Final report for LNE19-393R
Project Information
Tautog (Tautoga onitis) is a marine wrasse native to western Atlantic communities, and extends from Nova Scotia to South Carolina, with the highest abundances noted from south of Cape Cod to the Delaware Capes. Tautog exhibit strong site fidelity, low daily movement rates, and seasonal migrations, which naturally make this species vulnerable to robust fishing activity, and particularly difficult to rebuild impacted stocks. Regional markets have supported recreational and commercial fishing of tautog for over a century, and tautog is a highly sought after food fish, prized throughout its range for its firm white fillet, which commands a premium on both the processed, fish market and on the live market. Due to its life history characteristics, fish market potential, and fishing pressures, tautog have been identified as an ideal candidate for marine aquaculture. Laboratory studies of tautog conducted by the NOAA laboratory in Milford, CT have shown that adults can be conditioned to induce natural spawning, and successful larval rearing has been accomplished. A nutritional component may be contributing to low growth rates in juvenile tautog, however, other components of food and feeding behavior may also be factors.
The current Northeast SARE project was a collaboration between Ward Aquafarms and the University of Massachusetts Dartmouth which researched the potential of farming tautog, and investigating growout using wild-caught juveniles through three distinct feeding trials. It was hypothesized that slow growth rates determined from otolith samples of wild tautog, were due to resource competition and site fidelity through variable environmental conditions in southern New England waters. A series of trials were conducted to evaluate if tautog exhibit growth rates in an aquaculture setting that equal or surpass those found in the wild, and if to achieve those results, a natural diet would be required. Over three trials it was determined that tautog raised in recirculating aquaculture systems can indeed grow at high growth rates (0.77 mm/day, and growing from 7 grams to 400 grams in just 9 months), though the key component is not related to nutrition or flavor. Coating a commercial pellet with a feed attractant did not lead to increased growth, and utilizing a diet composed of homogenized crustacean meal did not lead to increased growth when compared to a commercially available pellet either.
Tautog in many of the treatments grew at a rapid rate, which equals or surpasses documented growth rates in the wild, and faster than previously published growth rates from aquaculture trials. The difference was within tanks as hierarchies developed, as some fish would out compete and continue to grow rapidly, while other fish would remain underfed due to lack of availability to feed, even when all tanks were fed to satiation. Future work must focus on behavioral and social aspects of tank design and culture criteria, as commercially available pellets were readily consumed and led to economically viable growth rates within the experimental populations. Research directions should focus on reducing in-tank variability through shelter design or feeding protocols, as tautog are an excellent candidate for commercial aquaculture with additional investigations.
The goal of the proposed project is to develop the strategy, diet and growout techniques necessary to farm tautog, which is a high-value fish in the Northeast, using a natural fish feed utilizing green crabs, which are a discarded invasive marine species. Diversifying product offerings is a critical development goal of marine aquaculture farmers, and industry adoption will be facilitated by demonstrating an economically and environmentally sustainable method of farming tautog to interested stakeholders.
The development of new aquaculture species is paramount to the expansion of the domestic aquaculture industry. Currently there are only a handful of well-developed aquaculture species (i.e. Atlantic salmon, trout, shrimp, tilapia, etc.), none of which are well suited to the southern New England environment. Developing local marine species will increase local production which will relieve pressure on wild populations, increase employment and economic opportunities as well as increase the overall production of seafood for local and export markets.
Tautog (Tautoga onitis), also known as blackfish, are a coastal wrasse whose range extends from Massachusetts to Virginia. Throughout their range, tautog is a highly sought-after species by commercial and recreational anglers. Tautog have a firm white fillet which commands a high market price (average $4.35/lb live weight ex-vessel price in Massachusetts in 2017, data from MADMF). While this is a lucrative fishery, supply is limited due to the current state of the resource. The 2016 stock assessment listed all four sub-populations of tautog as overfished, with overfishing occurring throughout much of the range due to the high demand (ASMFC, 2016). Abundance is at, or near historic lows, resulting in severe limitations on the wild fisheries. As such, availability has been limited to high-end restaurants and markets, with a live-market for small fish demanding the highest price.
Tautog (Tautoga onitis) had previously been identified as a candidate for regional aquaculture (Perry et al. 1998). Tautog are a robust and hardy fish, having a wide temperature (4⁰C - 30 ℃) and salinity tolerance. Tautog tolerate high-density culture due to their life history as a reef-oriented species with strong site fidelity and low movement rates (Bigelow and Schroeder, 1953, Olla et al. 1974, Steimle and Shaheen, 1999). As such there was early interest in the development of this species for aquaculture use. The early excitement of this species was due to previous ecological work which indicated that juveniles appeared to exhibit high growth rates (Cooper, 1967).
Spawning induction and larval culture had been successfully done in the laboratory (Perry et al. 1998, Perry et al. 2001, Perry et al. 2008). While the early life history work has been well studied, these trials documented relatively slow growth. Perry et al. (2001) was able to spawn and grow juvenile tautog for 490 days. Within this study, growth appeared to be linear from day 172 to 490 at a rate of 0.29 mm/day. Similar rates were observed at the University of Rhode Island (0.35 mm/day, Perry et al. 2001). Interestingly this contrasts with numerous studies of observed growth in the wild. Sogard et al. (1992) observed juvenile tautog growing at an average of 0.5 mm/day in New Jersey. Dorf et al. (1994) likewise observed growth rates of 0.54 mm/day in Narragansett Bay, Rhode Island. While Martin (1993) observed similar growth in northern fish (0.5 mm/day in New Jersey and Rhode Island), fish in Virginia displayed growth rates of 1 mm/day, double that of their northern counterparts and 3-4 times that of those grown in the lab. The difference in growth rates between northern and southern fish is theorized to be due an elongated growing season in warmer waters (Steimle and Shaheen, 1999, Mercaldo-Allen et al., 2006). The difference in growth between lab and wild fish is highly unusual. Perry et al. (2001) speculated that this could be due to an unknown missing nutritional component. Fish in the labs were fed a standard high protein commercial diet. These diets are typical for most commercial aquaculture species, however most commercial species are piscivorous, unlike tautog. Martin (1993) was able to grow young of the year tautog in a lab at a rate of 0.7 mm/day on a diet of mysids. This indicates that a natural feed may yield significantly improved growth rates.
