Progress report for LNC21-455
Project Information
To improve soil health in the North Central Region, a growing number of grass-fed beef producers are finishing cattle on plant-species diverse forage and/or cover crops. While beef provides many essential nutrients in the American diet, pilot data from our research team indicates that when livestock are raised and finished on plant-diverse pastures, additional health-promoting phytonutrients—terpenoids, phenols, carotenoids, and other anti-oxidants—concentrate in beef. While linkages among the plant-animal-human health continuum (“healthy plants, healthy animals, healthy humans”) are often touted as the reason why grass-fed beef may have additional health benefits, no studies have systematically assessed this.
Using novel metabolomics approaches, the goal of our work is to systematically detail the transfer of >500 biochemicals and phytochemicals from the forage/feed consumed by pasture-raised and feedlot-fed animals, to their beef, and into the body of consumers and determine metabolic health biomarkers in response to consumption.
Species composition of diets’ of cattle (grass-fed vs. grain-fed) will be characterized using DNA metabarcoding of cattle fecal samples, while phytochemical richness of forage and total mixed ration samples will be determined using metabolomics (Objective 1). Second, we will compare the presence of phytochemicals and biochemicals in both types of beef (Objective 2). To provide insight into consumer health (plasma metabolomes, inflammation, and gut microbiota communities), we will then perform a randomized controlled trial and obtain blood and stool samples from participants before and after three weeks of daily consumption of grass-fed vs grain-fed beef as part of identical diets (Objective 3). Our central hypothesis: consuming grass-fed beef results in inflammatory/metabolic signatures in the plasma and gut microbiome of indicative of improved metabolic health.
By overlaying the feed/forage metabolome (Objective 1), with the beef metabolome (Objective 2), with the consumer gut microbiome and plasma metabolome (Objective 3), our systems approach will provide an initial direct link between plant diversity, nutrient-density of beef, and human health.
Cooperating farmers will assist with sample collection, providing knowledge on grazed plants, and outreach. Findings will be shared with producers, consumers, and stakeholders in the North Central region and the US via workshops, tradeshows, articles in traditional and social media, and scientific publications. Outreach partners include the ARS-Northern Great Plains Extension program, grazing coalitions (South Dakota/North Dakota), and stakeholder groups including Understanding Ag, Thousand Hills Lifetime Grazed, Wisconsin Grass-fed beef Coop, and Carbon Cowboys. Project findings have potential for North Central grass-fed producers to improve economic viability while potentially enhancing consumer health.
Project Objectives:
(a) Determine if eating grass-fed beef from cattle that ate a plant species-diverse diet elicits more healthful inflammatory and metabolite profiles in adult people than eating grain-fed beef.
(b) Translate this information back to producers and consumers by partnering with producer coalitions and stakeholder groups.
Learning Outcomes:
Farmers will learn how finishing cattle on biodiverse forage influences the phytochemical richness of beef.
Consumers will learn if consuming such grass-fed beef impacts their metabolic health.
Action Outcomes:
Farmers can adopt biodiverse pastures and cover crops to increase healthfulness of their beef.
Consumers encouraged to seek out local grass-fed beef.
Beef is a popular food and provides many essential nutrients in the American diet. Grass-fed beef is growing in popularity as a type of beef consumed and pilot data from our research team indicates that when cattle are raised and finished on plant-diverse pastures, additional health-promoting phytonutrients—terpenoids, phenols, carotenoids, and other anti-oxidants—concentrate in beef. While linkages among the plant-animal-human health continuum (“healthy plants, healthy animals, healthy humans”) are often touted as the reason why grass-fed beef may have additional health benefits, no studies have systematically assessed this. Therefore, we will conduct fieldwork and a human feeding trial to study potential connections among plants and compounds in plants, cattle and human health. Then use a variety of educational and outreach efforts to share our findings will all interested people.
Cooperators
Research
Grass-fed beef produced on pastures with more diversity of plant species and plant parts available and consumed by cattle will have greater diversity of chemicals in this beef and will provide health benefits to consumers compared to conventional beef produced in feedlots with a high dietary level of concentrate feeds and less diversity of plant materials consumed by cattle.
