf fractions as treatments (1-5). Data is hatch percentage. BSD = Bare Soil Control; PRD = Potato Root Diffusate Control. The letter represent significance levels, with no significant difference at level P > 0.05 for treatments with same letters (N = 16). N represents the number of replications for statistical analysis.
Objective 3: Determine effect of Solanum sisymbriifolium on Globodera pallida reproduction and populations under Idaho field conditions.
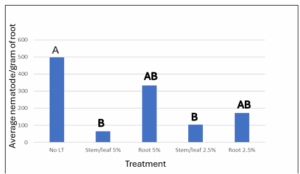
The effect of Solanum sisymbriifolium plant material on Globodera pallida has been very promising for control of G. pallida, producers are very interested in soil amendments with S. sisymbriifolium, and to satisfy these objectives for producers a Solanum sisymbriifolium dry plant material amendment was tested under greenhouse conditions. Two rounds of greenhouse trials were conducted using soil amendments of 0%, 2.5%, and 5% with both the stem/leaf material and the root material of S. sisymbriifolium. These were grown in 4-inch pots with the potato cultivar ‘Russet Burbank’ for 6 weeks then infection was analyzed through acid fuchsin staining. The average nematode per gram of root is shown in figure 4. The stem/leaf amendment at 5% of the soil volume had the most significant effect on infection with 87% reduction in root infection compared to the control (figure 4). The stem/leaf material at 2.5% amendment reduced infection by 79% (figure 4). Root material also had a significant effect on G. pallida infection. This experiment was repeated in Idaho field conditions in 2024.
Figure 4: Greenhouse experiment showing average G. pallida nematode per gram of root material 6 weeks post plant and amended with S. sisymbriifolium material with the control having no material added. (N=12; p<0.05).
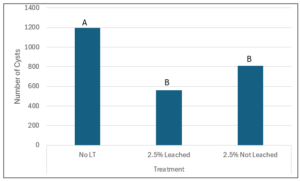
For the field experiment in Idaho field conditions there was not enough material to do a 5% by weight S. sisymbriifolium amendment treatment due to the large amount of soil in the 5-gallon microplots being used. Therefore, the treatments were 2.5% S. sisymbriifolium material by soil weight either leached or not leached of the toxic chemicals. Only stem/leaf S. sisymbriifolium material was used, as there was not a lot of evidence in the extracts, or greenhouse trials to warrant use of root material. Leached treatments were made by leaching chemicals from the plant material using 100% methanol then dried. Figure 5 shows the number of cysts per treatment when grown with potato cultivar ‘Russet Burbank.’ The treatments were not significantly different from each other but both treatments significantly reduced the number of cysts produced compared to the control (figure 5). This is very similar to what was shown in the greenhouse experiments. It is known that adding organic matter to soil helps reduce plant parasitic nematode populations, therefore it is hypothesized that the lignin or some other additive from the plant material is causing the reduction in nematodes, which was also seen in the greenhouse experiments and is seen in other plant parasitic nematode studies (Widmer et al., 2002).
Figure 5: Field reproduction experiment showing number of cysts reproduced on potato cultivar ‘Russet Burbank’ with treatments being 2.5% Solanum sisymbriifolium material by weight both leached of chemicals with methanol and without leaching of chemicals. The control is no Solanum sisymbriifolium material added.
Grower Recommendations:
This research shows the many possibilities of using the trap crop Solanum sisymbriifolium to manage the pale cyst nematode Globodera pallida. Based on this research producers can use organic matter at a rate of no less than 2.5% by weight of soil to reduce G. pallida populations. Additionally, there are exciting novel chemistries that are being discovered in this plant to develop new nematicides for producers to use. Glycoalkaloids are just one of the classes of chemicals that could potentially be used for G. pallida control.
References:
Dinan, L., Harmatha, J., and Lafont, R. 2001. Chromatographic procedures for the isolation of plant steroids. Journal of Chromatography A., 935(1-2):105-123.
Sánchez-Maldonado, A. F., Mudge, E., Gänzle, M. G., and Schieber, A. 2014. Extraction and fractionation of phenolic acids and glycoalkaloids from potato peels using acidified water/ethanol-based solvents. Food Research International, 65:27-34.
Widmer, T.L., Mitkowski, N.A., and Abawi, G.S. 2002. Soil organic matter and management of plant-parasitic nematodes. Journal of Nematology, 34(4), 289.
Research outcomes
The use of Solanum sisymbriifolium as a control method for Globodera pallida is incredibly important to sustainable agriculture. The use of nematicides to control G. pallida have been harmful to the environment. A new environmentally friendly way to control this economically devastating pest is needed. Here we show that soil amendments of S. sisymbriifolium have the ability to reduce infection of G. pallida by 87% compared to the controls. The use of organic soil amendments will provide a sustainable alternative for the control of G. pallida. It is recommended for farmers with G. pallida infestations to use dried S. sisymbriifolium stems/leaves as a soil amendment. This research shows the many possibilities of using the trap crop Solanum sisymbriifolium to manage the pale cyst nematode Globodera pallida. Based on this research producers can use organic matter at a rate of no less than 2.5% by weight of soil to reduce G. pallida populations. Additionally, there are exciting novel chemistries that are being discovered in this plant to develop new nematicides for producers to use. Glycoalkaloids are just one of the classes of chemicals that could potentially be used for G. pallida control.
Future research should focus on the chemical composition of the 1-butanol fractions. Additionally, the use of this material in alginate beads to deliver the toxin to the soil would be useful. Additionally, more research on soil amendments to determine the lowest level of amendment to add for reduced damage by plant parasitic nematodes is crucial.
Education and Outreach
Participation summary:
Objective 1: Utilize professional meetings and conferences to present findings and progress to stakeholders on nematicide development to help eradicate Globodera pallida populations in infested fields.
Presented research findings at Society of Nematologist Conference July 2023 and July 2024.
Objective 2: Evaluate stakeholder understanding through pre- and post-presentation surveys at professional meetings and conferences.
Presented research findings at Idaho Association of Plant Protection November 1, 2023 and November 2024. Stakeholders present included producers and researchers.
Objective 3: Publish a newsletter in Potato Growers magazine or Potato Country magazine to thoroughly explain in layman’s terms findings and progress of nematicide development to give stakeholders sustainable options for G. pallida eradication.
News article published on potatonematodes.org for stakeholder understanding of G. pallida.
Objective 1: Utilize professional meetings and conferences to present findings and progress to stakeholders on nematicide development to help eradicate Globodera pallida populations in infested fields.
Presented research findings at Society of Nematologist Conference July 2023 and 2024. This was a well attended professional conference with mostly other researchers.
Objective 2: Evaluate stakeholder understanding through pre- and post-presentation surveys at professional meetings and conferences.
Presented research findings at Idaho Association of Plant Protection November 1, 2023 and November 2024. Stakeholders present included producers and researchers. Producers reported learning more about G. pallida in Idaho and control methods for G. pallida in Idaho. WSARE surveys were handed out.
Objective 3: Publish a newsletter in Potato Growers magazine or Potato Country magazine to thoroughly explain in layman’s terms findings and progress of nematicide development to give stakeholders sustainable options for G. pallida eradication.
News article published on potatonematodes.org for stakeholder understanding of G. pallida biology.