Green crabs, Carcinus maenas, are an invasive species commonly found along the northeastern Atlantic and Pacific coasts. The establishment of this species has resulted in a significant regional ecological disruption causing damage to local mussel beds, oyster reefs and eelgrass beds (Hughes and Elner, 1979, Grosholz et al., 1995, Young et al. 2017). Most notable has been its local impact on the commercially important soft-shell clam, Mya arenaria. The green crab is a voracious predator of soft-shell clams consuming up to 21.8 clams/crab/day (Floyd and Williams, 2004). In Maine and Massachusetts the annual harvest of soft-shell clam has been shown to be inversely related to the abundance of green crabs (Young et al. 2017). As a result several communities in northern Massachusetts, in collaboration with the Massachusetts Division of Marine Fisheries (MADMF), have established green crab trapping programs. Additionally, the Maine Clammers Association started a “Five to Stay Alive” campaign which is encouraging all commercial clammers to actively fish 5 crab pots to reduce the green crab population. While there has been an effort to create a market for green crab products, there has been little success. The result is most of green crabs are thrown into landfills or composted. The goal of this project was to create an alternative fish feed using green crabs. Preliminary work at the University of Massachusetts Dartmouth has shown that juvenile tautog (as small as 10 g) readily eat chopped green crabs, have good survival and have exhibited good growth. Further improvements in growth are possible with increased feed rations and warmer temperatures (compared to the current ambient temperature), and results indicate that it would be feasible to raise tautog to market size (~1 kg) in 18-24 months.
Cooperators
- (Researcher)
- (Researcher)
- (Researcher)
Research
Hypothesis 1: Tautog fed a natural diet will exhibit higher growth rates compared to a commercial fish feed. Will a diet based solely on green crab, or a mixed diet of green crab and commercial feed provide the best growth?
Hypothesis 2: Tautog can be grown to market size in 18-24 months on commercial shellfish farms, in either tanks or netpens. Will both methods of growout be commercially viable, or will fish grown at a higher consistent temperature exhibit higher growth rates, leading to greater economic viability?
Part 1: Determining the impact of a natural diet on the growth and survival of Tautog (Tautoga onitis) in Recirculating Aquaculture Systems
During the month of April 2019, minnow traps baited with green crab were deployed in Megansett Harbor, MA (Figure 1, Location A). Traps were checked and rebaited every other day. However, the use of minnow traps proved highly inefficient with minimal to no weekly captures of tautog. Beach seining proved much more successful. During the months of May and June 2019 a series of beach seines were conducted at Megansett beach in North Falmouth, MA (Figure 1, Location A) and Monk’s Cove in Bourne, MA (Figure 1, Location B). Year 1 tautog ranging from 5.3 cm to 10.2 cm were kept from the beach seines and placed immediately in coolers upon capture. All bycatch was released back to the water at the respective sampling locations (Figure 1). Tautog within desired size rang (5 to 10 cm) were then transferred to lantern nets in Fiddlers Cove Marina in North Falmouth, MA, which in the same proximity as the Megansett Harbor sampling location (Figure 1, Location A).
All captured tautog were kept in lantern nets for at least 24 hours prior to transportation to UMASS Dartmouth wet lab location in New Bedford, MA. Until the required number of tautog were acquired (450 individuals) via beach seining, they were held in a flow through system at the wet lab facilities in New Bedford, MA. Once over 450 tautog had been transferred to the wet lab facilities, the tautog were divided into three separate treatment diets: 1) Natural: green crabs only; 2) Zeigler: commercially available marine finfish diet; 3) Natural + Zeigler: green crabs supplemented with commercial feed. At the initiation of the experiment, the 450 tautog used had a mean length of 7.9 cm (SE±0.1) ranging from 5.3 to 10.2 cm/individual and a mean weight of 7.3 grams (SE±0.2) ranging from 2 to 19 grams/individual. Each treatment was composed of three replicate 75L flow through tanks housing 50 tautog each. The replicate tanks had controlled temperature (~25oC), salinity (30-32 psu), lighting (12L:12D) and aeration. Every 30 days, all tautog from each replicate were sampled for total length (cm) and weight (g). Additionally, while the tautog were being measured from each replicate, the tanks housing the replicates being measured were scrubbed of algae and then drained to promote aquaria cleanliness. Once tanks had fully drained and had fresh saltwater flow, the sampled tautog were returned to the respective tanks.
Feeding amounts were determined based on satiation and administered at each feeding interval. Increases in feeding were decided based on percent growth and satiation. Satiation was determined by whether there was any left-over food left in the tanks after feeding. Table 1 shows the 3 different commercial pellet feeds that have been used up to this point in the study. From July to October, we used the ‘starter with Vpak’, and we fed 10 grams per feeding, twice a day. For the first two weeks in October, we used a half and half blend of the starter feed with the fingerling starter feed to ensure that the fish of different sizes were receiving food. From October 17, 2019 to January 4, 2020 the diet used was the ‘fingerling starter with Vpak’. The fish received 20 grams of this food, per feeding. Starting on January 5, 2020, we began to use the ‘Hi-Performance’ marine finfish food from Zeigler. The amount was 30 grams of food, twice a day. The amount of food was determined by 2% of the total body weight and observational record of uneaten food in the tanks.
|
Zeigler Commercial Marine Finfish Food |
||||||
|
|
Protein (%) |
Fat (%) |
Fiber (%) |
Moisture (%) |
Ash (%) |
Phosphorous (%) |
|
Starter w/ VPak (crumble #2) |
55 |
15 |
1 |
12 |
12 |
1.8 |
|
Fingerling starter w/ VPak (1.5-2.0 mm) |
50 |
15 |
1.5 |
12 |
12 |
1.8 |
|
Finfish Hi-Performance (2.5 mm) |
45 |
16 |
1.3 |
12 |
10 |
1.4 |
Table 1: Zeigler commercial feed composition and size used in this study
The amount of crab was determined by a total of 40% of the total weight of the fish, while also keeping track of whether or not all the food was being eaten in the tanks. Feeding to satiation while avoiding excess food in the tanks was important to ensure high water quality and growth. The amount of crab administered at each feeding, per month, is shown below in Table 2.
|
Month |
Crab (g) |
|
July |
200 |
|
September |
400 |
|
October |
650 |
|
November |
650 |
|
December |
1000 |
|
January |
1000 |
Table 2: amount of crab administered each month, per feeding
Statistical analyses were conducted in RStudio. Data of the mean daily growth rates, both in weight and length, of tautog from all three treatment groups for each sampling month were first tested for normal data distribution using a Shipiro-Wilks normality test. If data was normally distributed, an ANOVA was first conducted, and if a significant difference was present in the treatment groups, a Tukey post-hoc test was conducted. If the data was not normally distributed, then a Kruskal-Wallis test was conducted followed by Pair-Wise Wilcoxon post-hoc test.