Objective 1: Plant-species Diversity Characterization and Forage Metabolomics
Forage collection
Pastures on each cooperating farm (Brown’s Ranch, ND; Maier’s Farm, MN; Willow Creek Farm (Ofte), WI) will be sampled every 30 days during the last 3 months prior to slaughter. These efforts, led by Dr. Kronberg, will be performed in close collaboration with the farmers as they will know which fields have been grazed and need sampling. Plant samples will be collected in conical tubes, freeze-dried, powdered, and stored at -80°C for metabolomics analysis. Feedlot TMR is provided by Demkota (Aberdeen, SD). All samples (rations and forages) will be submitted for nutritional composition analysis by USDA-ARS, while phytochemical composition will be determined using metabolomics analysis as described in the Metabolomics subsection.
Fecal collection
At the same time as plant samples are being collected, the farmers and research team will collect fresh fecal samples (150 gram w:w) from cattle for DNA (fDNA) metabarcoding to quantify plant-species composition (%) of cattle’s diets (Scasta et al., 2019). Briefly, DNA will be extracted from fecal samples and amplified into amplicons using pairs of primers. Amplicons are sequenced and data will be bioinformatically processed to identify Operational Taxonomic Units (OTUs). OTUs will be referenced against a database to quantify plant species composition of cattle diets. Analyses will be performed by Jonah Ventures (https://jonahventures.com/plants/).
Climate Data collection
Records of weekly temperature and precipitation will be downloaded for an entire year from the Summer of 2021 to the Summer of 2022, which is the time that animals will be slaughtered.
Objective 2: Meat Biochemical Richness Characterization
Meat Sampling
Whole briskets will be collected from 10 cattle, slaughtered during the summer of 2022, from all three cooperative farmers and the feedlot and under the coordination of Dr. Kronberg (40 total; 10 from each farmer/feedlot). Brisket was chosen due to feasibility of receiving these from individual animals. Based on previous work it is expected that 46% of probed metabolites (167/353) are different (P≤ 0.05) between grass-fed and grain-fed beef (Carrillo et al., 2016); when we assume a P-value ≤ 0.05, Q-value ≤ 0.3, an N=10 cattle per farm is sufficient to provide true discovery rates ranging from 96-99 % assuming a fold difference of 1.1-1.25, respectively. The pasture-based farmers and the feedlot all raise/finish Black Angus breeds.