Part 2: The Effects of Feed Attractants on the Growth of Tautog (Tautoga onitis) in Recirculating Aquaculture Systems
Worldwide, aquaculture growth and development are greater than any other food production sector, while the FAO monitoring of marine fishery resources has shown that landings are continuing to decline (FAO 2018). With declining fishery resources, the world must face the challenge of feeding its growing population. The growing industry of aquaculture has a wide range of impacts, and contributes to food security, employment, enhanced resource management, and sustainable farm practices (Halwart et al., 2003) Sustainable farm practices are a key initiative for stakeholders, managers, and farmers moving forward. Limitations in production, high prices of fish meal and fish oil, and increasing concern for environmental impact have prompted a desire to seek alternatives to fuel the aquaculture industry (Nagel et al., 2017;Tantikitti 2014;). Feed alone contributes to more than 50% of the cost production in intensive aquaculture systems, and is often the single greatest expense in aquaculture systems (Pavadi et al., 2012; Fast et al., 1997) Aquaculture expansion is reliant on systems that favor readily available species that are preferred for local consumption (Halwart et al., 2003). In the United States, this is a challenge worth addressing in terms of ecological impacts and sustainability of the growing aquaculture initiative.
Tautog (Tautoga onitis) is a marine wrasse native to western Atlantic communities, and extends from Nova Scotia to South Carolina, with the highest abundances noted from south of Cape Cod to the Delaware Capes (Bigelow and Schroeder 1953). Tautog exhibit strong site fidelity, low daily movement rates, and seasonal migration (Olla et al., 1974). These life history characteristics make this species vulnerable to fishing activity. From the most recent stock assessment produced by the Atlantic States Marine Fisheries Commission, (ASMFC) tautog have been identified as a species that is recreationally and commercially overfished, and overfishing is occurring (ASMFC 2017). Regional markets have supported recreational and commercial fishing of tautog for over a century. Records have shown that tautog appeared in fish markets in Boston as early as 1814, and in Wellfleet, hook and line fisheries have existed at least since 1839 (Bigelow and Schroeder 1953).
Due to its life history characteristics, fish market potential, and fishing pressures, tautog have been identified as an ideal candidate for marine aquaculture (Perry et al., 1998). Laboratory studies of tautog have shown that adults can be conditioned to induce natural spawning, and success has been noted in multiple locations in Rhode Island and Connecticut (Perry et al., 1998). A year-round supply of high-quality, market preferred fish would boost regional economies, and could relieve fishing pressures. Growth rates in laboratory studies have been shown to be significantly lower than in the wild, and authors have acknowledged a potential missing component in the commercial feed (Perry et al., 2001). A nutritional component may be contributing to low growth rates in juvenile tautog, however, other components of food and feeding behavior may also be a factor.
Poor attract -ability and palatability may be responsible for poor ingestion rates in some fish species (Ajiboye et al., 2012). In order to enhance these aspects of the finfish diet at a commercial scale, feed additives may provide a solution. Micro-bound particles allow for nutrients to bind and create a stable feed, examples of these include alginate, agar, carrageenan, starch, zein, and gelatin. This approach offers the advantages of economic viability and the not using toxic ingredients in order to make the food (Langdon 2003). Feed attractants have the potential to reduce feed waste, promote growth, and subsequently enhance the economic success of finfish aquaculture (Pavadi et al., 2012).
Various studies have documented the trials of feed attractants on various species that have also been noted to have regional economic importance and market potential. In particular, hydrolysis has been employed as a promising way for the conversion of fish-by-products into new and suitable forms (Khosravi et al., 2015) Low molecular weight and well balanced amino acid profiles have made hydrolysates a viable chemo-attractant and fish meal replacement in aquafeeds(Aksenes et al., 2006a; Kolkovski et al., 2000). Gelatin bound squid hydrolysates have been used in the aquaculture of cod and octopus with success (Kvåle et al., 2006; Quintana et al., 2008). Hydrolysis breaks down large protein chains into peptides and free amino acids. Feeding behavior is stimulated by low molecular weight substances with higher solubility(Løkkeborg et al., 2014) Inclusion of hydrolysates as protein replacement may have a two-fold effect; one being that ingestion rates become higher and two being that there is higher assimilation due to the free amino acid and short peptides ( Kolkovski et al., 2000).
Olfactory and gustatory systems are the main centers for chemoreception in fish, odors may trigger food search, gustatory system may reject the object after tasting it (Løkkeborg et al., 2014). Feed attractants are chemicals that orient an animal towards the source of the chemicals (Pavadi et al., 2012). Feed palatability in carnivorous aquaculture species shares a strong relationship with attractants that are associated with the prey components under wild conditions (Tantikitti 2014). When considering potential hydrolysates for tautog aquaculture, green crab may provide a solution, due to natural feeding habits. Dietary extracts from the tissues of prey species have been shown to stimulate feeding behavior (Løkkeborg et al., 2014). Further supporting this are studies documenting the success of crustacean meals in several aquaculture species, exhibiting positive results on palatability and growth performance (Toppe et al., 2006; Tibbets & Lall, 2013). In addition, attractants may provide a useful use of an otherwise underutilized marine resource.
Research Objective
The goals of the project were to determine the role of feed attractants in the growth of tautog in recirculating aquaculture systems. In order to achieve this research goal, the project identified alternative sources of protein to inform the aquaculture community of potential replacements for fish meal and fish oil as a primary protein source. This portion of the project has also being used for the MSc thesis for graduate student Michael Coute to be completed at the University of Massachusetts Dartmouth School for Marine Science & Technology.
Methods
In June 2020, 450 year-1 (5-15 g) tautog were collected locally from Buzzards Bay, which hosts a relatively large population of fish which can be obtained by beach seine. The fish were brought to UMASS Saltwater Facility (New Bedford, MA) and introduced to a tank with flow-through seawater to acclimate. After 14 days, the fish were introduced to a heated saltwater recirculating system of 300-gallon aquaria with controlled temperature (20° C), lighting (12L:12D), constant salinity (30-32 psu) and aeration. The fish were separated into one of the three treatments (135 fish per treatment), each tank with 45 fish maintained in triplicate. The tautog were fed to satiation twice daily (08:00, and 16:00), with one of the three diets. Once all the fish were placed into tanks randomly, they were assigned one of three possible feeding treatments: crab hydrolysate, squid hydrolysate, or commercial feed. This resulted in triplicates of each diet. Feed was created on site through the process of hydrolysis. All feed treatments consisted of a 1.5mm standard commercial pellet purchased from Ziegler fish foods (Table 1). The pellets were coated in a non-flavored gelatin to ensure that the test variable is the hydrolysate. The designed feeding trials took place over 12 weeks, with growth measurements for total length and weight taken biweekly to assess growth over time.
Part 3: Determining the performance of Tautog (Tautoga onitis) fed a shrimp-meal based diet.
The first component of this project documented improved growth and survival of tautog when fed a diet of green crab. However, the combination of cost, handling and the negative impacts on water quality made this diet commercially infeasible and non-scalable. This component of the project attempted to address these issues by evaluating an alternative crustacean-based diet. An experimental diet was created using dried shrimp meal. Shrimp meal is a fisheries byproduct in which the carapace of commercial shrimp are dried and ground. Shrimp meal is relatively cheap, accessible, and shelf-stable making it a potential candidate for fish meal replacement. The goal of this experiment was to compare the growth and survival of juvenile tautog fed a shrimp meal-based diet compared to the commercial finfish diet.
Methods
Diet formulation
Dried shrimp meal was procured from Blum and Bergeron, Inc. (Houma, LA). The shrimp meal was produced as a byproduct of the commercial “Gulf shrimp” fishery which may consist of pink shrimp (Penaeus duorarum), brown shrimp (Penaeus aztecus) or white shrimp (Penaeus setiferus). The shrimp meal was formed into shrimp cakes to provide a desired texture and consistency for the fish. Fish cakes were produced by combining 20 grams of dried shrimp meal with 80 grams of water and gelatin. The water and gelatin were used at a ratio of 1 g dried powdered gelatin (Knox Unflavored Gelatin) to 17 g of water. The mixture was placed into silicon molds and was allowed to set in a refrigerator overnight before use. A commercial fish pellet (Zeigler Finfish Starter 3 mm pellet) was used as a control diet. A sample of each diet was submitted to a laboratory (Eurofins Microbiology Laboratories, North Kingstown, RI) for proximate analysis. The nutritional information for each diet can be found in Table 3.
|
|
Marine Finfish Diet |
|
Shrimp Meal |
|
||||||||
|
Mean |
SE |
|
Mean |
SE |
|
|||||||
|
Ash (%) |
6.98 |
0.01 |
|
22.48 |
0.19 |
|
|
|||||
|
Protein (%) |
44.40 |
0.11 |
|
50.34 |
0.45 |
|
|
|||||
|
Nitrogen (%) |
7.10 |
0.02 |
|
8.05 |
0.07 |
|
|
|||||
|
Moisture (%) |
6.27 |
0.03 |
|
12.27 |
0.03 |
|
|
|||||
|
Crude Fat (%) |
17.21 |
0.03 |
|
3.39 |
0.02 |
|
|
|||||
|
Crude Fiber (%) |
2.10 |
0.10 |
|
9.50 |
0.06 |
|
|
|||||
|
NFE (%) |
23.04 |
0.17 |
|
1.48 |
0.25 |
|
|
|||||
|
Calories (kcal/kg) |
3823.67 |
4.41 |
|
2148.33 |
8.41 |
|
|
|||||
Table 3: Nutritional composition of each diet obtained by proximate analysis. NFE stands for “Nitrogen-Free Extract” which represents soluble carbohydrates.
Experimental Design
One hundred and twenty juvenile tautog were selected for use from the previous experiment. The population was restricted to provide a relatively uniform distribution of length and weight. From this population, tautog were randomly placed into one of six tanks (389 liters). Each tank housed 20 tautog and was randomly assigned one of the two feeding treatments (shrimp meal or control). All tanks were on a recirculating seawater system providing consistent and uniform water conditions with respect to temperature (20⁰C) and salinity (35 psu). Dissolved oxygen was monitored daily. Several sections of PVC pipe (4” diameter x 12” length) were placed in the tanks to provide structure which tautog prefer to reside in.
Tautog were fed to satiation twice daily (0900 and 1600). Satiation was determined by observing excess food remaining in the tank during subsequent feedings. Feeding rates were adjusted after each sampling period. Initial feeding rates were based on the consumption observed in previous trials. All excess feed was siphoned out of the tank every morning.
Data Collection and Analysis
To evaluate the growth performance, the length and weight of all fish were measured biweekly with the feeding trials lasting six weeks. A one-way ANOVA was used to evaluate the effects of the treatment on starting weight and length, finishing weight and length, and growth.
Part 1.
Results:
Mean daily growth rates (weight)
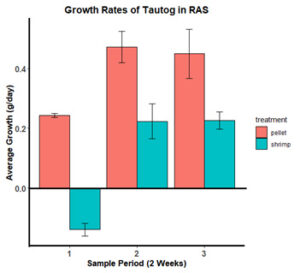
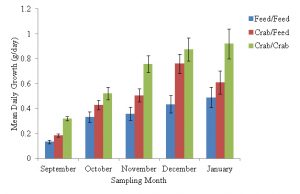
For sampling period one (July 2019 – September 2019), observed mean daily growth rates in weight (g) were 0.32 g/day (SE±0.02) for tautog fed a green crab only diet, 0.19 g/day (SE±0.01) for tautog fed a diet of both green crab and feed and 0.10 g/day (SE±0.01) for tautog fed a feed only diet, which was significantly less than the growth rate observed in the green crab only diet (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p = 0.026, Figure 2). For sampling period two (September 2019 – October 2019), the observed mean daily tautog growth rate was 0.33 g/day (SE±0.04) for the feed only treatment, which was significantly lower than 0.52 g/day (SE±0.05) for the green crab only treatment (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p = 0.026, Figure 2). The mean daily growth rate of 0.43 g/day (SE±0.4) observed for the tautog fed the green crab/feed treatment in sampling period two did not significantly vary from the other two feeding treatments (Figure 2). Mean daily growth rate for sampling period three was 0.76 g/day (SE±0.07) for tautog fed a green crab only diet, 0.51 g/day (SE±0.5) for tautog fed a diet of crab and feed and 0.36 g/day (SE±0.05) for tautog fed a feed only diet, which was significantly lower than the observed tautog growth rates in the crab only and mixed crab/feed treatments (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p < 0.05, Figure 2). In the fourth sampling period (November 2019 – December 2019), tautog fed a feed only diet had a mean daily growth rate of 0.44 g/day (SE±0.07), which was significantly lower than a mean daily growth rate of 0.88 g/day (SE±0.09) for tautog fed a green crab only diet (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, P = 0.0044, Figure 2) and 0.76 g/day (SE±0.08) for tautog fed a mixed diet of green crab and feed (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, P = 0.014, Figure 2). Tautog fed a diet of only green crab in sampling period five (December 2019 – January 2020) had a mean daily growth rate of 0.92 g/day (SE±0.12), which was significantly higher (Tukey HSE, α=0.05, df =2, 410, P = 0.008) than 0.49 g/day (SE±0.08) observed in tautog fed a diet of only feed (Figure 2). Tautog fed a mixed diet of green crab and feed in sampling period five had a mean daily growth rate 0.61 g/day (SE±0.09), which was not significantly different from the growth rates observed in the two other feeding treatments (Figure 2).