Meat Analysis
Beef samples will be analyzed for proximate composition and macronutrient evaluation (Microbac Laboratories, Warrendale, PA). Beef samples will be evaluated for biochemical richness—vitamin and mineral derivatives, fatty acids, organic acids, antioxidants, phytochemicals, xenobiotics, and other bioactive compounds—via nontargeted metabolomics (see Metabolomics subsection below for methods description). The forage and beef metabolome data will be overlaid with the plasma metabolome of participants in the human intervention trials to directly establish the impact of biochemical richness of the meat on the human metabolome (Figure 4). 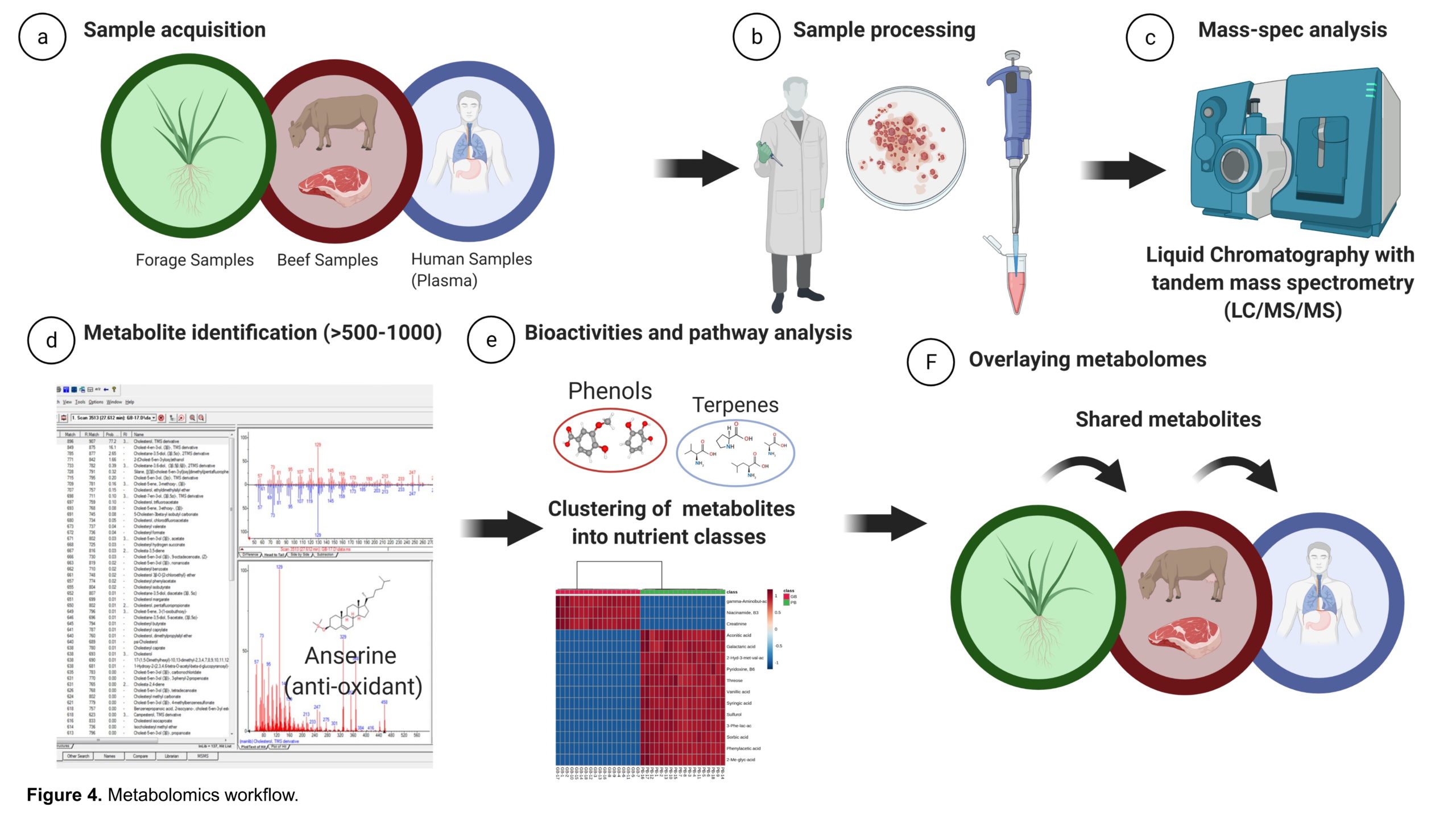
Objective 3: Human Intervention Trials
Participants
Twenty (N=20) middle-aged adults (35-65 years) off all races and ethnicities who are overweight (Body Mass Index [BMI] ≥25 and <35 kg/m2) and without chronic disease will be recruited to participate. The selected age and BMI range represents a population at increased risk for low-grade systemic inflammation and future development of chronic disease. They will benefit most from risk-reducing dietary interventions. Before entering the study, all volunteers will provide written informed consent. They will be screened carefully for medical-, dietary-, and physical activity history and will undergo blood tests to determine eligibility using the following inclusion and exclusion criteria.
Inclusion criteria: • Non-diabetic (fasting blood glucose <126 mg/dl; Hb1Ac ≤5.7%) • Weight stable (±6 lbs in last 3 months) • Consistent physical activity levels (but <150 min of structured exercise per week) • Stable medication use for 3 months prior to study.
Exclusion criteria: • Congestive heart failure, cancer, chronic obstructive pulmonary disease, thyroid disease, and/or other metabolic disorders that influence metabolism • Use of tobacco products, dietary supplements, and/or medications (e.g., NSAIDs, corticosteroids etc.) that can affect inflammation • Use of antibiotics 90 days prior to study entry • Consumption of >25 g of alcohol per day • Inflammatory diseases (e.g., autoimmune diseases, coeliac disease, hepatitis, inflammatory bowel disease, etc.) • Strict dietary patterns (e.g., vegan, keto, etc.) • Self-reported sleep <5 h per night • Pregnant or lactating women.
Study Design
We will use a randomized, double-blind, cross-over design. Subjects will consume each source of beef (grass-fed vs grain-fed) as part of a controlled diet for 21-days with a 7-day washout period. The interventional diets will be identical in all other foods (fruits, vegetables, grains etc.) and differ only in the source of beef consumed (grass-fed vs grain-fed). Blood samples will be taken in the morning after an overnight fast on days 1 and 21 of both intervention periods. Upon collection, blood samples will be processed immediately for plasma isolation. First-of-the-day stool samples will be collected on days 1 and 21 of each intervention period using validated stool collection kits (Omni-gut 200, DNA Genotek, Ottawa, CA). Plasma and stool samples will be stored at − 80 °C until further analysis (see Analysis subsection).
On day 1 off each intervention phase, we will determine the acute meal response (“acute meal challenge”) to grass-fed vs grain-fed beef. Based on previous work (van Vliet et al., 2017;Pimentel et al., 2018), we expect to see biochemicals from the beef appear in the blood within hours. The acute meal study will inform the identification of the metabolites and metabolic pathways that are most likely to change after prolonged, 21-day intake. Participants will report to the lab at the Stedman Center for Nutrition and Metabolism at Duke University after a 12h overnight fast. After fasted blood sample collection, participants will ingest a single 10 oz (224 g) dose of ground beef (brisket). For the next 20 days, participants will be instructed to consume 10 oz of ground beef per day (spread out over lunch and dinner) from their treatment (grass-fed vs grain-fed). The study flow is provided in Figure 5.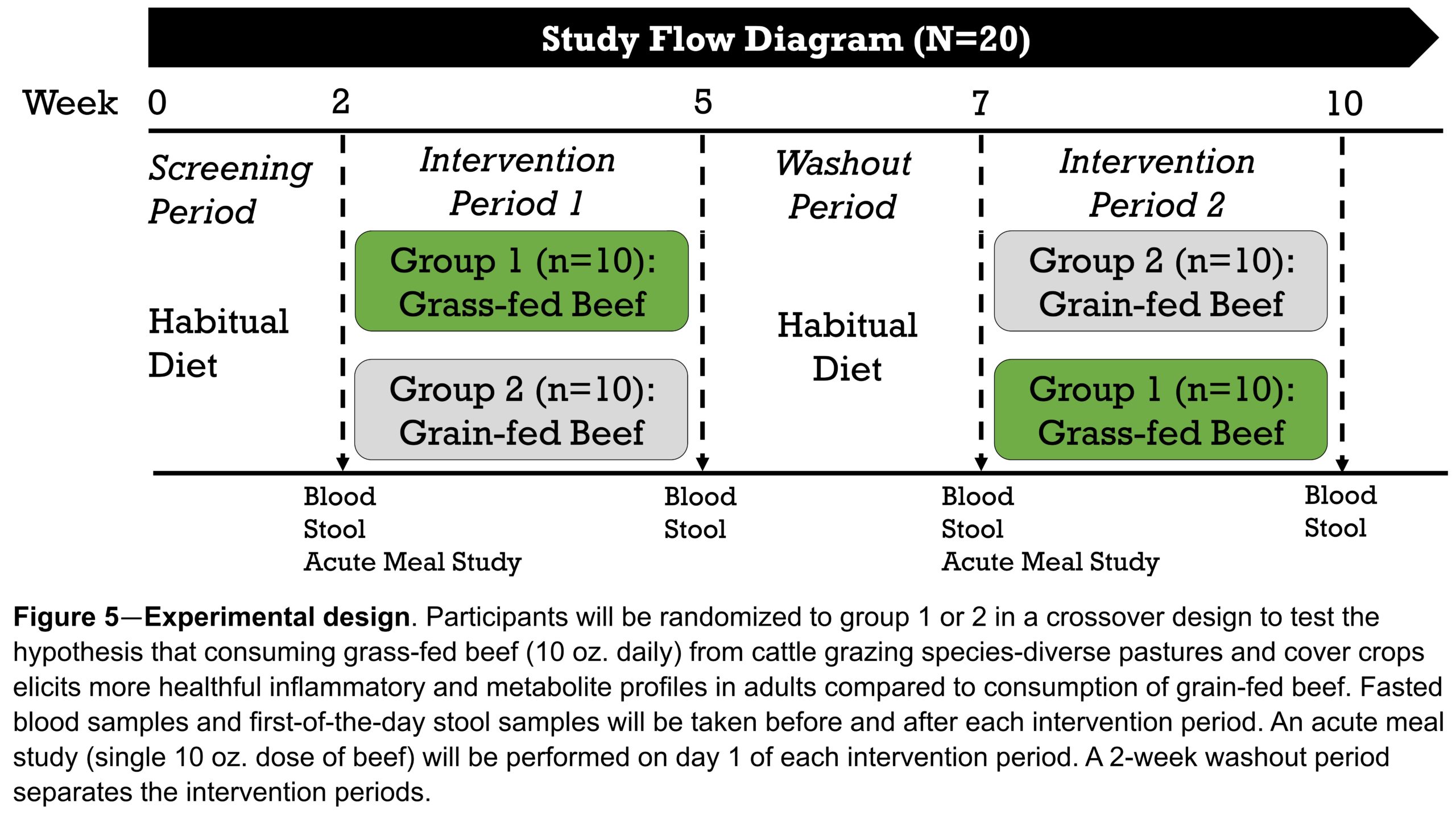
Design Rationale
A 21-day intervention period is adequate to observe dietary-induced changes in plasma metabolome and inflammatory profiles(Telle-Hansen et al., 2017;Pimentel et al., 2018), and the gut microbiota of participants (Johnson et al., 2019).. Plasma metabolites and inflammatory markers can change after a single meal (Manning et al., 2008;Pimentel et al., 2018), but health effects cannot be inferred from such acute work. Dietary-induced changes in the gut microbiome have already been observed after several days (Johnson et al., 2019). A 14-day washout period is adequate for all plasma and gut microbiota markers to return to baseline values (Telle-Hansen et al., 2017;Pimentel et al., 2018;Johnson et al., 2019).
Dietary and Activity Control