Mean daily growth rates (length)
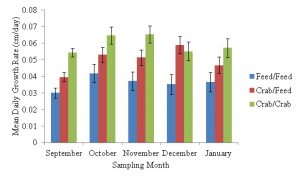
Mean daily growth rate in length (cm/day) for sampling period one (July 2019 – September 2019) did not significantly vary (Kruskal-Wallis Rank Sum Test, χ2 = 5.58, df = 2, n = 410, p = 0.06153, Figure 3). Mean daily growth rates for sampling period one were 0.05 cm/day (SE±0.00) for tautog fed a diet of only green crab, 0.04 cm/day (SE±0.00) for tautog fed both green crab and feed and 0.03 cm/day (SE±0.00) for tautog fed an feed only diet (Figure 3). The mean daily tautog growth rate in length for the second sampling period (September 2019 – October 2019) was 0.06 cm/day (SE±0.01) for the green crab only diet, which was significantly greater (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p = 0.017) than 0.04 cm/day (SE±0.01) observed for tautog fed the feed only diet (Figure 3). However, the observed mean daily growth rate of 0.05 cm/day (SE±0.00) observed for tautog fed the crab/feed diet did not significantly vary from tautog fed a diet of green crab only, and feed only (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p = 0.235, Figure 3). For sampling period three (October – November 2019), the mean daily growth rate was 0.07 cm/day (SE±0.01) for tautog fed a diet of only green crab, which was significantly greater (Pairwise Wilcoxon Rank Sum Test, df = 2, n = 410, p = 0.0048) than 0.04 cm/day (SE±0.01) for tautog fed a feed only diet (Figure 3). Tautog in sampling period three that were fed a mixed diet of green crab and feed had a mean daily growth rate 0.05 cm/day (SE±0.00), which was not significantly different than the growth rates observed in the other two treatments (Figure 3). The mean daily growth rates for tautog fed a green crab only diet and a diet of green crab and feed in the fourth sampling period (November 2019 – December 2019) were both 0.06 cm/day (SE±0.01) and both of which were significantly higher (Tukey HSE, α=0.05, df =2, 410, P < 0.05) than 0.04 cm/day (SE±0.00) for tautog fed a feed only diet (Figure 3). For sampling period five (December 2019 – January 2020), tautog fed a diet of only green crab had mean daily growth rate of 0.06 cm/day (SE±0.01), which was significantly greater (Tukey HSE, α=0.05, df =2, 410, P = 0.0260) than 0.04 cm/day (SE±0.01) observed in tautog fed a diet of only feed (Figure 3). Tautog fed a mixed diet in sampling period five had a mean daily growth rate of 0.05 cm/day (SE±0.00), which was not significantly different the mean daily growth rates observed in tautog fed either a diet of only green crab or a diet of only feed (Figure 3).
Discussion:
Results from this study indicate that the use of green crab in the diet of aquacultured tautog may be a successful method at significantly increasing production yield compared to a diet comprised of currently available commercial fish feeds. In every sampling month, form September 2019 through January 2020, tautog fed a diet of comprised of only green crab had significantly higher daily growth rates in weight (g) than tautog fed comprised diet of only commercially available fish feed. The mean daily growth rates (g/day) of tautog fed a mixed diet of commercially available fish feed and green crab were less than those fed a green crab only diet. However, the growth rates (g/day) of tautog fed a mixed diet were higher than tautog fed a diet of only commercially available fish feed, reinforcing the observation that a diet of green crab may promoting increased production in tautog aquaculture. The significant increase in weight gain (g/day) in tautog fed a green crab only diet compared to tautog fed a diet of only commercially available fish feed may be due to a variety of factors. Factors leading to higher growth rates in tautog fed a green crab diet could be due to the green crab having a higher nutritional value, a more attractive scent, a longer period in which it is desirable for consumption compared commercial pellet food, or a variety of other factors. Instances of mortality have been much higher for tautog fed a diet solely consisting of commercial fish feed. This supports the argument that green crab may have a higher nutritional value. Further research will be needed to elucidate what exactly is leading to increased growth when tautog are fed a green crab diet as well as a way to make processing green crab viable on a commercial production scale.
Part 2:
Weight & Length
Preliminary results were generated using R Programming Language, and Analysis of Variance (ANOVA) tests were used to determine if the weight (g) and length (mm) were significantly different at the end of the feeding trial.
ANOVA tests revealed that length (mm) was not significantly different among tanks (DF=8, Sum Sq= 3259, Mean Sq= 407.4, F Value= 1.145, Pr(>F)= 0.333). Additionally, weight (g) was not significantly different among tanks after running an ANOVA on the final recorded values (DF= 8, Sum Sq= 3827, Mean Sq= 478.4, F Value= 1.335, Pr(>F)= 0.226).
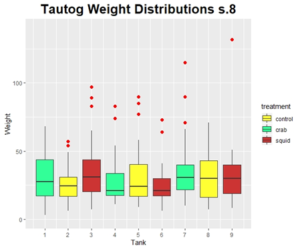
Growth Rates
ANOVA tests were used to confirm no significant differences in the growth rate of tautog in length (mm/day) (DF=8, Sum Sq= 0.0784, Mean Sq= 0.009798, F Value= 0.333, Pr(>F)0.949). The same test was performed for weight gain (g/day) and resulted in no significant differences (DF=8, Sum Sq= 0.0805, Mean Sq= 0.01006, F Value= 0.891, Pr(>F)= 0.53).
Discussion:
Results were determined to not be significant among any of the weights or lengths at the terminus of the feeding trial. It appears that feed attractants do not play a significant role in influencing the growth rates of tautog in recirculating aquaculture systems. Through both parts of our feeding trials so far, we have learned that using green crab as a natural diet is one way to significantly improve the growth rates of juvenile tautog in aquaculture systems, however the amount required of green crab required to produce market quality deliverables would be damaging to water quality in the natural environment or in the recirculating system. Using green crab at this time does not appear to be commercially viable, and further steps must be taken in order to explore and refine the farming techniques for tautog.
Part 3.
Results
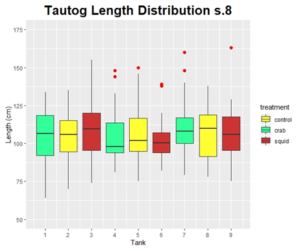
The populations of tautog were not significantly different between treatments at the start of the experiment with respect to weight (F= 0.046, p<0.83) or length (F= 0.034, p<0.86). Fish in the control treatment had a starting length and weight of 146.7 ± 12.6 mm and 69.0 ± 20.3 g, respectively (Table 4). Similarly, fish fed the shrimp diet had a starting length and weight of 146.2 ± 12.3 mm and 68.2 ± 19.6 g, respectively (Figure 8 & 9). A uniform feeding rate was maintained in the control group throughout the trials, as excessive food was observed in each tank throughout the duration of the experiment (Table 4). Tautog exhibited modest feeding attraction to the pellets with the heaviest feeding activity occurring within 30 minutes of introducing food. Conversely, tautog exhibited a strong feeding attraction to the shrimp meal. The feeding rate was increased from 40 g of shrimp meal at the onset of the trials to 60 g during the second sampling period. A final feeding rate of 80 g was used during the final sampling period. No mortality was observed in either treatment.
|
Sampling Period |
Shrimp Treatment |
Control Treatment |
||||||||
|
Weight (g) |
Length (mm) |
Feeding Rate (g/day) |
Feeding Rate (% BW) |
Weight (g) |
Length (mm) |
Feeding Rate (g/day) |
Feeding Rate (% BW) |
|
||
|
Start |
68.2 ± 19.6 |
146.2 ± 12.3 |
- |
- |
69.0 ± 20.3 |
146.7 ± 12.6 |
- |
- |
|
|
|
1 |
66.0 ± 18.6 |
147.2 ± 12.4 |
40 g |
58% |
72.9 ± 22.6 |
149.6 ± 13.7 |
40 g |
58% |
|
|
|
2 |
69.1 ± 19.8 |
148.7 ± 14.2 |
60 g |
91% |
79.6 ± 25.3 |
153.3 ± 14.2 |
40 g |
54% |
|
|
|
3 |
72.2 ± 21.2 |
151.1 ± 13.1 |
80 g |
116% |
85.8 ± 27.7 |
157.2 ± 15.0 |
40 g |
50% |
|
|
Table 4: Average length, weight and feeding rates for the two feeding treatments,
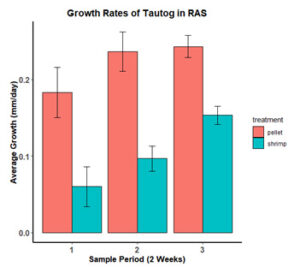
Over the duration of the experiment growth rates averaged 0.39 ± 0.14 g/day or 0.22 ± 0.05 mm/day in the control group and 0.10 ± 0.19 g/day or 0.10 ± 0.05 mm/day in the experimental treatment (p <0.01). Fish in the control group exhibited moderate growth (0.24 g/day and 0.18 mm/day) after the first sampling period with growth rates increasing and remaining consistent between the second (0.47 g/day and 0.23 mm/day) and third sampling periods (0.45 g/day and 0.24 mm/day; Figure 8 & 9). Individuals fed the shrimp diet lost weight (-0.14 g/day) and had reduced growth in length (0.06 mm/day) during the first sampling period. Growth rates increased and remained consistent in both treatments for the remainder of the study period with the experimental treatment growing at half the rate of the control fish (Figures 8 & 9). At the end of the experiment, fish in the control group were significantly larger in both length (F = 5.457, p<0.022) and weight (F = 9.113, p<0.0032) then those fed the shrimp diet.
Figure 8: Average growth rates (g/day) of tautog fed a commercial fish pellet and a shrimp meal diet.
Figure 9: Average growth rates (mm/day) of tautog fed a commercial fish pellet and a shrimp meal diet.
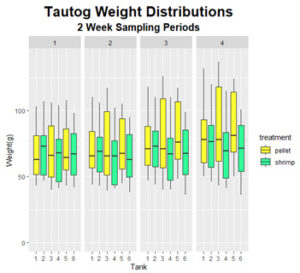
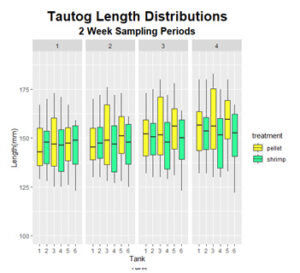
Final weight of fish in the control group ranged from 43 to 137 g with the coefficient of variation (CV) increasing from 0.29, at the start of the experiment, to 0.32, at the end of the experiment. Final fish lengths ranged from 130 to 183 mm with the CV increasing from 0.09 to 0.1. This indicates that the population of fish in the control group grew larger and more variable (Figure 3 & 4). Fish fed the shrimp diet ranged in weight from 36 to 120 g at the end of the experiment with CV’s remaining constant at 0.29. Similarly, final lengths ranged from 122 to 180 mm with the CV’s remaining constant at 0.09. This indicates that the population of fish fed the shrimp diet had moderate but consistent growth between individuals.
Figure 10: Distribution of fish weights observed throughout the feeding trials.
Figure 11: Distribution of fish lengths observed throughout the feeding trials.
Discussion
Overall, the shrimp-based diet elicited a strong feed attraction in tautog. Tautog are a shy, benthic dwelling fish primarily observed residing in the provided shelters. During feeding, fish would aggressively feed in the open and at the top of the tank. Additionally, fish were observed foraging on the shrimp diet several hours after feeding. Despite generating a strong feeding response, the growth of tautog fed the shrimp-based diet was modest. While the same feeding activity was not generated for the commercial fish pellet, the overall growth rates were significantly higher.