Participants will be asked to complete a 5-day food record prior to the start of the study to provide insight into their habitual diet. In accordance with established protocols (Porter Starr et al., 2016), our Registered Dietitian will provide individualized meal plans with a macronutrient distribution of 20% protein, 30% fat, and 50% carbohydrate based on each individual’s daily energy requirement (1.4-2.0 [depending on physical activity levels] x measured resting energy expenditure). The prescribed meal plans will adhere to healthy eating patterns recommended by the Dietary Guidelines for Americans. Participants will fill out daily food logs and provide these to the research dietitian on a bi-weekly basis to promote compliance to their meal plans (defined as >90% of prescribed food consumed).
We will ask participants to discontinue the use of nutritional supplements, nonprescription medication, and alcohol for 14 days prior to and throughout the intervention and washout periods. Participants will be instructed to maintain habitual physical activity levels, body, weight, sleep patterns, which we will monitor in real-time using activity monitors and scales (Garmin International Inc, Olathe, KS). No blood draws will occur when participants report changes in health on any of the test-days. Instead, participants will be kept on the diet several days longer, if needed, to ensure conditions of testing are similar.
Analysis
Inflammatory Markers and Blood Work
Routine bloodwork: fasting insulin, glucose, blood urea nitrogen, carbon dioxide, chloride, creatinine, estimated glomerular filtration rate, cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and very low-density lipoprotein (VLDL) cholesterol will be determined by enzymatic methods (LabCorp Inc). Concentrations of plasma inflammatory biomarkers—interleukin-6, tumor necrosis factor-α, and c-reactive protein—will be measured by multiplex electro-chemiluminescence according to the manufacturer’s instructions (V-PLEX Human Biomarker Kits, Meso Scale Discovery) at the Duke Molecular Physiology Institute. Analysis will occur on samples collected before and after each 21-day dietary intervention and the acute meal challenges. Change-scores (baseline – post meal) will be created for all inflammatory biomarkers, and differences between the grass-fed and grain-fed beef group will be determined using paired t-Tests at 5% significance (P<0.05).
Gut Microbiota Profiling (16S rRNA Gene Sequencing)
DNA will be extracted (~10 mg) from stool samples using a QIAamp Fast DNA Stool Mini Kit (Allen et al., 2019). Amplified PCR libraries will be sequenced from both ends of the 250 nt region of the V4-V5 16S rRNA hypervariable region using an Illumina MiSeq. DADA2 and Quantitative Insights into Microbial Ecology [QIIME] 2.0 will be utilized for amplicon processing, quality control, and downstream taxonomic assignment using the ribosomal RNA database SILVA. Gut bacterial proportions will be normalized by log transformation after genus level taxonomy, and analyzed for differences between diets using paired t-Tests at 5% significance (P<0.05) (Allen et al., 2019).