The low growth rates observed could have resulted from a lack of adequate nutrition. During the initial sampling period fish exhibited weight loss. The initial feeding rates were based on our previous work with a green crab diet. The shrimp meal was provided at a rate (~50% body weight per day) comparable to those which exhibited high growth in the green crab diet. Data from the nutritional analysis indicated that the shrimp meal contained similar quantities of protein but significantly lower quantities of lipids and carbohydrates resulting in lower caloric intake (Table 5). Increasing the feeding rates to 80 grams per day provided an iso-caloric diet, compared to pellets, but still provided significantly lower levels of lipids and carbohydrates. While growth rates increased, the average rates were still significantly lower than observed in the commercial diet. Additional research should be conducted on the role of lipids and carbohydrates on growth of tautog.
|
Pellet 40g |
Shrimp 40 g |
Shrimp 60 g |
Shrimp 80 g |
|
|
ash(g) |
2.79 |
8.99 |
13.49 |
17.98 |
|
protein(g) |
17.76 |
20.13 |
30.20 |
40.27 |
|
nitrogen(g) |
2.84 |
3.22 |
4.83 |
6.44 |
|
moisture/volatiles(g) |
2.51 |
4.91 |
7.36 |
9.81 |
|
calories(kcal) |
152.95 |
85.93 |
128.90 |
171.87 |
|
crude fat(g) |
6.89 |
1.57 |
2.36 |
3.15 |
|
crude fiber(g) |
0.84 |
3.80 |
5.70 |
7.60 |
|
NFE(g) |
9.22 |
0.59 |
0.89 |
1.19 |
Table 5: Quantities of nutritional components in each of the trial diets.
Additionally, further research should be conducted on improving the feed delivery system. In the wild, tautog feed on crustaceans, shellfish and barnacles. Tautog are observed to bite into prey working the food in and out of their mouths to remove the shell material. The use of gelatin bound the shrimp meal into a semi-solid texture which provided a consistency like the tissue of prey items. While this system proved adequate, fish were observed working the shrimp meal back into a fine powder which littered the tank and remained uneaten. Improving the binding of the shrimp meal may facilitate higher quantities of the food entering the digestive system.
In summary these trials showed that shrimp meal could be a feasible fish meal replacement for tautog however additional research would be required to improve growth rates. Tautog fed the shrimp meal diet displayed a strong feeding response but exhibited modest growth. No mortality was observed in either diet. Future research should focus on providing an enriched diet to further improve growth. Additionally, improving the feed delivery system would be required to reduce waste, reduce cost, and enhance growth.
In the northeastern United States, aside from salmonids, finfish aquaculture expansion has been slow for many decades, and marine finfish research and development, has not translated into a commercial industry. Numerous species have been thoroughly investigated, such as black sea bass, summer flounder, cobia, Atlantic cod, among others, with research into many aspects of culture methods. However, there is at least one primary issue with raising each one of those species commercially; from Atlantic cod taking too long to grow to market size, to cobia costing too much to keep warm, to summer flounder frequently getting disease, and black sea bass being abundant in the wild and driving down market price. There is good reason there is not more marine finfish aquaculture in the northeastern US, and it all comes down to economics, and if the species raised can be cultured at a high density, with low disease, at temperate water temperatures; and critically, if the market demands the product and there is not an abundant supply available.
While many species fit the biological requirements; if there is not a high market demand, and low supply availability, there will not be a commercial opportunity. Black sea bass are a viable aquaculture species from a biological perspective, but they are abundant in the wild, with current boat price in Woods Hole, Massachusetts at $1.25 per pound (July 10, 2020), which would be below the cost of production of even juvenile fish in an aquaculture setting. Tautog (Tautoga onitis) is a marine wrasse native to western Atlantic communities, and extends from Nova Scotia to South Carolina, with the highest abundances noted from south of Cape Cod to the Delaware Capes (Bigelow and Schroeder 1953). Tautog exhibit strong site fidelity, low daily movement rates, and seasonal migrations, which naturally make this species vulnerable to robust fishing activity, and particularly difficult to rebuild impacted stocks (Olla et al., 1974). From the most recent stock assessment produced by the Atlantic States Marine Fisheries Commission, (ASMFC) tautog have been identified as a species that is recreationally and commercially overfished, and overfishing is occurring in 3 of the 4 distinct management areas (ASMFC 2017). Regional markets have supported recreational and commercial fishing of tautog for over a century. Tautog is a highly sought after food fish, prized throughout its range for its firm white fillet, which commands a premium on both the processed, fish market ($15-$22 retail) and on the live market ($11 per pound live).
Due to its life history characteristics, fish market potential, and fishing pressures, tautog have been identified as a promising candidate for marine aquaculture (Perry et al., 1998). Laboratory studies of tautog conducted by the NOAA laboratory in Milford, CT have shown that adults can be conditioned to induce natural spawning, and success has been noted in multiple locations in Rhode Island and Connecticut (Perry et al., 1998). NOAA identified key issues in tautog early life history with transitioning from natural diets of artemia and rotifers to pellet diets. A nutritional component may be contributing to low growth rates in juvenile tautog, however, other components of food and feeding behavior may also be factors. Growth rates in laboratory studies have been shown to be significantly lower than in the wild, and authors have acknowledged a potential missing component in the commercial feed (Perry et al., 2001).
The current project was a collaboration between Ward Aquafarms and the University of Massachusetts Dartmouth which researched the potential of farming tautog, and investigating growout using wild-caught juveniles. It was hypothesized that slow growth rates determined from otolith samples of wild tautog, were due to resource competition and site fidelity through variable environmental conditions in southern New England waters. Over the past year a trial was conducted comparing growth rates of juvenile (5 g) tautog fed one of three diets: a natural diet consisting of green crab (Carcinus maenas), a commercial marine finfish pellet (Ziegler Bros, PA), or a 50:50 ratio of the two diets. The natural diet, and the hybrid diets were very successful in demonstrating a fast growth rate in recirculating systems, as long as feed is sufficient, and water temperature is maintained at 20 C. Juvenile tautog in the natural diet treatment achieved growth rates as high as 0.77 mm/day, and growing from 7 grams to 400 grams in just 9 months. However, water quality suffered as the natural diet was prone to disintegrate in the water and led to adverse health of the fish within the tanks.
A second trial was initiated based on the insights gained in the first trial, which was to use the fact that the tautog showed higher feeding activity when exposed to the natural diet, though the commercial pelletized diet led to greater water quality. Covering the commercial pellets with a hydrolysate coating would change the flavor profile, and the hypothesis was to encourage greater feeding of the commercial pellets when coated with a crustacean or squid flavor. The hydrolysate-coated feeds did not lead to greater feed consumption as compared to controls, though the fish fed commercial pellets did grow at a much faster rate compared to the first trials, while the size variation within groups remained large.