Metabolomics
In partnership with Duke University (Durham, NC) and ARS, Metabolon Inc (Cary, NC) will perform the untargeted metabolomics analysis of forage, beef, and plasma samples (mView+ global metabolomics, see letter of support). Untargeted metabolomics will provide information on various metabolites—terpenoids, phenolics, carotenoids, tocopherols, nucleotides, xenobiotics, vitamins, minerals, lipids, amino acids, sugars, peptides, and organic acids—in feed/forage (feed metabolome), beef (food metabolome), and the plasma of consumers (human metabolome). Two types of statistical analyses will be conducted: (1) significance tests and (2) classification analysis. Following log transformation, Benjamini-Hochberg adjusted p-values at 5%, to account for false discovery rates will identify metabolites that differ significantly between sample metabolome profiles. Classification analyses will include principal component analysis (PCA) and hierarchical clustering (heatmap analysis) to determine the primary metabolites contributing to the discrimination between groups. Bioactivities and potential health effects of annotated metabolites from the metabolomes will be explored by using the FooDB and/or PubChem databases, while metabolic pathway identification will use the Kyoto Encyclopedia of Genes and Genomes (KEGG). Statistical analyses will be performed in SAS 9.4 or R (cran.r‐project.org/).
Power Calculations
Using nontargeted metabolomics for our power calculations, we assume a P-value ≤ 0.05, a Q-value ≤ 0.3, and a standard deviation of 0.3 based on previous work (Haro et al., 2016;Pimentel et al., 2018). We typically find 800 metabolites in similar studies and it is expected that between 80 and 120 will be significantly different with a mean of 0.2624 (30% change). An n=16 per group is expected to provide true discovery rates ranging from 74-90 % assuming differences in 80-120 metabolites. Assuming a drop-out of 20%, the final number of participants we will recruit is N=20.
Objective 4: Outreach
Our proposed efforts to disseminate the findings to producers, consumers, and the scientific community are described in detail in the Outreach subsection. All farmers provided key input to the design and rationale of this study, and farmer cooperators will have important roles in disseminating the findings to peers.
References
Allen, J.M., (2019). Frontiers in immunology 10, 1774-1774.10.3389/fimmu.2019.01774
Haro, C., (2016). The Journal of Clinical Endocrinology & Metabolism 101, 233-242.10.1210/jc.2015-3351
Johnson, A.J.,. (2019). Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 25, 789-802.e785.10.1016/j.chom.2019.05.005
Manning, P.J., (2008). Postprandial Cytokine Concentrations and Meal Composition in Obese and Lean Women. Obesity 16, 2046-2052.10.1038/oby.2008.334
Pimentel, G., (2018). Metabolic Footprinting of Fermented Milk Consumption in Serum of Healthy Men. J Nutr 148, 851-860.10.1093/jn/nxy053
Porter Starr, K.N., (2016). The journals of gerontology. Series A, Biological sciences and medical sciences 71, 1369-1375. doi: 1310.1093/gerona/glv1210
Scasta, J.D., (2019). Animal Feed Science and Technology 255, 114219. doi: 114210.111016/j.anifeedsci.112019.114219
Telle-Hansen, V.H., Genes & Nutrition 12, 26.10.1186/s12263-017-0580-4
Van Vliet, S., The American Journal of Clinical Nutrition, 1401-1412. doi: 1410.3945/ajcn.1117.159855.10.3945/ajcn.117.159855
2023 Update
All soil samples, which were collected from the three regenerative agriculture style farms and adjacent conventional style farmland, were analyzed for many conventional and soil health-related attributes and all forage samples have been freeze dried and will be analyzed soon. All grass-fed beef was procured from the three cooperating farms and is now in storage in a freezer at Utah State University. Preparations are underway to start the human feeding trial including obtaining approval from the Institutional Review Board at Utah State University to conduct the trial.
Project Activities
Educational & Outreach Activities
Participation Summary:
No educational or outreach activities were conducted in the first year of this project.