For the third and final trial, it was hypothesized that solidified diet based on crustacean meal would both solve the water quality issues of utilizing a natural diet without any binders, while continuing to build off of the increased feeding behavior noted in fish fed a natural diet as opposed to a commercial pelletized diet. In the third trial, while all fish were fed to satiation, the fish fed the pelletized commercial diet grew faster in both length and weight throughout the trial, and growth rates were comparable to those fish from the natural diet fed in trial 1. The shrimp meal-based diet did not lead to greater increased in growth when fed to the juvenile tautog, and also led to deterioration in water quality.
At the outset of this project, it was hypothesized that the reason previous aquaculture trials of growing tautog did not see adequate growth rates when feeding commercial fish feed pellets had to do with one of two reasons: 1) the existing commercial diets was lacking a nutritional component, or 2) the tautog did not like the flavor of commercial pellets, because they are comprised of fish meal and oil from offshore pelagic fish species, which tautog are unlikely to encounter in their demersal habitat close to shore. In the first trial we determined that growth rates of tautog can be quite high, and as high as other finfish species in development as commercial aquaculture species, though it did appear as though at least part of their required diet had to be from natural sources. However, this find, while encouraging, did not lead us to determine if one of our original hypotheses were correct. In the second trial, we were able to rule out an olfactory reason for choosing the natural diet, as there were no differences in growth between treatments, when coating the commercial pellets with a hydrolysate of the types of food species they would be likely to encounter. The third diet led us to investigate if the reason we did not see equal growth in the first trial was due to a missing nutritional component. By utilizing a homogenized shrimp meal pellet, and having the diets analyzed for proximate composition, we were able to determine the missing component is likely not nutritional either, as the fish fed the commercial pellets grew significantly faster than the shrimp-meal diet.
Throughout the three trials (and a fourth trial funded by a Northeast SARE Graduate Student grant to Michael Coute), all investigators noted significant behavioral differences within the tanks, which changed depending on tank density and variability in fish size. There are clear hierarchies that develop within the tanks, and paradoxically, when at higher densities, the feed anticipatory behavior is higher, and pellet consumption rates are higher. Within each trial, there were fish that grew at or above the average growth rate found in the first trial when feeding a natural diet, and there were also fish that were essentially the same size as the start of the trial at the end of the trial, which is indicative of a lack of feeding. The behavioral interactions regarding feeding preference, shelter preference, and feeding location were apparent, and would dissipate when the fish were all similar sizes when first introduced to the tanks, and would increase over time as hierarchies developed. This was not the anticipated result at the beginning of this work, but is incredibly important for future research.
Tautog were proposed to be a good candidate for commercial aquaculture, and at the conclusion of the project, they have been determined to be an excellent candidate for commercial aquaculture. Tautog are very hardy, and not prone to disease concerns. Their natural slime coat and thick skin makes them conducive to handling without generating abrasions or legions. Average growth rate may be adequate, but those values obscure the wide variability within each tank of those fish with very fast growth rates, and those fish that did not grow at all. The variability in each tank is an artifact of performing research trials over a period of time, without subsequent grading and homogenization of size classes within each tank. Size grading in terrestrial and aquatic agriculture is standard practice, and is the way farmers ensure equal growth across the animals, and avoid a small proportion of very large fish and a large proportion of smaller fish that have not been feeding as robustly. Future work will include evaluating shelter design, hierarchy development, sex differences, tank design changes, and grading protocols to address the behavioral component of tautog farming that leads to size variation. The trials throughout this project were very successful, led to new insights regarding the viability of commercial tautog farming, and have informed the subsequent research goals of the next round of research leading to commercial production.
Education & Outreach Activities and Participation Summary
Educational activities:
Participation Summary:
Outreach activities occurred throughout the project, and were able to occur even with the pandemic-related restrictions on in person tours and meetings. Research assistant Michael Coute was able to produce a poster on the tautog aquaculture work occurring at the University of Massachusetts, and present the poster remotely in fall of 2020. In February of 2021, Dr. Ward presented the results of the project as part of a Massachusetts Institute of Technology senior design class presentation, explaining current aquaculture research and development at Ward Aquafarms, LLC. The Scallop Bay Aquaculture group expressed interest in future aquaculture species under development, and they were given a presentation by Dr. Ward of the results of the current tautog research, and directions for future work. Dr. Ward was able to present the results from the tautog research to a group of NOAA interns and their mentors in July of 2021, and the feedback from the group was good and interest in future tautog work was very high. The Northeast Aquaculture Conference and Expo was canceled in 2020 and 2021 due to the pandemic, and the conference has been rescheduled for January 2022. The results of the work funded by Northeast SARE will be presented at NACE in 2022 as well.
Learning Outcomes
The research proposed in this project was novel, in that tautog had not been previously grown out in recirculating systems to evaluate growth rates, survival and overall potential for growout success beyond the early post-larval stages. Since the outcome was uncertain at the start of the project, the farmers involved in collecting the juvenile tautog, fishermen helping with site selection, and researchers and educators involved in trial design, were all optimistic, but were unsure of how tautog would perform in an aquaculture setting. Not only did the tautog perform well in the trials, but the growth rates and survival throughout all of the trials were higher than anticipated, and certainly higher in the later trials for the fish fed commercially-produced pelleted feeds. Most of the researchers and farmers anticipated that the juvenile tautog would feed and grow well on the natural diet of green crabs, since that at least partially comprises their natural diet in the wild. What was surprising to the farmers and researchers, was how quickly the tautog would take to pelleted feeds once they were acclimated, and how growth rates of the fastest growing fish fed pellets was similar if not higher than the growth rates of fish fed a crustacean-based diet in subsequent trials. The farmers surveyed at the end of the project were encouraged that tautog survive and grow through 400 g at a rate comparable to other cultured marine fishes, and with additional development, tautog may be a viable secondary crop to many farmers throughout New England.
Project Outcomes
An oyster farmer from Cape Cod has been working in the commercial shellfish industry for many years, and has been frustrated by the recent seasonal booms and busts in oyster demand, and the revenue disruption that uncertainty brings to his farm. He was very encouraged by the results of this project, and he is excited at the prospect of adding another crop to his farm to smooth out the revenue swings, and make his entire operation more sustainable.
Information Products
- Coute NESARE tautog poster (Conference/Presentation Material)